Highly Efficient and Sustainable HT@NC/Pd Catalysts for Suzuki Coupling and Their Application in Elacestrant Synthesis
Abstract
:1. Introduction
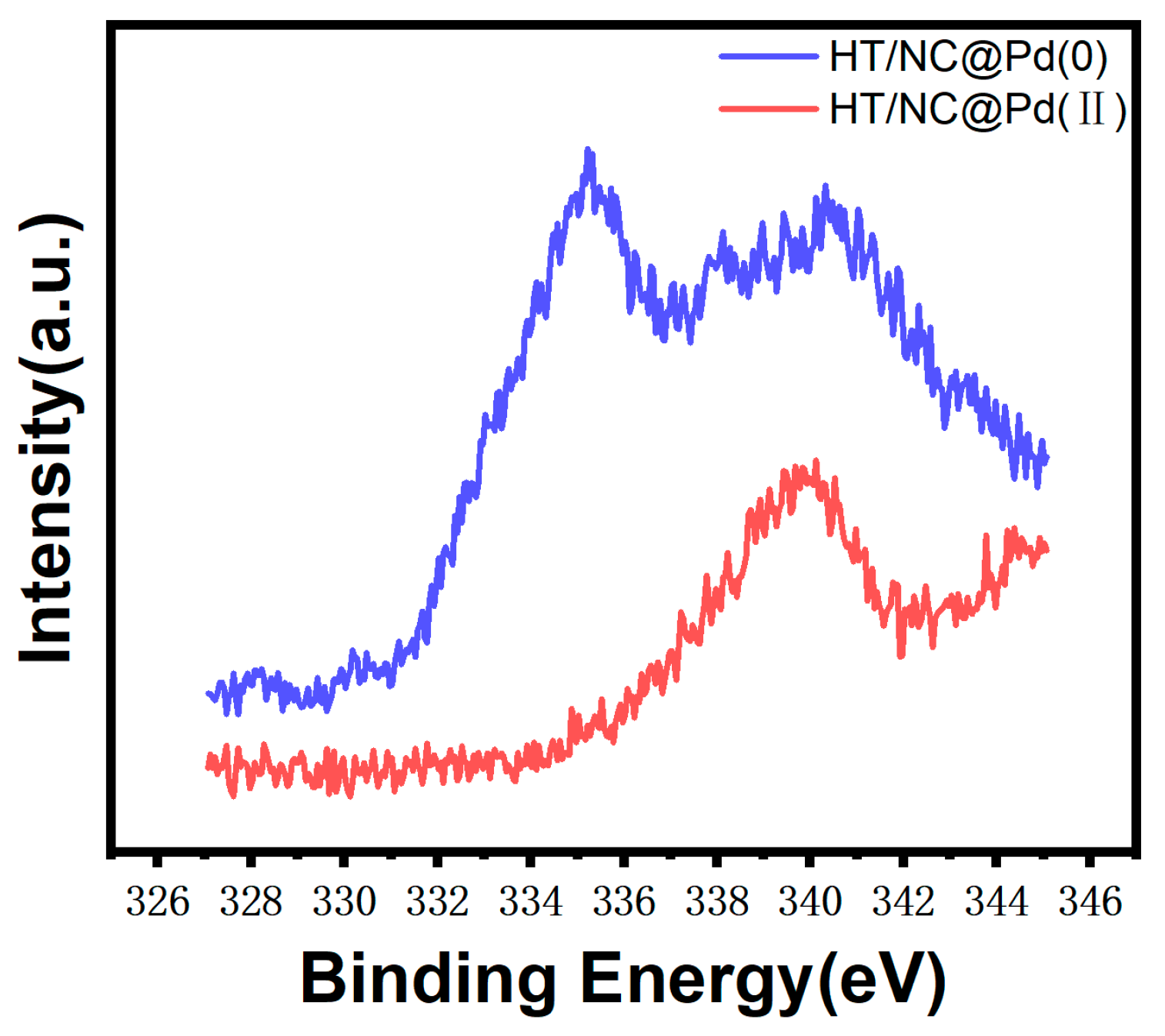
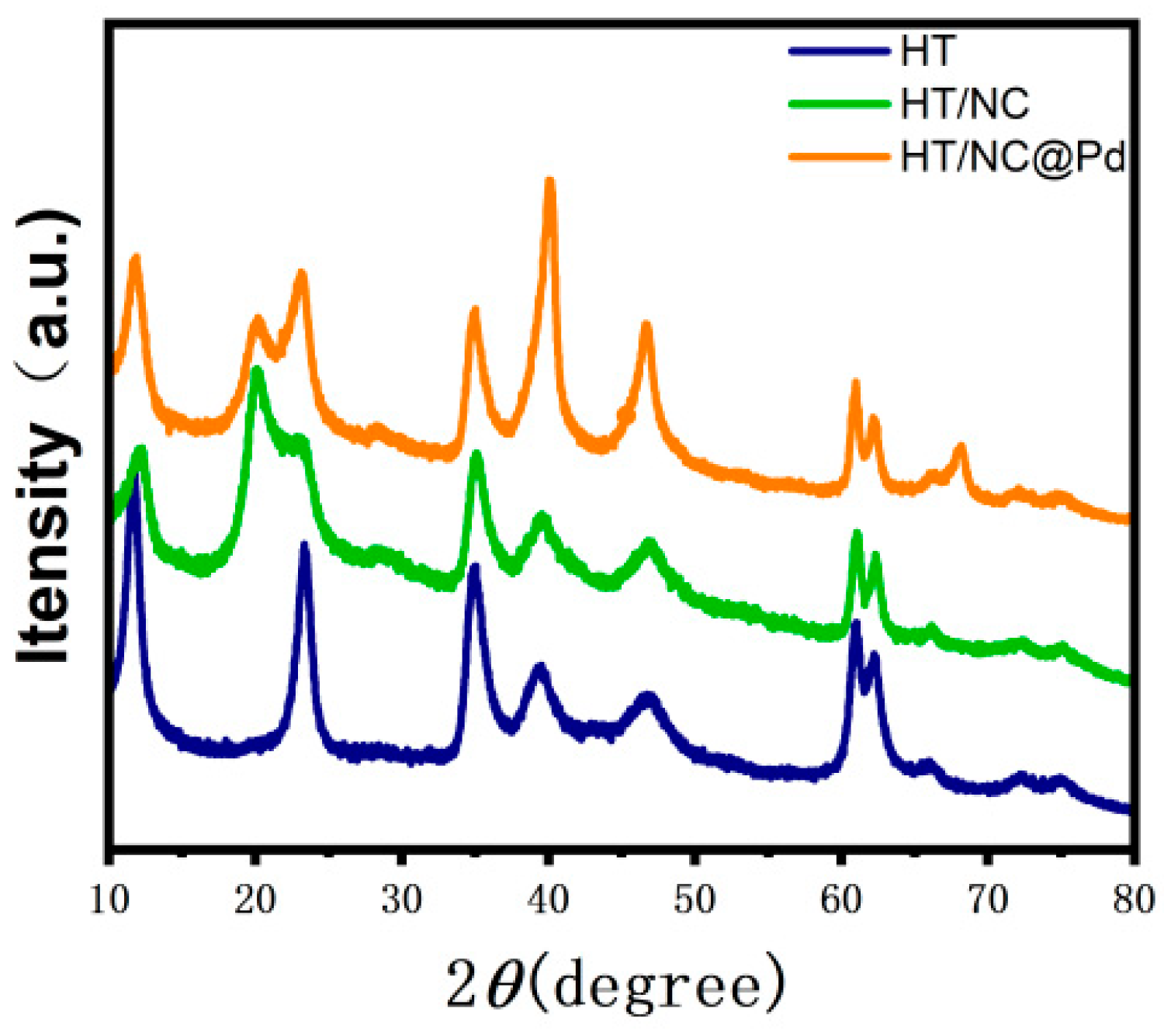
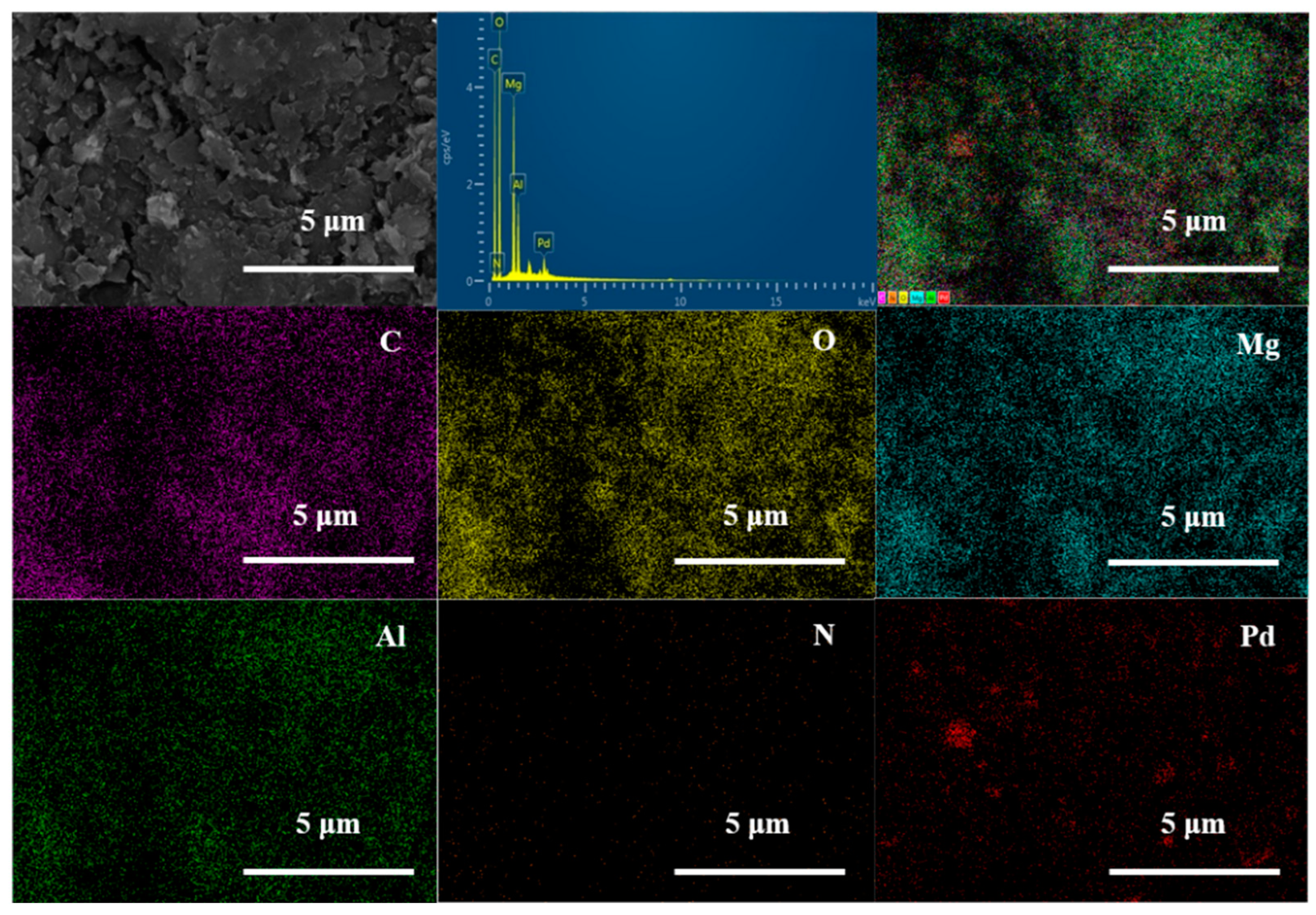
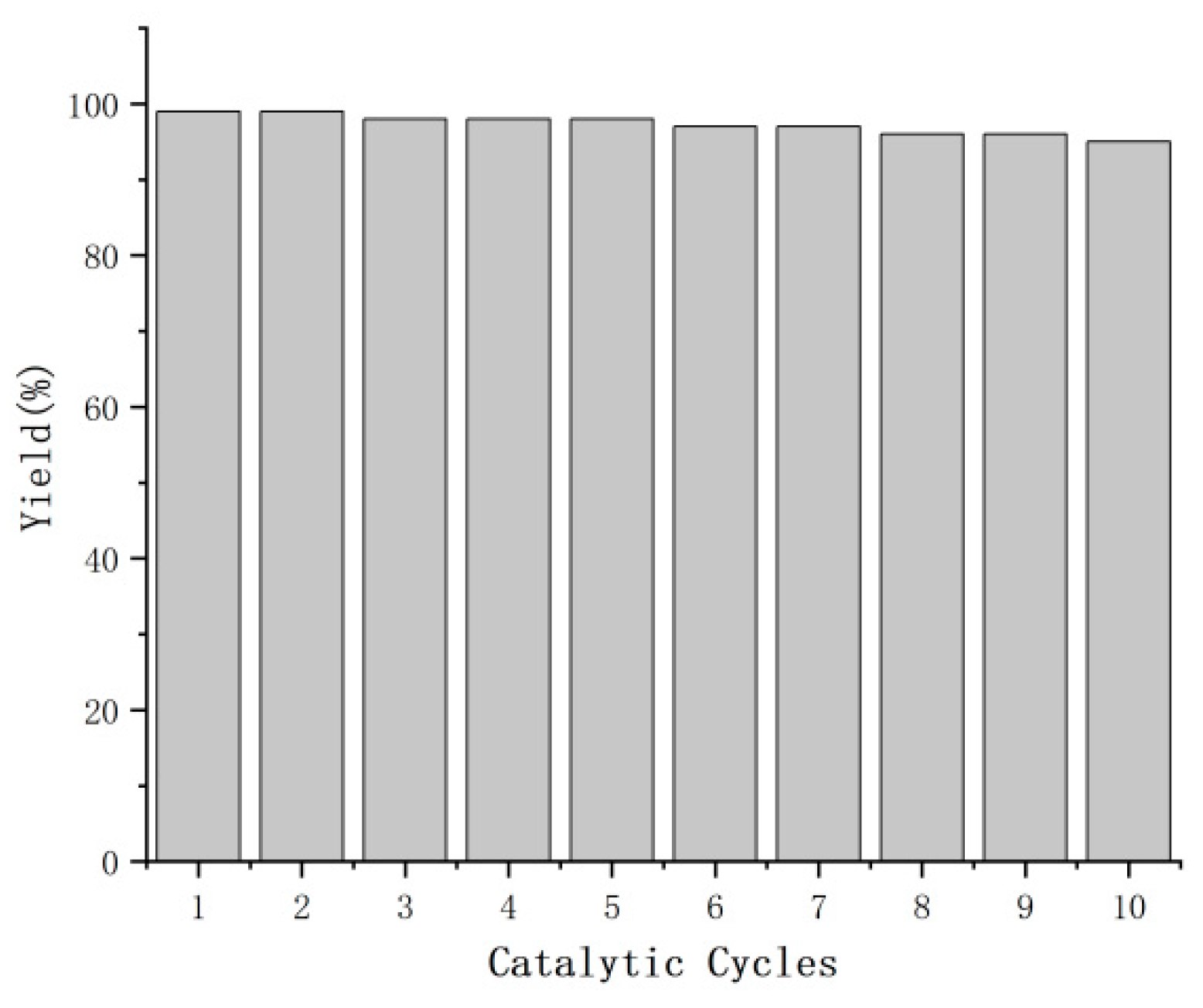
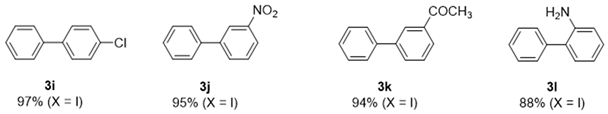
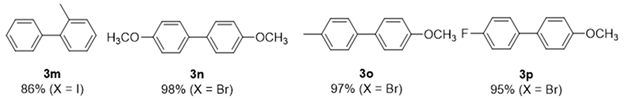
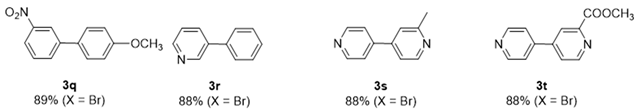
2. Results and Discussion
3. Experimental Materials
3.1. Preparation of HT Particles
3.2. Preparation of HT/Pd Particles
3.3. Preparation of HT@NC Particles
3.4. Preparation of HT@NC/Pd Catalyst
3.5. General Procedure for the Suzuki Coupling Reactions
3.6. General Procedure for Catalyst Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salih, K.S.M.; Baqi, Y. Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond. Catalysts 2019, 10, 4. [Google Scholar] [CrossRef]
- Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Reusable N-Heterocyclic Carbene Complex Catalysts and Beyond: A Perspective on Recycling Strategies. Chem. Rev. 2018, 118, 9843–9929. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhou, H.; Ding, Y.; Wågberg, T.; Han, X. Advances in Bridging Homogeneous and Heterogeneous Water Oxidation Catalysis by Insolubilized Polyoxometalate Clusters. ACS Catal. 2024, 14, 5898–5910. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, Y.; Cao, J. Carbon-Based Material-Supported Palladium Nanocatalysts in Coupling Reactions: Discussion on their Stability and Heterogeneity. Appl. Organomet. Chem. 2020, 34, e5539. [Google Scholar] [CrossRef]
- Xue, J.; Han, R.; Li, Y.; Zhang, J.; Liu, J.; Yang, Y. Advances in multiple reinforcement strategies and applications for silica aerogel. J. Mater. Sci. 2023, 58, 14255–14283. [Google Scholar] [CrossRef]
- Evans, A.M.; Strauss, M.J.; Corcos, A.R.; Hirani, Z.; Ji, W.; Hamachi, L.S.; Aguilar-Enriquez, X.; Chavez, A.D.; Smith, B.J.; Dichtel, W.R. Two-Dimensional Polymers and Polymerizations. Chem. Rev. 2022, 122, 442–564. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Fan, T.; Li, X.; Ji, J.; Dong, P.; Baines, R.; Shen, J.; Ye, M. Magnetic Core-Shell to Yolk-Shell structures in pal-ladium catalyzed Suzuki-Miyaura reactions: Heterogeneous versus homogeneous nature. ChemPlusChem 2016, 81, 564–573. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Qian, K.; Huang, W. Metal–Support Interactions in Metal/Oxide Catalysts and Oxide–Metal Interactions in Oxide/Metal Inverse Catalysts. ACS Catal. 2022, 12, 1268–1287. [Google Scholar] [CrossRef]
- Kamal, N.A.; Pungot, N.H.; Soh, S.K.C.; Tajuddin, N.A. Facile and green hydrothermal synthesis of MgAl/NiAl/ZnAl layered double hydroxide nanosheets: A physiochemical comparison. Pure Appl. Chem. 2024, 96, 1667–1682. [Google Scholar] [CrossRef]
- Liu, F.; Wang, E.; Wu, C.; Sun, D.; Li, J. Ultrathin CoNi-layered double hydroxide grown on nickel foam as high-performance current collector for lithium-sulfur batteries. J. Solid State Electrochem. 2021, 25, 2033–2039. [Google Scholar] [CrossRef]
- Dong, Z.; Gao, P.; Xiao, Y.; Chen, J.; Wang, W. Pd–Co Nanoparticles Supported on Calcined Mg–Fe Hydrotalcites for the Suzuki–Miyaura Reaction in Water with High Turnover Numbers. Catalysts 2019, 9, 1061. [Google Scholar] [CrossRef]
- Mora, M.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Heterogeneous Suzuki cross-coupling reactions over palladium/hydrotalcite catalysts. J. Colloid Interface Sci. 2006, 302, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, L.; Zhao, M.; Dai, L.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Bamboo charcoal fused with polyurethane foam for efficiently removing organic solvents from wastewater: Experimental and simulation. Biochar 2022, 4, 1–16. [Google Scholar] [CrossRef]
- Mei, Y.; Yang, J.; Lu, Y.; Hao, F.; Xu, D.; Pan, H.; Wang, J. BP–ANN Model Coupled with Particle Swarm Optimization for the Efficient Prediction of 2-Chlorophenol Removal in an Electro-Oxidation System. Int. J. Environ. Res. Public Health 2019, 16, 2454. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Liu, H.; Hrynshpan, D.; Savitskaya, T.; Chen, J.; Chen, J. Effects of carbon nanotube on denitrification performance of Alcaligenes sp. TB: Promotion of electron generation, transportation and consumption. Ecotoxicol. Environ. Saf. 2019, 183, 109507. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, J.; Pan, H.; Hao, F.; Yao, J. Electrochemical oxidation of triclosan using Ti/TiO2 NTs/Al–PbO2 electrode: Reaction mechanism and toxicity evaluation. Environ. Sci. Pollut. Res. 2021, 28, 26479–26487. [Google Scholar] [CrossRef]
- Niu, L.; Liu, W.; Juhasz, A.; Chen, J.; Ma, L. Emerging contaminants antibiotic resistance genes and microplastics in the environment: Introduction to 21 review articles published in CREST during 2018–2022. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4135–4146. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, L.; Yao, J.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Enhanced adsorption and reduction performance of nitrate by Fe–Pd–Fe3O4 embedded multi-walled carbon nanotubes. Chemosphere 2021, 281, 130718. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Liu, H.; Hrynshpan, D.; Savitskaya, T.; Chen, J.; Chen, J. Enhanced denitrification performance of Alcaligenes sp. TB by Pd stimulating to produce membrane adaptation mechanism coupled with nanoscale zero-valent iron. Sci. Total Environ. 2020, 708, 135063. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, W.; Hu, L.; Zhao, M.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Improvement of electron transfer efficiency during denitrification process by Fe-Pd/multi-walled carbon nanotubes: Possessed redox characteristics and secreted endogenous electron mediator. Sci. Total Environ. 2021, 781, 146686. [Google Scholar] [CrossRef]
- Guo, T.; Yue, H.; Ma, C.; Li, S.; Chen, J.; Li, W.; Zhao, J. A mesoscopic fluidic reactor for studying the bioreduction ability of biofilm in a chemical absorption-biological reduction system for NO removal. Biorem. J. 2022, 26, 171–177. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Yuan, B.; Zheng, F.; Wang, Z.; Chen, J. Microbial co-culture mediated by intercellular nanotubes during DMAC degradation: Microbial interaction, communication mode, and degradation mechanism. Environ. Res. 2023, 241, 117613. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Z.; Guo, T.; Hu, L.; Chen, Y.; Chen, C.; Yu, G.; Ma, L.Q.; Chen, J. Pollution characteristics and source analysis of microplastics in the Qiantang River in southeastern China. Chemosphere 2022, 293, 133576. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Mei, Y.; Xia, G.; Lu, Y.; Xu, D.; Sun, N.; Wang, J.; Chen, J. Process Optimization of Electrochemical Oxidation of Ammonia to Nitrogen for Actual Dyeing Wastewater Treatment. Int. J. Environ. Res. Public Health 2019, 16, 2931. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zheng, F.; Wang, Z.; Hayat, K.; Veiga, M.C.; Kennes, C.; Chen, J. Unveiling novel pathways and key contributors in the nitrogen cycle: Validation of enrichment and taxonomic characterization of oxygenic denitrifying microorganisms in environmental samples. Sci. Total Environ. 2023, 908, 168339. [Google Scholar] [CrossRef]
- Yao, J.; Lv, S.; Wang, Z.; Hu, L.; Chen, J. Variation of current density with time as a novel method for efficient electrochemical treatment of real dyeing wastewater with energy savings. Environ. Sci. Pollut. Res. 2022, 29, 49976–49984. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, K.; Zhu, J.; Chen, H.; Zhang, P.; Shen, C.; Cao, J. Self-catalytic photochemical three-component reaction for the synthesis of multifunctional 3,3-disubstituted oxindoles. Mol. Catal. 2024, 565, 114379. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, Z.; Wang, Y.; Chen, C.; Shen, C. Photo-Induced Oxidative C-H Esterification of Quinoxalinones with Arylaldehydes under Mild Conditions. Adv. Synth. Catal. 2025. [Google Scholar] [CrossRef]
- Zheng, K.; He, J.; Zhang, L.; Wang, Z.; Zhang, P.; Cao, J.; Shen, C. Photoelectrochemical upcycling of PVC plastic waste for the synthesis of chlorinated quinolinone derivatives. Org. Chem. Front. 2025. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, J.; Qiao, J.; Tong, J.; Shen, C. Facile Fabrication of Glycosylpyridyl-Triazole@Nickel Nanoparticles as Recyclable Nanocatalyst for Acylation of Amines in Water. Catalysts 2020, 10, 230. [Google Scholar] [CrossRef]
- Shen, C.; Shen, H.; Yang, M.; Xia, C.; Zhang, P. A novel d-glucosamine-derived pyridyl-triazole@palladium catalyst for solvent-free Mizoroki–Heck reactions and its application in the synthesis of Axitinib. Green Chem. 2014, 17, 225–230. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Yu, W.; Zhang, P. A highly active and easily recoverable chitosan@copper catalyst for the C–S coupling and its application in the synthesis of zolimidine. Green Chem. 2014, 16, 3007–3012. [Google Scholar] [CrossRef]
- Yan, X.; Wang, J.; Chen, C.; Zheng, K.; Zhang, P.; Shen, C. Remote Sulfonylation of Anilines with Sodium Sulfifinates Using Biomass-Derived Copper Catalyst. Molecules 2024, 29, 4815. [Google Scholar] [CrossRef]
- Hong, Y.; Xu, J.; Chen, A.; Du, Y.; Wang, G.; Shen, J.; Zhang, P. Visible-Light-Induced Divergent C–H Esterification/Alkylation of Quinoxalin-2(1H)-ones with Aldehydes under Mild Conditions. Org. Lett. 2025, 27, 2526–2531. [Google Scholar] [CrossRef]
- Zheng, K.; Shen, C.; Qiao, J.; Tong, J.; Jin, J.; Zhang, P. Novel Magnetically-Recyclable, Nitrogen-Doped Fe3O4@Pd NPs for Suzuki–Miyaura Coupling and Their Application in the Synthesis of Crizotinib. Catalysts 2018, 8, 443. [Google Scholar] [CrossRef]
- Shen, C.; Qiao, J.; Zhao, L.; Zheng, K.; Jin, J.; Zhang, P. An efficient silica supported Chitosan@vanadium catalyst for asymmetric sulfoxidation and its application in the synthesis of esomeprazole. Catal. Commun. 2017, 92, 114–118. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, E.; Zhang, L.; Zhang, L.; Yu, W.; Xu, H.; Shen, C. Catalyst controlled remote C–H activation of 8-aminoquinolines with NFSI for C N versus C F coupling. Catal. Commun. 2021, 158, 106336. [Google Scholar] [CrossRef]
- Lee, G.; Kang, J.Y.; Yan, N.; Suh, Y.-W.; Jung, J.C. Simple preparation method for Mg–Al hydrotalcites as base catalysts. J. Mol. Catal. A Chem. 2016, 423, 347–355. [Google Scholar] [CrossRef]
- Yang, P.; Ma, R.; Bian, F. Palladium Supported on Metformin-Functionalized Magnetic Polymer Nanocomposites: A Highly Efficient and Reusable Catalyst for the Suzuki–Miyaura Coupling Reaction. Chem. Cat. Chem. 2016, 8, 3746–3754. [Google Scholar] [CrossRef]
- Bihani, T.; Patel, H.K.; Arlt, H.; Tao, N.; Jiang, H.; Brown, J.L.; Purandare, D.M.; Hattersley, G.; Garner, F. Elacestrant (RAD1901), a Selective Estrogen Receptor Degrader (SERD), Has Antitumor Activity in Multiple ER+ Breast Cancer Patient derived Xenograft Models. Clin. Cancer Res. 2017, 23, 4793–4804. [Google Scholar] [CrossRef]
- Yao, J.; Tao, Y.; Hu, Z.; Li, J.; Xue, Z.; Zhang, Y.; Lei, Y. Optimization of small molecule degraders and antagonists for targeting estrogen receptor based on breast cancer: Current status and future. Front. Pharmacol. 2023, 14, 1225951. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Shi, Z.; Li, B.; Yang, L.; Luo, L.; Yang, X. Adsorption of Cr(VI) and Phosphate on Mg–Al Hydrotalcite Supported Kaolin Clay Prepared by Ultrasound-Assisted Coprecipitation Method Using Batch and Fixed-Bed Systems. Ind. Eng. Chem. Res. 2014, 53, 7746–7757. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, K.; Lei, D.; Si, W.; Feng, Y.; Lou, L.-L.; Liu, S. Basicity-Tuned Hydrotalcite-Supported Pd Catalysts for Aerobic Oxidation of 5-Hydroxymethyl-2-furfural under Mild Conditions. ACS Sustain. Chem. Eng. 2016, 4, 4752–4761. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Luo, L.; Chang, Z.; Sun, X. One-pot synthesis and catalyst support application of mesoporous N-doped carbonaceous materials. J. Mater. Chem. 2012, 22, 12149–12154. [Google Scholar] [CrossRef]
- Kumar, B.S.; Amali, A.J.; Pitchumani, K. Mesoporous Microcapsules through d-Glucose Promoted Hydrothermal Self-Assembly of Colloidal Silica: Reusable Catalytic Containers for Palladium Catalyzed Hydrogenation Reactions. ACS Sustain. Chem. Eng. 2016, 5, 667–674. [Google Scholar] [CrossRef]
- Paterova, I.; Slana, M.; Gorlova, O. New efficient catalysts based on hydrotalcite for synthesis of sandalwood-type fragrances. Catal. Today 2024, 427, 114405. [Google Scholar] [CrossRef]
- Ji, W.T.; Yang, J.J.; He, J.; Wang, Y.; Wen, X.P.; Wang, Y. Preparation and characterization of flower-like Mg-Al hydrotalcite powder for suppressing methane explosion. J. Loss Prev. Process Ind. 2022, 80, 104858. [Google Scholar] [CrossRef]


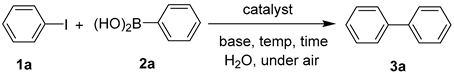 | |||||
| Entry | Catalyst | Base | Temp (°C) | Time (h) | Yield b(%) |
| 1 | - | K2CO3 | 90 | 0.5 | - |
| 2 | HT | K2CO3 | 90 | 0.5 | - |
| 3 | HT@NC | K2CO3 | 90 | 0.5 | - |
| 4 | HT/Pd | K2CO3 | 90 | 0.5 | 95 |
| 5 | HT@NC/Pd | K2CO3 | 90 | 0.5 | 93 |
| 6 | HT@NC/Pd | KOH | 90 | 0.5 | 96 |
| 7 | HT@NC/Pd | K3PO4 | 90 | 0.5 | 92 |
| 8 | HT@NC/Pd | NaOH | 90 | 0.5 | 94 |
| 9 | HT@NC/Pd | Na2CO3 | 90 | 0.5 | 92 |
| 10 | HT@NC/Pd | Et3N | 90 | 0.5 | 71 |
| 11 | HT@NC/Pd | Cs2CO3 | 90 | 0.5 | 83 |
| 12 | HT@NC/Pd | KOH | rt | 0.5 | 66 |
| 13 | HT@NC/Pd | KOH | 50 | 0.5 | 75 |
| 14 | HT@NC/Pd | KOH | 70 | 0.5 | 90 |
| 15 | HT@NC/Pd | KOH | 100 | 0.5 | 95 |
| 16 | HT@NC/Pd | KOH | 90 | 0.5 | 95 c |
| 17 | HT@NC/Pd | KOH | 90 | 1 | 96 |
| 18 | HT@NC/Pd | KOH | 90 | 0.5 | 72 d |
| 19 | HT@NC/Pd | KOH | 90 | 0.5 | 37 e |
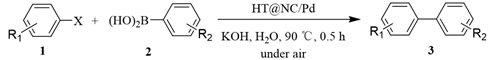 |
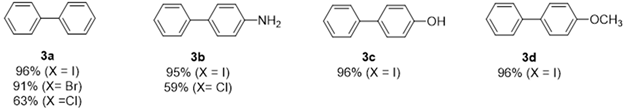 |
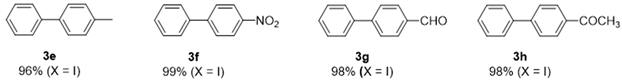 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Liu, M.; Chen, C.; Li, G.; Zheng, K.; Shen, C. Highly Efficient and Sustainable HT@NC/Pd Catalysts for Suzuki Coupling and Their Application in Elacestrant Synthesis. Catalysts 2025, 15, 389. https://doi.org/10.3390/catal15040389
He J, Liu M, Chen C, Li G, Zheng K, Shen C. Highly Efficient and Sustainable HT@NC/Pd Catalysts for Suzuki Coupling and Their Application in Elacestrant Synthesis. Catalysts. 2025; 15(4):389. https://doi.org/10.3390/catal15040389
Chicago/Turabian StyleHe, Jiajun, Muwei Liu, Chao Chen, Guozhang Li, Kai Zheng, and Chao Shen. 2025. "Highly Efficient and Sustainable HT@NC/Pd Catalysts for Suzuki Coupling and Their Application in Elacestrant Synthesis" Catalysts 15, no. 4: 389. https://doi.org/10.3390/catal15040389
APA StyleHe, J., Liu, M., Chen, C., Li, G., Zheng, K., & Shen, C. (2025). Highly Efficient and Sustainable HT@NC/Pd Catalysts for Suzuki Coupling and Their Application in Elacestrant Synthesis. Catalysts, 15(4), 389. https://doi.org/10.3390/catal15040389







