Effective and High-Performance MgFe2O4/Mg-MOF Composite for Direct Methanol Fuel Cells
Abstract
:1. Introduction
2. Results and Discussions
2.1. X-Ray Diffraction Analysis
2.2. Fourier Transform Infrared Spectroscopy Analysis
2.3. Magnetic Properties
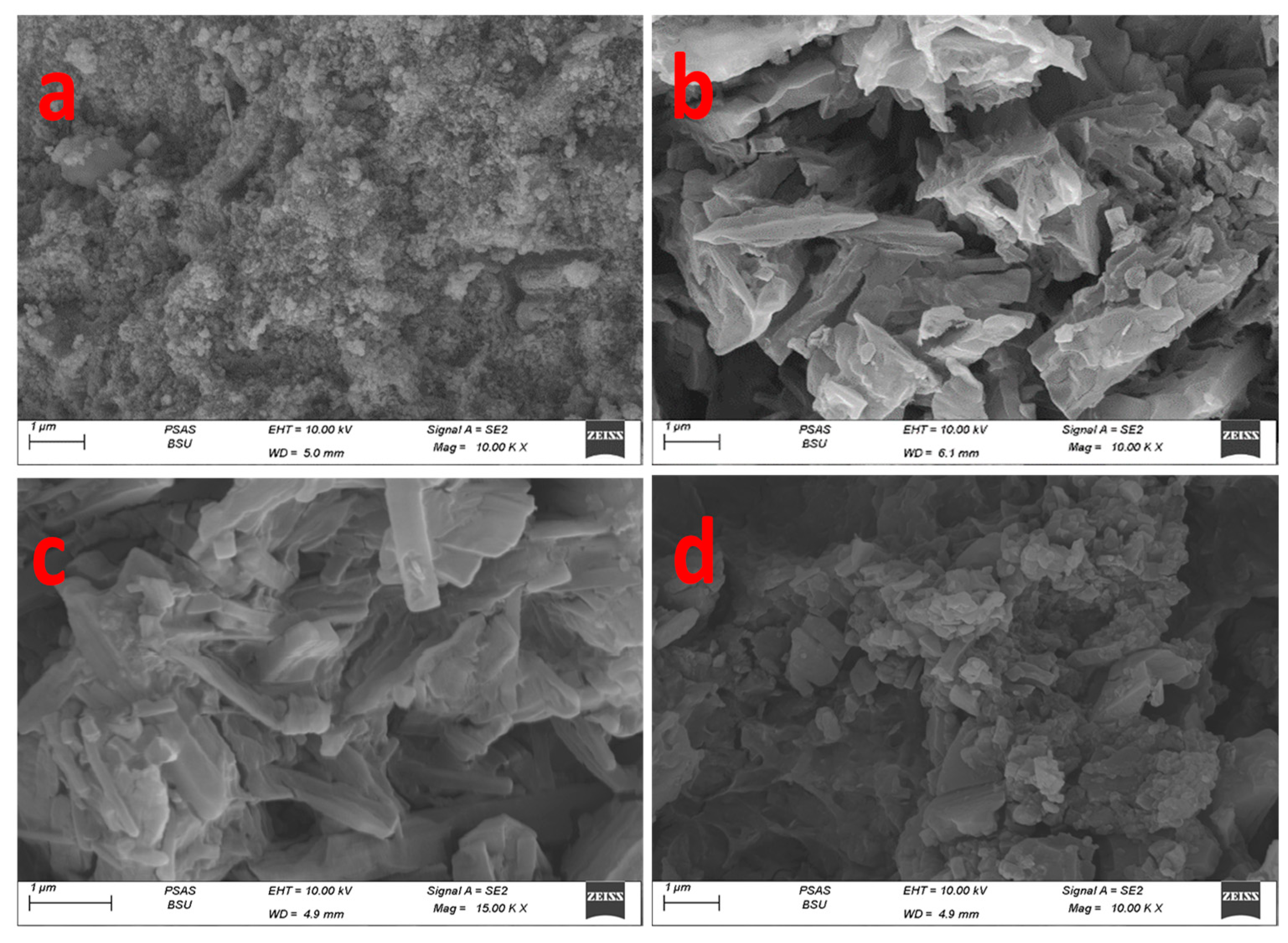
2.4. Morphological Characterization
2.5. BET Surface Area Measurements
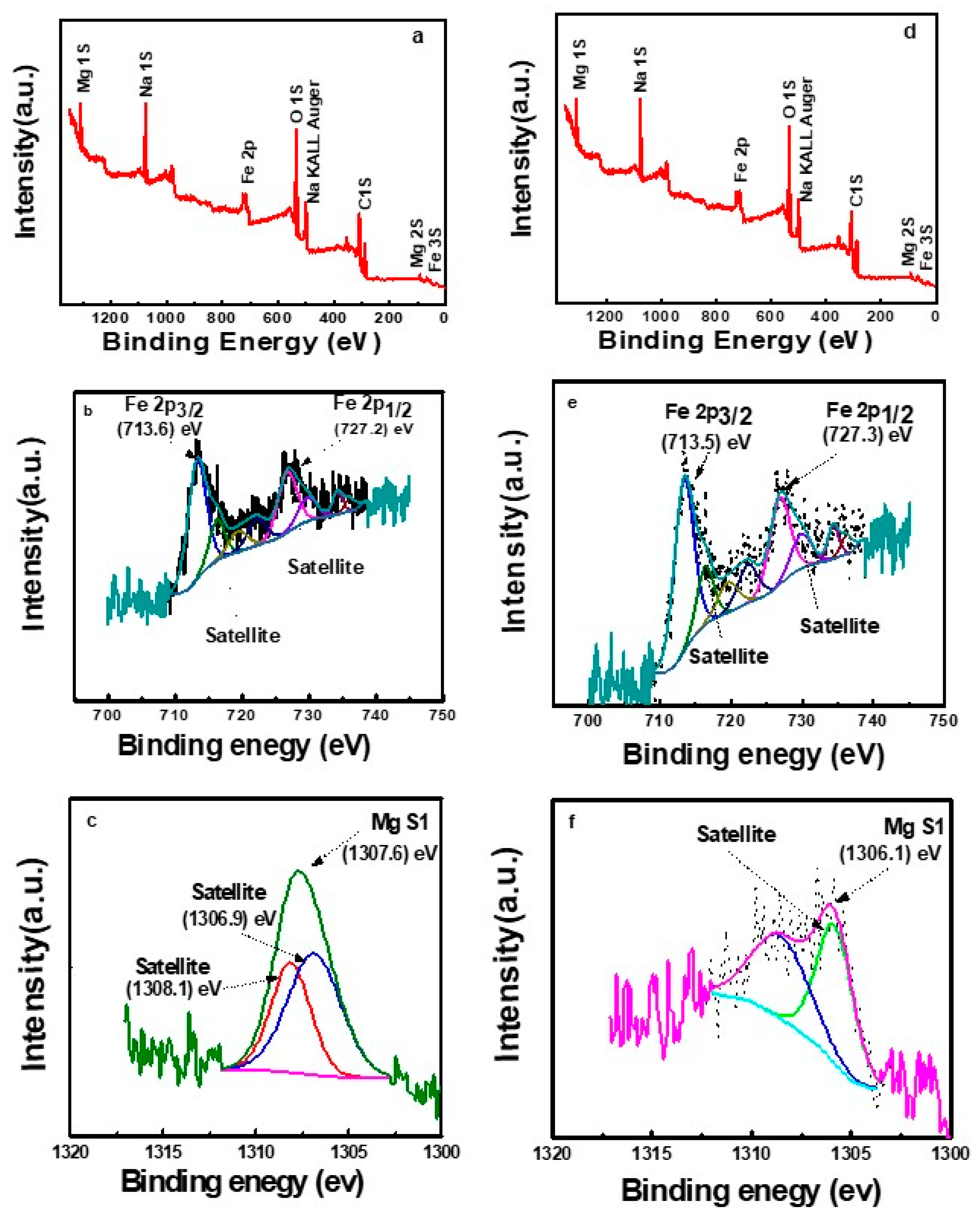
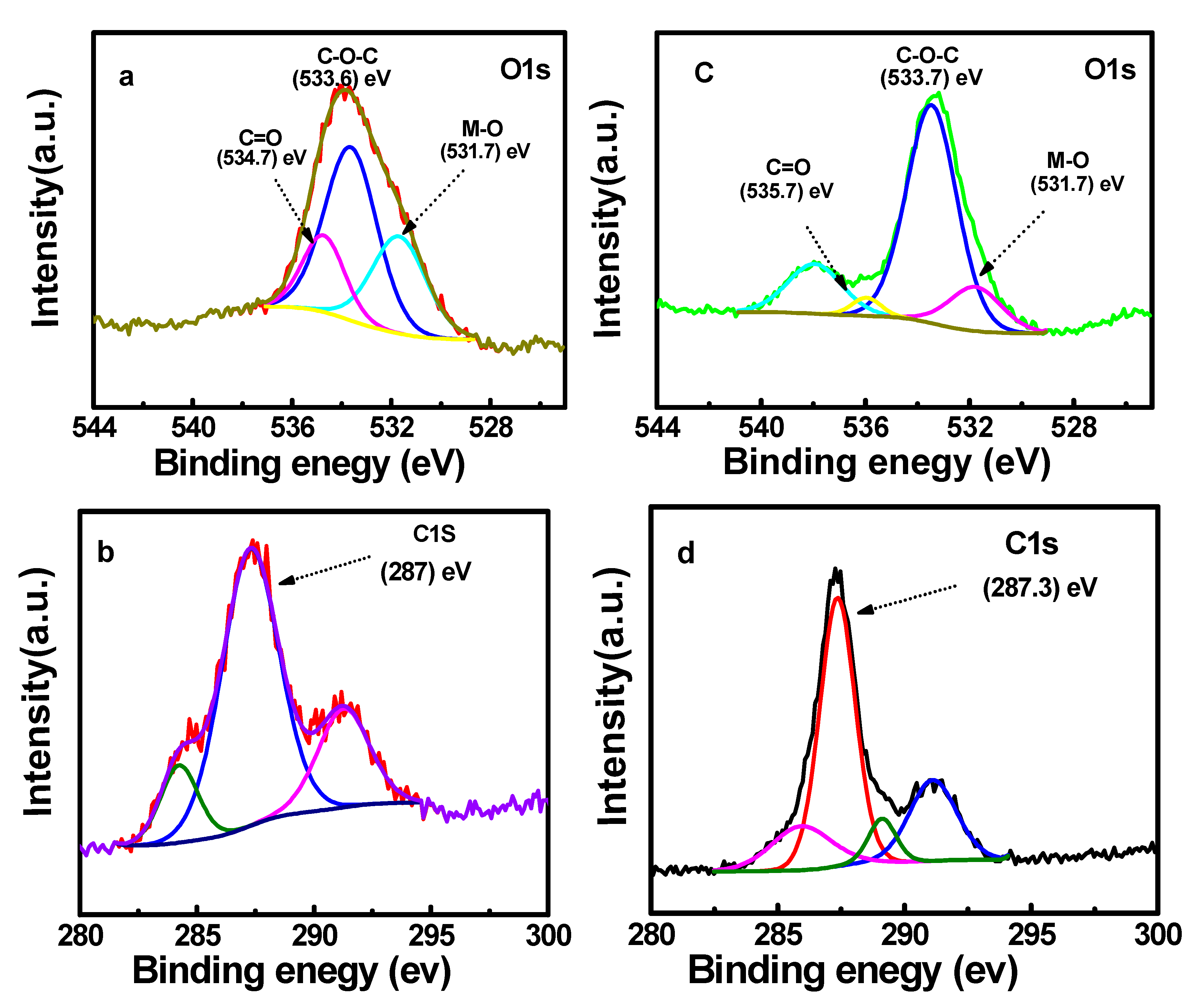
2.6. XPS Spectra Investigation
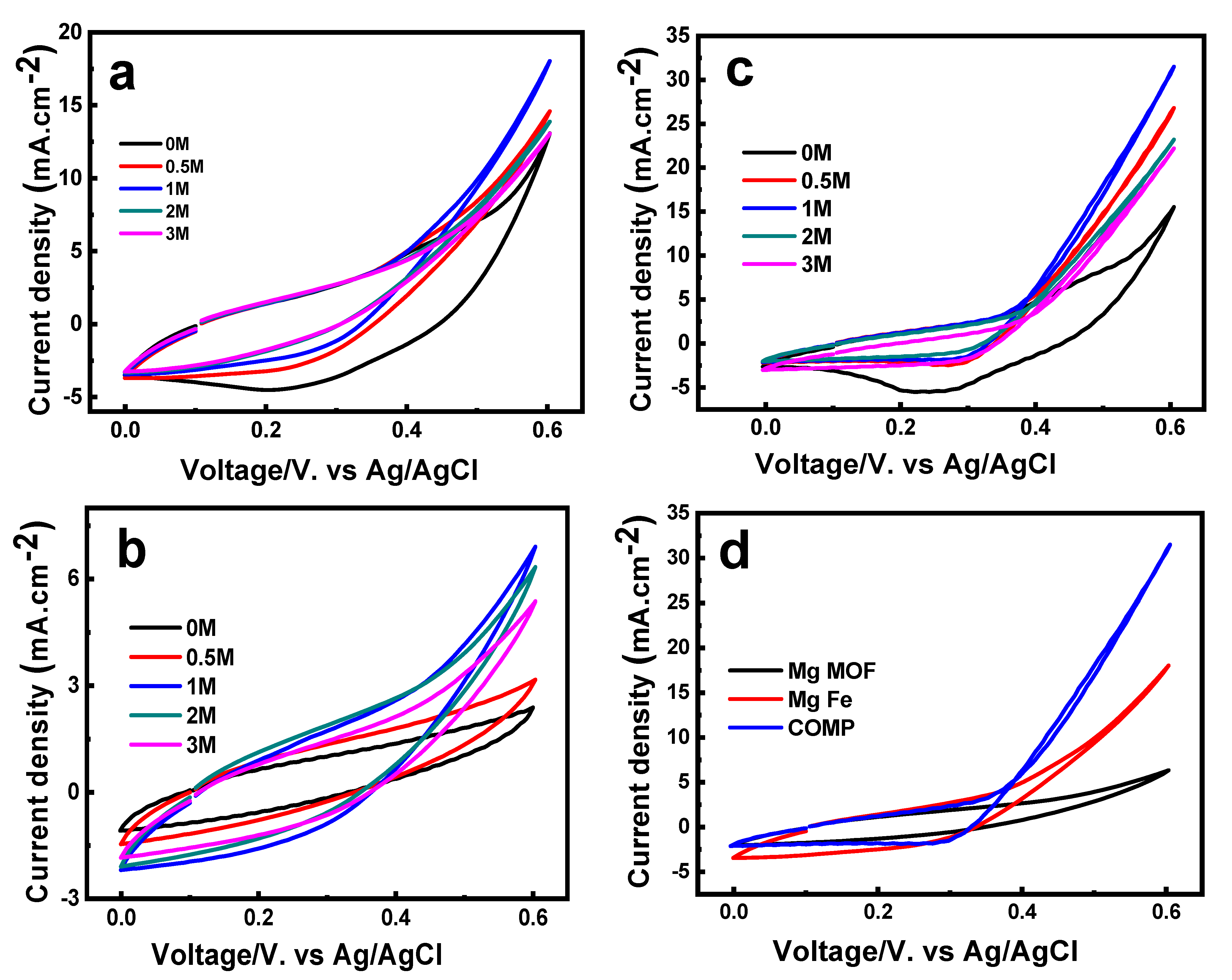
2.7. The Electrochemical Reactions of Prepared Electrodes
2.8. Electrochemical Oxidation Reactions of Methanol (MOR)
2.9. The Electrochemical Reaction and Stability
| Materials | Synthesis Method | Scan Rate (mV/s) | Current Density (mA∙cm−2) | Onset Potential (V) | Reference |
|---|---|---|---|---|---|
| Ni-MOF-74/CPE | Hydrothermal | 25 | 13.46 | 0.70 | [64] |
| MnCo2O4/NiCo2O4/rGO | Hummers Method | 20 | 24.76 | 0.58 | [65] |
| MOF-74(Ni)/NiOOH | Self-Sacrificing Template | 50 | 27.62 | - | [66] |
| Co-MOF-71@GO | Hydrothermal | 50 | 29.1 | 0.60 | [67] |
| Cu@AC(1:1) | Carbonized at 700 °C | 50 | 2.11 | 0.9 | [68] |
| MWCNTs@Ni3S2/CuxS | Solvothermal | 50 | 22.3 | - | [69] |
| Mg-MOF | Ultrasound-Assisted | 100 | 6.9 | 0.360 | This Work |
| Mg Fe2O4 | Co-Precipitation | 100 | 18 | 0.41 | This Work |
| Composite (1:1) | Physical Mixing | 100 | 31.5 | 0.340 | This Work |
3. Experimental Section
3.1. Material
3.2. Synthesis of Magnesium Ferrite (MgFe2O4)
3.3. Synthesis of Mg-MOF
3.4. Synthesis of Magnesium Ferrite (MgFe2O4) and Mg-MOF Composite
3.5. Characterization Techniques
3.6. Fabrication of the Working Electrode
3.7. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahsavari, A.; Akbari, M. Potential of solar energy in developing countries for reducing energy-related emissions. Renew. Sustain. Energy Rev. 2018, 90, 275–291. [Google Scholar] [CrossRef]
- Kumar, A.; Bhansali, S.; Gupta, N.; Sharma, M. Bioenergy and Climate Change: Greenhouse Gas Mitigation. In Prospects of Renewable Bioprocessing in Future Energy Systems. Biofuel and Biorefinery Technologies; Rastegari, A., Yadav, A., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 10, pp. 269–289. [Google Scholar] [CrossRef]
- Adanta, D.; Sari, D.P. Harnessing renewable energy for a sustainable future. Indones. J. Eng. Sci. 2024, 5, 105–107. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Goor, M.; Menkin, S.; Peled, E. High power direct methanol fuel cell for mobility and portable applications. Int. J. Hydrogen Energy 2019, 44, 3138–3143. [Google Scholar] [CrossRef]
- Moreira, C.S.; Pinto, A.M.F.R.; Oliveira, V.B. The Effect of a Reduction in the Catalyst Loading on a Mini Passive Direct Methanol Fuel Cell. Energies 2024, 17, 5174. [Google Scholar] [CrossRef]
- Shi, X.; Dai, Y.; Esan, O.C.; Huo, X.; An, L.; Zhao, T. A Passive Fuel Cell Fed with an Electrically Rechargeable Liquid Fuel. ACS Appl. Mater. Interfaces 2021, 13, 48795–48800. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Tong, Y.; Yan, X.; Liang, J.; Dou, S.X. Metal-Based Electrocatalysts for Methanol Electro-Oxidation: Progress, Opportunities, and Challenges. Small 2019, 17, e1904126. [Google Scholar] [CrossRef]
- Fu, X.; Wan, C.; Huang, Y.; Duan, X. Noble Metal Based Electrocatalysts for Alcohol Oxidation Reactions in Alkaline Media. Adv. Funct. Mater. 2022, 32, 2106401. [Google Scholar] [CrossRef]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-performance Platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep. 2018, 8, 3591. [Google Scholar] [CrossRef]
- Royko, M.M.; Howell, S.; Faegh, E.; Mustain, W.; Lauterbach, J. Influence of Preparation Conditions on Platinum and Palladium Catalysts Supported on Anodically Oxidized Stainless Steel Wire Meshes for CO Oxidation. Emiss. Control. Sci. Technol. 2021, 7, 210–221. [Google Scholar] [CrossRef]
- Lee, D.; Gok, S.; Kim, Y.; Sung, Y.-E.; Lee, E.; Jang, J.-H.; Hwang, J.Y.; Kwon, O.J.; Lim, T. Methanol Tolerant Pt–C Core–Shell Cathode Catalyst for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 44588–44596. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, D.Y. Toward the scaling up of microfluidic fuel cells, investigation and optimization of the aggravated cathode flooding problem. Electrochimica Acta 2016, 222, 312–322. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhai, Y.; Tu, M.; Huang, X.; Shu, M.; Guo, X.; Ying, Y.; Wu, Y.; Wen, Y.; Yang, H. Bimetallic alloy and semiconductor support synergistic interaction effects for superior electrochemical catalysis. Nanoscale 2020, 12, 4719–4728. [Google Scholar] [CrossRef]
- Qin, H.; He, Y.; Xu, P.; Huang, D.; Wang, Z.; Wang, H.; Wang, Z.; Zhao, Y.; Tian, Q.; Wang, C. Spinel ferrites (MFe2O4): Synthesis, improvement and catalytic application in environment and energy field. Adv. Colloid Interface Sci. 2021, 294, 102486. [Google Scholar] [CrossRef] [PubMed]
- Gabal, M.A.; Katowah, D.F.; Hussein, M.A.; Al-Juaid, A.A.; Awad, A.; Abdel-Daiem, A.M.; Saeed, A.; Hessien, M.M.; Asiri, A.M. Structural and Magnetoelectrical Properties of MFe2O4 (M = Co, Ni, Cu, Mg, and Zn) Ferrospinels Synthesized via an Egg-White Biotemplate. ACS Omega 2021, 6, 22180–22187. [Google Scholar] [CrossRef]
- Abraham, A.G.; Manikandan, A.; Manikandan, E.; Manikandan, E.; Vadivel, S.; Jaganathan, S.; Baykal, A.; Renganathan, P.S. Enhanced magneto-optical and photo-catalytic properties of transition metal cobalt (Co2+ ions) doped spinel MgFe2O4 ferrite nanocomposites. J. Magn. Magn. Mater. 2018, 452, 380–388. [Google Scholar] [CrossRef]
- Kannan, Y.B.; Saravanan, R.; Srinivasan, N.; Praveena, K.; Sadhana, K. Synthesis and characterization of some ferrite nanoparticles prepared by co-precipitation method. J. Mater. Sci. Mater. Electron. 2016, 27, 12000–12008. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A.; Zadeh, M.H.R.; Beitollahi, H.; Tajik, S. Hierarchical nanostructures of MgCo2O4 on reduced graphene oxide as a high-performance catalyst for methanol electro-oxidation. Ceram. Int. 2021, 47, 16079–16085. [Google Scholar] [CrossRef]
- Khan, N.; Anwer, A.H.; Khan, M.D.; Azam, A.; Ibhadon, A. Magnesium ferrite spinels as anode modifier for the treatment of Congo red and energy recovery in a single chambered microbial fuel cell. J. Hazard. Mater. 2020, 410, 124561. [Google Scholar] [CrossRef]
- Aglan, H.; Ali, I.A.; Ali, B.M.; Kandil, S.A. Impact of Gd, Pr, Yb, and Nd doping on the magnetic properties of Mg-ferrite nanoparticles. Journal of materials science. Mater. Med. 2025, 36, 17. [Google Scholar] [CrossRef]
- Prasanna, D.; Selvaraj, V. Cyclophosphazene based conductive polymer-carbon nanotube composite as novel supporting material for methanol fuel cell applications. J. Colloid Interface Sci. 2016, 472, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.H.; Kamarudin, S.K.; Basri, S.; Karim, N.A. Potential of 3D Hierarchical Porous TiO2-Graphene Aerogel (TiO2-GA) as Electrocatalyst Support for Direct Methanol Fuel Cells. Nanomaterials 2023, 13, 1819. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, N.; Narjinari, H.; Sharma, H.; Dhole, S.; Jasra, R.V.; Kumar, A. Electrocatalytic Oxidation of Methanol and Ethanol with 3d-Metal Based Anodic Electrocatalysts in Alkaline Media Using Carbon Based Electrode Assembly. Inorg. Chem. 2024, 63, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, Q.; Li, G.; Qian, L.; Zeng, Y. Metal organic framework-derived transition metal-doped CoSx nanocage for enhanced visible light-assisted methanol electrocatalytic oxidation. Phys. Chem. Chem. Phys. 2023, 25, 27331–27341. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, Y.; Zhang, X.; Zhao, M.; Chen, Q.; Wu, Y.; Yu, J.; Pan, Q.; Yang, F.; Lin, H. Construction of Ternary PtRuPd Alloy Supported on Carbon Nanotubes for Concentrated Methanol Oxidation. ChemCatChem 2023, 15, e202300255. [Google Scholar] [CrossRef]
- Narsimulu, D.; Rao, B.N.; Venkateswarlu, M.; Srinadhu, E.; Satyanarayana, N. Electrical and electrochemical studies of nanocrystalline mesoporous MgFe2O4 as anode material for lithium battery applications. Ceram. Int. 2016, 42, 16789–16797. [Google Scholar] [CrossRef]
- Hazri, N.S.; Basri, S.; Kamarudin, S.K.; Zainoodin, A.M. Critical review on development of magnesium alloy as anode inMg-Airfuel cell and additives in electrolyte. Int. J. Energy Res. 2021, 45, 15739–15759. [Google Scholar] [CrossRef]
- Tayyaba; Batool, M.; Nazar, M.F.; Zafar, M.N.; Tahir, A.A. Applications of metal ferrites as photocatalyst for solar fuel production, water splitting and carbon dioxide reduction. In Photoelectrochemical Engineering for Solar Harvesting; Elsevier: Amsterdam, The Netherlands, 2024; pp. 109–139. [Google Scholar]
- Al-Maswari, B.M.; Al-Zaqri, N.; Alkanad, K.; AlOstoot, F.H.; Boshaala, A.; Radhika, R.T.; Venkatesha, B.M. Magnesium Bismuth Ferrite Nitrogen-Doped Carbon Nanomagnetic Perovskite: Synthesis and Characterization as a High-Performance Electrode in a Supercapacitor for Energy Storage. ACS Omega 2023, 8, 16145–16157. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Gong, M.; Chen, A.; Li, L.; Zhang, Z.; Liu, Y.; Dan, N.; Li, Z. Core-shell structure Mg-MOF-74@MSiO2 with mesoporous silica shell having efficiently sustained release ability of magnesium ions potential for bone repair application. J. Non-Crystalline Solids 2022, 600, 122018. [Google Scholar] [CrossRef]
- Li, L. Controlled Memristic Behavior of Metal-Organic Framework as a Promising Memory Device. Nanomaterials 2023, 13, 2736. [Google Scholar] [CrossRef]
- Lu, J.; Nieckarz, D.; Jiang, H.; Zhu, Z.; Yan, Y.; Zheng, F.; Rżysko, W.; Lisiecki, J.; Szabelski, P.; Sun, Q. Order–Disorder Transition of Two-Dimensional Molecular Networks through a Stoichiometric Design. ACS Nano 2023, 17, 20194–20202. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Goodwin, A.L.; Dove, M.T.; Keen, D.A.; Tucker, M.G.; Barney, E.R.; Soper, A.K.; Bithell, E.G.; Tan, J.-C.; Cheetham, A.K. Structure and Properties of an Amorphous Metal-Organic Framework. Phys. Rev. Lett. 2010, 104, 115503. [Google Scholar] [CrossRef]
- D’Angelo, P.; Tavani, F.; Tofoni, A.; Vandone, M.; Busato, M.; Braglia, L.; Torelli, P.; Stanzione, M.G.; Armstrong, R.; Morris, R.; et al. A Combined Soft X-Ray and Theoretical Investigation Discloses the Water Harvesting Behaviour of Mg MOF-74 at the Crystal Surface. 2024. Available online: https://www.researchsquare.com/article/rs-5433792/v1 (accessed on 14 November 2024).
- Waheed, I.F.; Al-Abady, F.M.; Ali, B.M. Synthesis of Mg-Ferrite nanoparticles via auto combustion method and investigation their structural, morphological and magnetic properties. Tikrit J. Pure Sci. 2019, 24, 52–58. [Google Scholar] [CrossRef]
- Bamzai, K.; Kour, G.; Kaur, B.; Arora, M.; Pant, R. Infrared spectroscopic and electron paramagnetic resonance studies on Dy substituted magnesium ferrite. J. Magn. Magn. Mater. 2013, 345, 255–260. [Google Scholar] [CrossRef]
- Gopi, S.; Ramu, A.G.; Yun, K. A highly stable mesoporous spinel ferrite (CoxFe3−xO4) derived from CoFe-MOF for efficient adsorption of ultratrace As(III) ions from aqueous solution. J. Environ. Chem. Eng. 2023, 11, 110106. [Google Scholar] [CrossRef]
- Araújo, J.; Araujo-Barbosa, S.; Souza, A.; Iglesias, C.; Xavier, J.; Souza, P.; Cid, C.P.; Azevedo, S.; da Silva, R.; Correa, M.; et al. Tuning structural, magnetic, electrical, and dielectric properties of MgFe2O4 synthesized by sol-gel followed by heat treatment. J. Phys. Chem. Solids 2021, 154, 110051. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. A designable magnetic MOF composite and facile coordination-based post-synthetic strategy for the enhanced removal of Hg2+ from water. J. Mater. Chem. A 2015, 3, 11587–11595. [Google Scholar] [CrossRef]
- Sarmah, S.; Patra, K.; Maji, P.; Ravi, S.; Bora, T. A comparative study on the structural, magnetic and dielectric properties of magnesium substituted cobalt ferrites. Ceram. Int. 2022, 49, 1444–1463. [Google Scholar] [CrossRef]
- Muratović, S.; Martinez, V.; Karadeniz, B.; Pajić, D.; Brekalo, I.; Arhangelskis, M.; Mazaj, M.; Mali, G.; Etter, M.; Friščić, T.; et al. Low-Dimensional Magnetism in Multivariate Copper/Zinc MOF-74 Materials Formed via Different Mechanochemical Methods. Inorg. Chem. 2022, 61, 18181–18192. [Google Scholar] [CrossRef]
- Kang, M.G.; Kang, H.B.; Clavel, M.; Maurya, D.; Gollapudi, S.; Hudait, M.; Sanghadasa, M.; Priya, S. Magnetic Field Sensing by Exploiting Giant Nonstrain-Mediated Magnetodielectric Response in Epitaxial Composites. Nano Lett. 2018, 18, 2835–2843. [Google Scholar] [CrossRef]
- Yadav, S.; Dixit, R.; Sharma, S.; Dutta, S.; Solanki, K.; Sharma, R.K. Magnetic metal–organic framework composites: Structurally advanced catalytic materials for organic transformations. Mater. Adv. 2021, 2, 2153–2187. [Google Scholar] [CrossRef]
- Geng, B.; Ding, Z.; Ma, Y. Unraveling the correlation between the remanence ratio and the dipolar field in magnetic nanoparticles by tuning concentration, moment, and anisotropy. Nano Res. 2016, 9, 2772–2781. [Google Scholar] [CrossRef]
- Azqandi, M.; Ramavandi, B.; Nasseh, N.; Zaarei, D.; Fanaei, F. Green synthesis of manganese ferrite magnetic nanoparticle and its modification with metallic-organic frameworks for the tetracycline adsorption from aqueous solutions: A mathematical study of kinetics, isotherms, and thermodynamics. Environ. Res. 2024, 256, 118957. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Maji, T.K. Mg-MOF-74@SBA-15 hybrids: Synthesis, characterization, and adsorption properties. APL Mater. 2014, 2, 124107. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. ChemInform Abstract: Mesoporous Metal—Organic Framework Materials. ChemInform 2012, 43. [Google Scholar] [CrossRef]
- Noor, T.; Mohtashim, M.; Iqbal, N.; Naqvi, S.R.; Zaman, N.; Rasheed, L.; Yousuf, M. Graphene based FeO/NiO MOF composites for methanol oxidation reaction. J. Electroanal. Chem. 2021, 890, 115249. [Google Scholar] [CrossRef]
- Shi, K.-X.; Qiu, F.; Wang, P.; Li, H.; Wang, C.-C. Magnetic MgFe2O4/MIL-88A catalyst for photo-Fenton sulfamethoxazole decomposition under visible light. Sep. Purif. Technol. 2022, 301, 121965. [Google Scholar] [CrossRef]
- Mittal, V.; Bera, S.; Nithya, R.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Solid state synthesis of Mg–Ni ferrite and characterization by XRD and XPS. J. Nucl. Mater. 2004, 335, 302–310. [Google Scholar] [CrossRef]
- Mitani, H.; Xu, Y.; Hirano, T.; Demura, M.; Tamura, R. Catalytic properties of Ni-Fe-Mg alloy nanoparticle catalysts for methanol decomposition. Catal. Today 2017, 281, 669–676. [Google Scholar] [CrossRef]
- Glisenti, A.; Favero, G.; Granozzi, G. Reactivity of simple alcohols on Fe2O3powders An XPS and FTIR study. J. Chem. Soc. Faraday Trans. 1998, 94, 173–182. [Google Scholar] [CrossRef]
- Benedet, M.; Rizzi, G.A.; Barreca, D.; Gasparotto, A.; Maccato, C. XPS analysis of graphitic carbon nitride functionalized with CoO and CoFe2O4. Surf. Sci. Spectra 2023, 30, 014004. [Google Scholar] [CrossRef]
- Ullah, N.; Ullah, S.; Khan, S.; Guziejewski, D.; Mirceski, V. A review: Metal-organic framework based electrocatalysts for methanol electro-oxidation reaction. Int. J. Hydrogen Energy 2022, 48, 3340–3354. [Google Scholar] [CrossRef]
- Shi, Q.; He, Y.; Bai, X.; Wang, M.; Cullen, D.A.; Lucero, M.; Zhao, X.; More, K.L.; Zhou, H.; Feng, Z.; et al. Methanol tolerance of atomically dispersed single metal site catalysts: Mechanistic understanding and high-performance direct methanol fuel cells. Energy Environ. Sci. 2020, 13, 3544–3555. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Jiannakoudakis, A.; Sotiropoulos, S. Amperometric detection of dissolved methanol using microelectrodes. Sens. Electroanal. 2010, 5, 153–161. [Google Scholar]
- Lv, J.; Liao, Y.; Lin, X.; Zuo, J.; He, G. Construction of high-efficiency Fe–MnO@Fe electrocatalyst for methanol and ethanol oxidation in alkaline medium. 2D Mater. 2024, 11, 045022. [Google Scholar] [CrossRef]
- Yang, J.-H.; Song, X.; Zhao, X.; Wang, Y.; Yang, Y.; Gao, L. Nickel phosphate materials regulated by doping cobalt for urea and methanol electro-oxidation. Int. J. Hydrogen Energy 2019, 44, 16305–16314. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E.; Scarpa, D. PtRh and PtRh/MoS2 nano-electrocatalysts for methanol oxidation and hydrogen evo-lution reactions. Chem. Eng. J. 2019, 377, 120600. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, J.; Liu, M.; Guo, Y. Fabrication of a novel PtPbBi/C catalyst for ethanol electro-oxidation in alkaline medium. Electrochim. Acta 2012, 83, 1. [Google Scholar] [CrossRef]
- Askari, M.B.; Rozati, S.M.; Seifi, M.; Beheshti-Marnani, A. Mo0.25Co1.257W0.25S3 hybridized with graphene oxide as a nanocatalyst based on transition metal dichalcogenides for methanol electro-oxidation. Chem. Phys. Lett. 2018, 708, 146–152. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Chu, Z.; Zhang, J.; Du, P.; Zhang, Q.; Cao, F.; Liu, J. Electrocatalytic Oxidation of Methanol over An Electrode with Ni-MOF-74 Catalyst. ChemCatChem 2021, 13, 4824–4832. [Google Scholar] [CrossRef]
- Askari, M.B.; Azizi, S.; Moghadam, M.T.T.; Seifi, M.; Rozati, S.M.; Di Bartolomeo, A. MnCo2O4/NiCo2O4/rGO as a Catalyst Based on Binary Transition Metal Oxide for the Methanol Oxidation Reaction. Nanomaterials 2022, 12, 4072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-Q.; Xi, B.-J.; Chang, X.-W.; Wang, B.; Wu, X.-Q.; Li, S.; Wu, Y.-P.; Li, D.-S. Facile in Situ Transformation of NiOOH into MOF-74(Ni)/NiO OH Heterogeneous Composite for Enchancing Electrocatalytic Methanol Oxidation. Molecules 2022, 27, 2113. [Google Scholar] [CrossRef] [PubMed]
- Mehek, R.; Iqbal, N.; Noor, T.; Nasir, H.; Mehmood, Y.; Ahmed, S. Novel Co-MOF/Graphene Oxide Electrocatalyst for Methanol Oxidation. Electrochimica Acta 2017, 255, 195–204. [Google Scholar] [CrossRef]
- Rizvi, S.A.M.; Iqbal, N.; Haider, M.D.; Noor, T.; Anwar, R.; Hanif, S. Synthesis and Characterization of Cu-MOF Derived Cu@AC Electrocatalyst for Oxygen Reduction Reaction in PEMFC. Catal. Lett. 2019, 150, 1397–1407. [Google Scholar] [CrossRef]
- Abbasi, Y.; Jalali, F.; Sheikhi, S. Preparation of nickel/copper sulfides from metal-organic frameworks. Applications to energy storage in a symmetric supercapacitor and electrocatalytic methanol oxidation. J. Alloys Compd. 2023, 938, 168450. [Google Scholar] [CrossRef]






| Materials | Ms (emu/g) | Mr (emu/g) | Hc (G) | (Mr/Ms) |
|---|---|---|---|---|
| Mg Ferrite | 24.7 | 1.81 | 23.15 | 0.07 |
| Mg Mof | 0.45 | 0.189 | 1695.3 | 0.42 |
| Composite (1:1) | 15.07 | 2.72 | 114.22 | 0.17 |
| Materials | BET m2∙g−1 | Total Pore Volume cc.g−1 | Pore Width nm |
|---|---|---|---|
| Mg Ferrite | 8.684 | 10.05 | 2.3 |
| Mg-Mof | 89.318 | 10.44 | 4.69 |
| Composite (1:1) | 18.887 | 10.11 | 3.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, M.R.; Kotp, A.A.; Elsayed, E.M.; Elseman, A.M.; Abdel-wahab, M.S. Effective and High-Performance MgFe2O4/Mg-MOF Composite for Direct Methanol Fuel Cells. Catalysts 2025, 15, 394. https://doi.org/10.3390/catal15040394
Hussein MR, Kotp AA, Elsayed EM, Elseman AM, Abdel-wahab MS. Effective and High-Performance MgFe2O4/Mg-MOF Composite for Direct Methanol Fuel Cells. Catalysts. 2025; 15(4):394. https://doi.org/10.3390/catal15040394
Chicago/Turabian StyleHussein, M. R., Amna A. Kotp, E. M. Elsayed, A. M. Elseman, and Mohamed Sh. Abdel-wahab. 2025. "Effective and High-Performance MgFe2O4/Mg-MOF Composite for Direct Methanol Fuel Cells" Catalysts 15, no. 4: 394. https://doi.org/10.3390/catal15040394
APA StyleHussein, M. R., Kotp, A. A., Elsayed, E. M., Elseman, A. M., & Abdel-wahab, M. S. (2025). Effective and High-Performance MgFe2O4/Mg-MOF Composite for Direct Methanol Fuel Cells. Catalysts, 15(4), 394. https://doi.org/10.3390/catal15040394







