Effects of the Intrinsic Structures of Graphite Felt and Carbon Cloth on the Working Condition of Iron-Chromium Redox Flow Batteries
Abstract
:1. Introduction
2. Results and Discussion
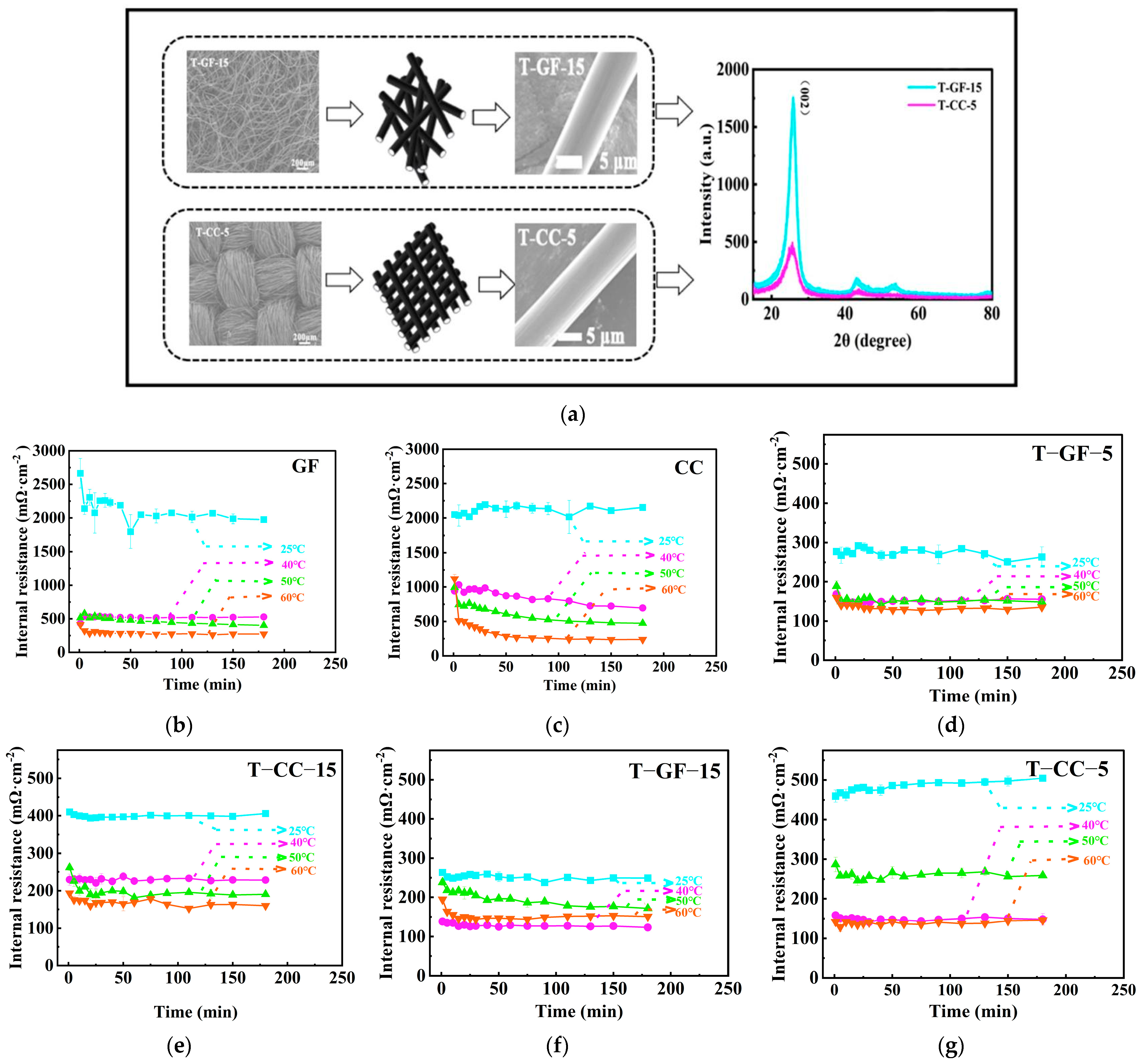
2.1. Initial Cell Resistance
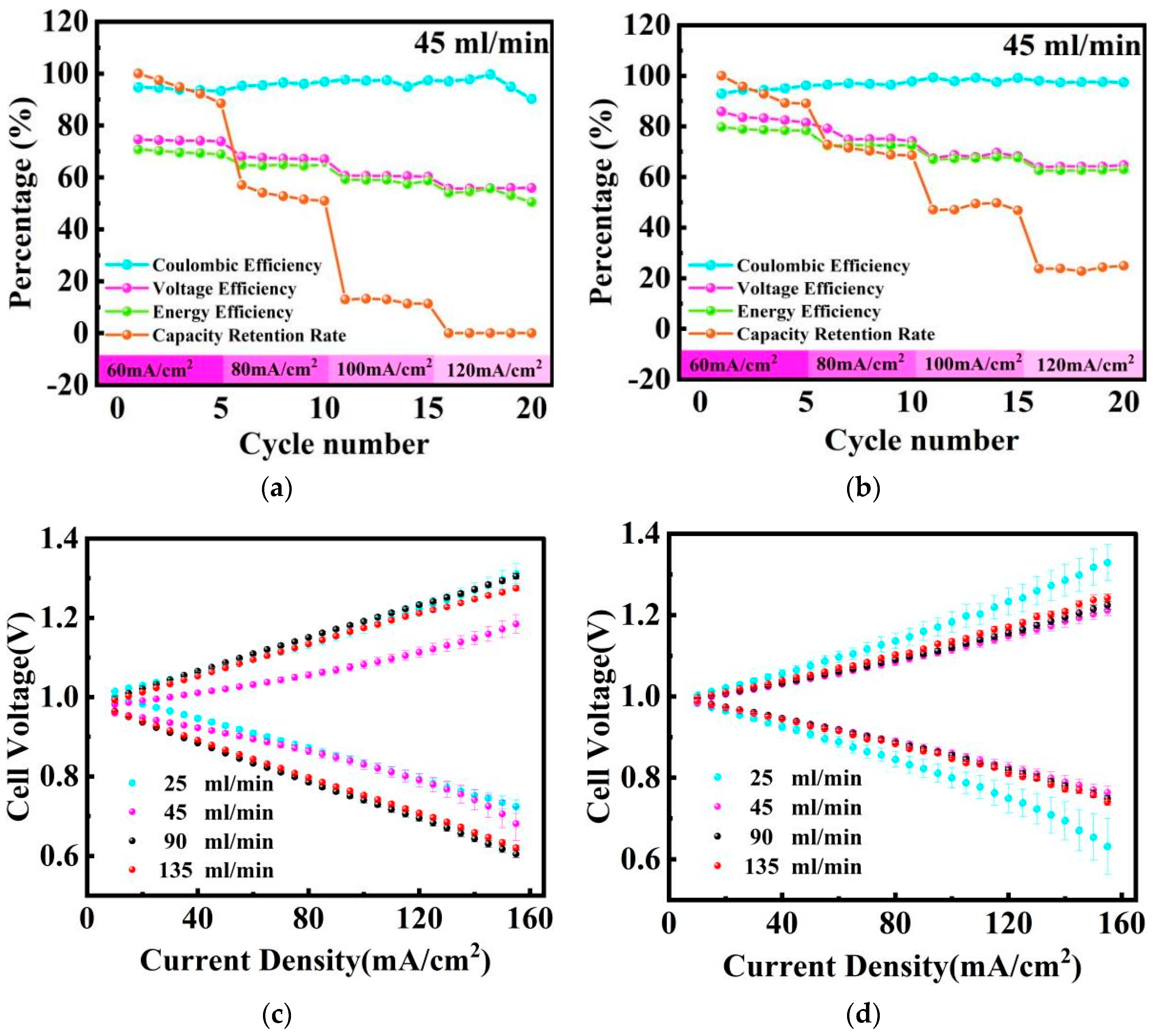
2.2. Electrolyte Flow Rate
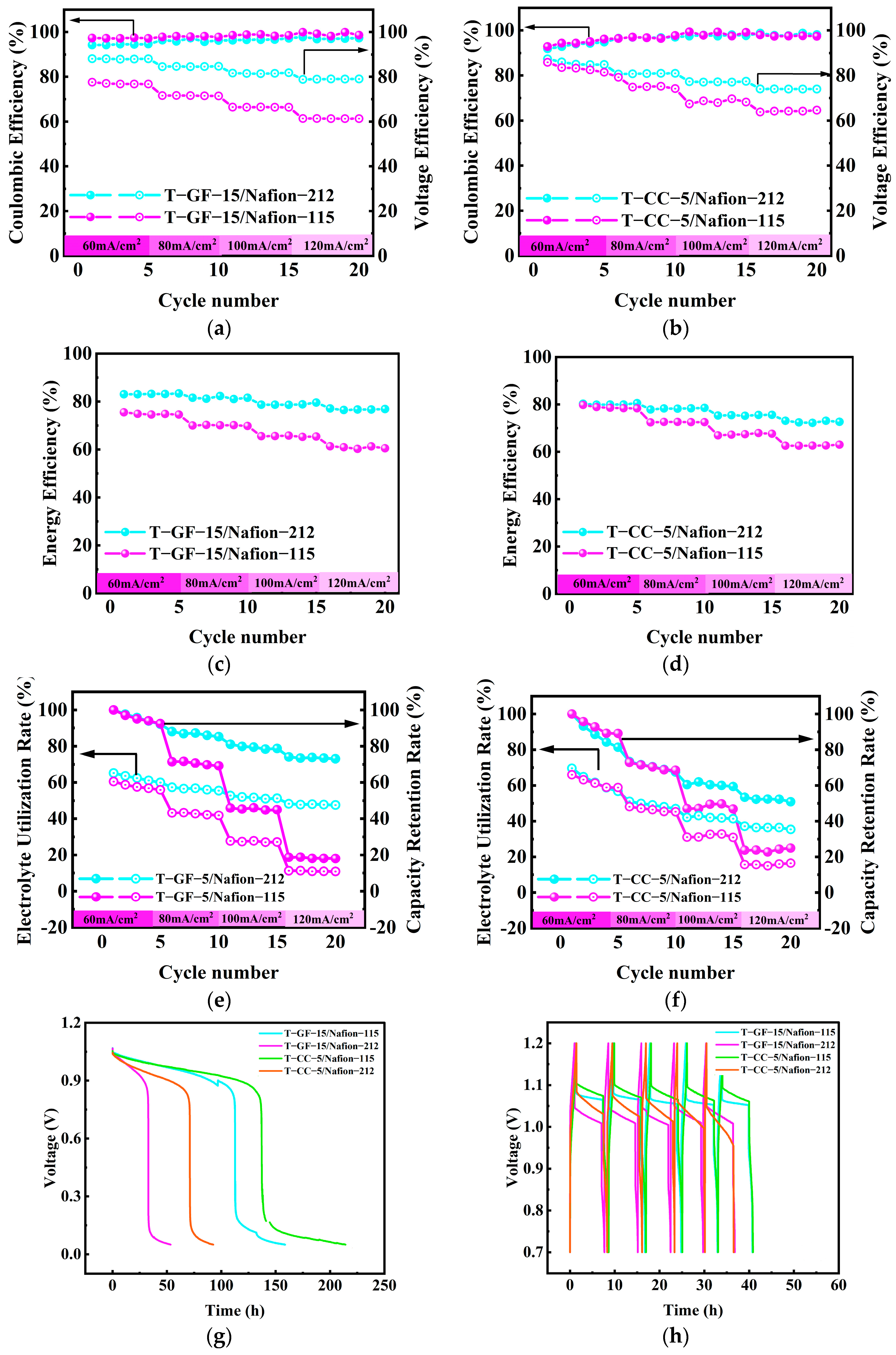
2.3. Membrane
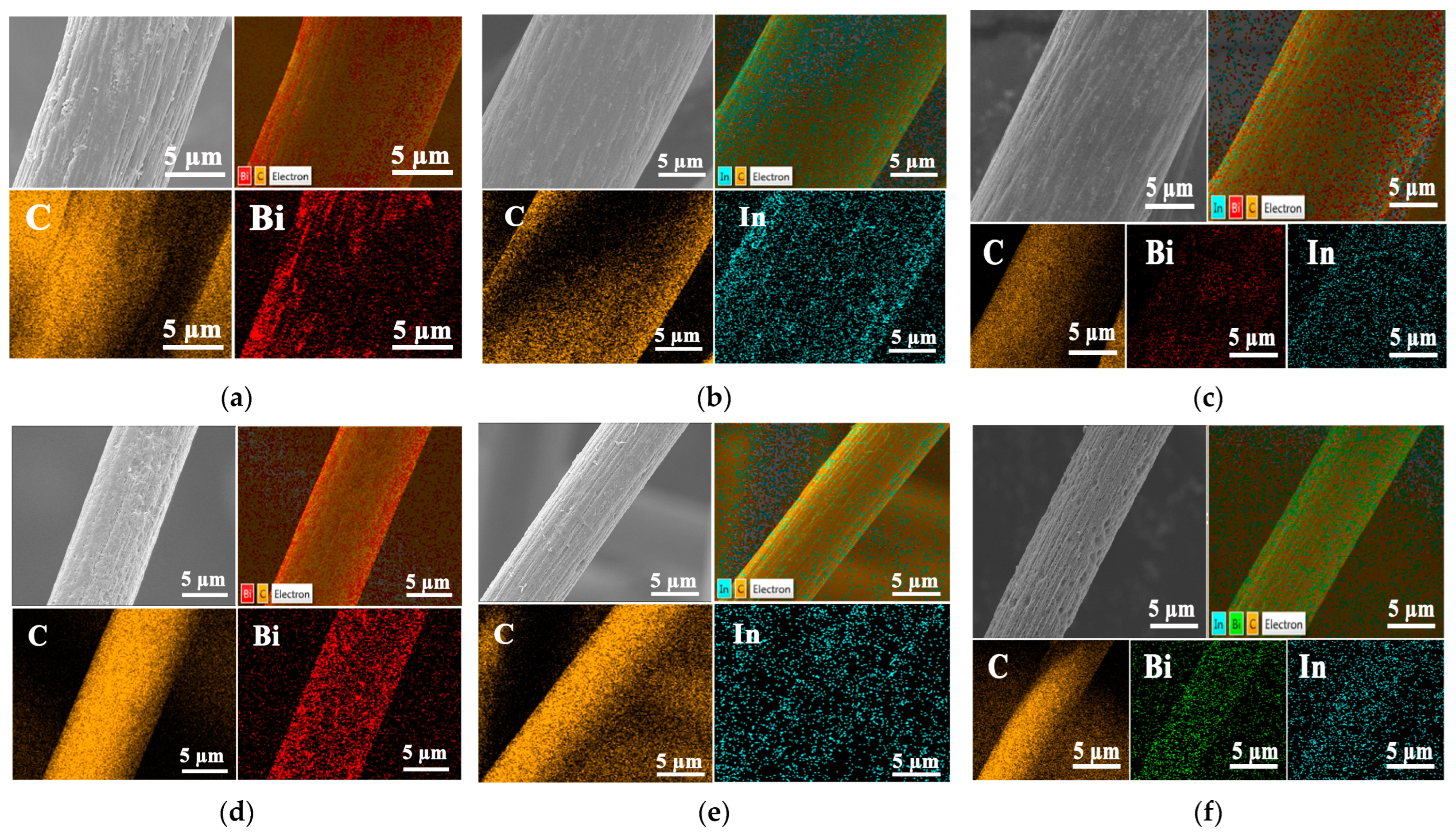
2.4. Bi, in Catalyst
3. Materials and Methods
3.1. Electrode Materials
3.2. Electrochemical Testing
3.3. Battery Assembly and Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Niu, X.; Liu, X.; Wu, C.; Duan, X.; Ma, Z.; Xu, Y.; Li, H.; Yang, W. Unraveling the coordination behavior and transformation mechanism of Cr3+ in Fe–Cr redox flow battery electrolytes. Mater. Rep. Energy 2024, 4, 100271. [Google Scholar] [CrossRef]
- Niu, S.; Sun, S.; Chu, F.; Liu, Y.; Lai, Q.; Cheng, Y. Chelation approach to long-lived and reversible chromium anolytes for aqueous flow batteries. J. Energy Storage 2024, 100, 113720. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Ge, M. Progress in profitable Fe-based flow batteries for broad-scale energy storage. WIREs Energy Environ. 2024, 13, e541. [Google Scholar]
- Niu, Y.; Heydari, A.; Qiu, W.; Guo, C.; Liu, Y.; Xu, C.; Zhou, T.; Xu, Q. Machine learning-enabled performance prediction and optimization for iron–chromium redox flow batteries. Nanoscale 2024, 16, 3994–4003. [Google Scholar] [CrossRef]
- Wan, C.T.-C.; Rodby, K.E.; Perry, M.L.; Chiang, Y.-M.; Brushett, F.R. Hydrogen evolution mitigation in iron-chromium redox flow batteries via electrochemical purification of the electrolyte. J. Power Sources 2023, 554, 232248. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Review of the development of first-generation redox flow batteries iron-chromium system. ChemSusChem 2022, 15, e202101798. [Google Scholar]
- Zeng, Y.K.; Zhao, T.S.; Zhou, X.L.; Zeng, L.; Wei, L. The effects of design parameters on the charge-discharge performance of iron-chromium redox flow batteries. Appl. Energy 2016, 182, 204–209. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, N.; Sun, C.; Luo, X. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery. Int. J. Energy Res. 2019, 44, 3839–3853. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Luo, X.D.; Sun, C.Y. SiO2-decorated graphite felt electrode by silicic acid etching for iron-chromium redox flow battery. Electrochim. Acta 2020, 336, 135646. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, Y.; Guo, C.; Qu, F.; Liu, Z.; Zhou, X.; Guo, W.; Xu, C.; Xu, Q. Nitrogen-doped bismuth oxide-modified carbon cloth as a bifunctional electrocatalyst for iron-chromium redox flow batteries. Energy Fuels 2024, 38, 12202–12211. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Z.; Wu, X.; Zhao, H.; Zhao, J.; Liu, J.; Yan, C.; Fan, X. Excellent stability and electrochemical performance of the electrolyte with indium ion for iron–chromium flow battery. Electrochim. Acta 2021, 368, 137524. [Google Scholar] [CrossRef]
- Xie, C.; Yan, H.; Song, Y.; Song, Y.; Yan, C.; Tang, A. Catalyzing anode Cr2+/Cr3+ redox chemistry with bimetallic electrocatalyst for high-performance iron-chromium flow batteries. J. Power Sources 2023, 564, 232860. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, S.; Xu, C.; Chen, X.; Zeng, S.; Li, C.; Zhou, Y.; Zhou, T.; Niu, Y. Synergistic effect of electrode defect regulation and Bi catalyst deposition on the performance of iron–chromium redox flow battery. Chin. Chem. Lett. 2023, 34, 108188. [Google Scholar] [CrossRef]
- Xing, F.; Fu, Q.; Xing, F.; Zhao, J.; Long, H.; Liu, T.; Li, X. Bismuth single atoms regulated graphite felt electrode boosting high power density vanadium flow batteries. J. Am. Chem. Soc. 2024, 146, 26024–26033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Valencia, A.; Li, W.; Ao, K.; Shi, J.; Yue, X.; Zhang, R.; Daoud, W.A. Decoupling activation and transport by electron-regulated atomic-Bi harnessed surface-to-pore interface for vanadium redox flow battery. Adv. Mater. 2023, 36, 2305415. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, Z.; Sun, J.; Guo, Z.; Liu, B.; Fan, X.; Zhao, T. In-situ electrodeposition of homogeneous and dense bismuth nanoparticles onto scale-up graphite felt anodes for vanadium redox flow batteries. J. Power Sources 2023, 586, 233655. [Google Scholar] [CrossRef]
- Zheng, Y.; Qu, D.; Yang, F.; Chen, D. Study on performance improvement of vanadium redox flow batteries focused on electrode fabrication parameters and compression strategies. J. Energy Storage 2025, 109, 115142. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Sun, J.; Guo, Z.; Wei, L.; Fan, X.; Zhao, T. Characterizations and selections of electrodes with optimal performance for large-scale vanadium redox flow batteries through lab-scale experiments. J. Power Sources 2022, 549, 232094. [Google Scholar] [CrossRef]
- Xiong, J.; Song, Y.; Wang, S.; Li, X.; Liu, J.; Yan, C.; Tang, A. Evaluation of the influence of clamping force in electrochemical performance and reliability of vanadium redox flow battery. J. Power Sources 2019, 431, 170–181. [Google Scholar] [CrossRef]
- Charvát, J.; Mazúr, P.; Dundálek, J.; Pocedič, J.; Vrána, J.; Mrlík, J.; Kosek, J.; Dinter, S. Performance enhancement of vanadium redox flow battery by optimized electrode compression and operational conditions. J. Energy Storage 2020, 30, 101468. [Google Scholar] [CrossRef]
- Ma, T.; Huang, Z.; Li, B.; Xie, X.; Huang, C.; Lin, T.; Guo, Z. Effect of operating conditions on the capacity of vanadium redox flow batteries. J. Electrochem. Soc. 2024, 171, 60503. [Google Scholar] [CrossRef]
- Chai, Y.; Qu, D.; Fan, L.; Zheng, Y.; Yang, F. A comprehensive study in experiments combined with simulations for vanadium redox flow batteries at different temperatures. J. Energy Storage 2024, 99, 113405. [Google Scholar] [CrossRef]
- Ye, Q.; Dai, J.; Cheng, P.; Zhao, T. Gas evolution induced vicious cycle between bubble trapping and flow choking in redox flow battery stacks. Int. J. Heat Mass Transf. 2024, 51, 125100. [Google Scholar] [CrossRef]
- Dai, J.; Ye, Q.; Zhao, T. Increased electrolyte flow resistance and blockage due to hydrogen evolution in a flow battery single cell under stack electrolyte feeding conditions. J. Power Sources 2025, 628, 235940. [Google Scholar] [CrossRef]
- Zeng, Y.K.; Zhou, X.L.; Zeng, L.; Yan, X.; Zhao, T. Performance enhancement of iron-chromium redox flow batteries by employing interdigitated flow fields. J. Power Sources 2016, 327, 258–264. [Google Scholar] [CrossRef]
- Pan, L.; Sun, J.; Qi, H.; Han, M.; Dai, Q.; Xu, J.; Yao, S.; Li, Q.; Wei, L.; Zhao, T. Dead-zone-compensated design as general method of flow field optimization for redox flow batteries. Proc. Natl. Acad. Sci. USA 2023, 120, 1989395176. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, J.; Wan, S.; Wang, Z.; Ren, J.; Pan, L.; Wei, L.; Fan, X.; Zhao, T. Deep neural network-assisted fast and precise simulations of electrolyte flows in redox flow batteries. Appl. Energy 2025, 379, 124910. [Google Scholar] [CrossRef]
- Sun, J.; Yang, P.; Lv, M.; Zhao, M.; Xiao, Q.; Zhang, W.; Wang, J.; Zhang, L. Localized acoustic streaming-mediated efficiency enhancement for vanadium redox flow battery. J. Energy Storage 2025, 110, 115108. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.; Wei, L. Critical transport issues for improving the performance of aqueous redox flow batteries. J. Power Sources 2017, 339, 1–12. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, M.; Fan, X.; Chao, C.; Zhao, T. Aligned hierarchical electrodes for high-performance aqueous redox flow battery. Appl. Energy 2020, 271, 115235. [Google Scholar] [CrossRef]
- Li, J.-C.; Lu, M.-Y.; Yang, W.-W.; Zhang, R.-Z.; Su, J.-W.; Xu, Q. Development of high-performance and ultra-stability hierarchical nested-network-pore carbon electrode for vanadium redox flow batteries. J. Energy Storage 2024, 97, 112987. [Google Scholar] [CrossRef]
- Park, S.H.; Ha, J.; Kim, D.W.; Hwang, C.; Choi, J.-I.; Park, H.S.; Kim, Y. Mesoporous graphite felt electrode prepared via thermal oxidative etching on all-vanadium redox flow batteries. Chem. Eng. J. 2024, 500, 157238. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Q.; Ma, Z. Effects of trace amount of copper, nickel and bismuth on hydrogen evolution reaction of the carbon felt electrode in a hydrochloride-based flow battery. J. Energy Storage 2024, 103, 114329. [Google Scholar] [CrossRef]
- Cecchetti, M.; Messaggi, M.; Donazzi, A.; Facibeni, A.; Russo, V.; Casari, C.; Bassi, A.L.; Casalegno, A.; Zago, M. A combined morphological and electrochemical characterization of carbon electrodes in vanadium redox flow batteries: Insights into positive and negative electrode performance. Electrochim. Acta 2020, 329, 135143. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.R.; Zhang, B.W.; Chao, C.; Zhao, T. Towards uniform distributions of reactants via the aligned electrode design for vanadium redox flow batteries. Appl. Energy 2019, 259, 114198. [Google Scholar] [CrossRef]
- Trovo, A.; Zamboni, W.; Marini, G.; Poli, N.; Guarnieri, M. A flow battery cell testing facility for versatile active material characterization: Features and operations. J. Power Sources 2025, 625, 235679. [Google Scholar] [CrossRef]
- Saraidaridis, J.D.; Darling, R.M.; Yang, Z.; Shovlin, C.; Fortin, M.E.; Robb, B.H.; Waters, S.E.; Marshak, M.P. Transport of ligand coordinated iron and chromium through cation-exchange membranes. J. Electrochem. Soc. 2022, 169, 60532. [Google Scholar] [CrossRef]
- du Toit, J.P.; Krieg, H.M.; Mans, N.; van der Westhuizen, D.J. UV–Vis spectrophotometric analytical technique for monitoring Fe2+ positive electrolyte of an ICRFB. J. Power Sources 2023, 553, 232178. [Google Scholar] [CrossRef]
- Farooq, M.O.; Furquan, M.; Munteshari, O.; Qamar, M. An operando investigation of temperature distribution behaviour in full-cell vanadium-redox flow batteries. Electrochim. Acta 2025, 513, 145572. [Google Scholar] [CrossRef]
- Ghimire, P.C.; Schweiss, R.; Scherer, G.G.; Lim, T.M.; Wai, N.; Bhattarai, A.; Yan, Q. Optimization of thermal oxidation of electrodes for the performance enhancement in all-vanadium redox flow battery. Carbon 2019, 155, 176–185. [Google Scholar] [CrossRef]
- Caiado, A.A.; Chaurasia, S.; Aravamuthan, S.R.; Roy, A.; Inalpolat, M.; Agar, E. Binder-coated carbon cloth electrodes for all-vanadium redox flow batteries. J. Electrochem. Soc. 2024, 171, 120524. [Google Scholar] [CrossRef]
- Hou, Y.; Sheng, Z.; Zhang, M.; Lin, K.; Kong, J.; Zhang, X. Hierarchically porous carbon colloidal aerogels for highly efficient flow cells. Adv. Funct. Mater. 2024, 2418721. [Google Scholar] [CrossRef]
- Wu, M.; Nan, M.; Ye, Y.; Yang, M.; Qiao, L.; Zhang, H.; Ma, X. A highly active electrolyte for high-capacity iron-chromium flow batteries. Appl. Energy 2024, 358, 122534. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Clement, J.T.; Veith, G.M.; Zawodzinski, T.A.; Mench, M.M. High performance electrodes in vanadium redox flow batteries through oxygen-enriched thermal activation. J. Power Sources 2015, 294, 333–338. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, T.; Li, X.; Liu, Z. Carbon paper coated with supported tungsten trioxide as novel electrode for all-vanadium flow battery. J. Power Sources 2012, 218, 455–461. [Google Scholar] [CrossRef]
- Schafner, K.; Becker, M.; Turek, T. Membrane resistance of different separator materials in a vanadium redox flow battery. J. Membr. Sci. 2019, 586, 106–114. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An Efficient Barrier toward Vanadium Crossover in Redox Flow Batteries: The Bilayer [Nafion/(WO3)x] Hybrid Inorganic-Organic Membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- du Toit, J.P.; Krieg, H.M. The feasibility of microporous separators in iron-chromium flow batteries. J. Energy Storage 2025, 107, 115008. [Google Scholar] [CrossRef]
- Wu, Q.; Lv, Y.; Lin, L.; Zhang, X.; Liu, Y.; Zhou, X. An improved thin-film electrode for vanadium redox flow batteries enabled by a dual layered structure. J. Power Sources 2019, 410, 152–161. [Google Scholar] [CrossRef]
- Alhammadi, A.; Fetyan, A.; Susantyoko, R.A.; Mustafa, I.; Bamgbopa, M.O. Understanding characteristic electrochemical impedance spectral data of redox flow batteries with multiphysics modeling. J. Energy Chem. 2025, 102, 329–339. [Google Scholar] [CrossRef]
- Monnens, W.; Deferm, C.; Binnemans, K.; Fransaer, J. Indium electrodeposition from indium(III) methanesulfonate in DMSO. Phys. Chem. Chem. Phys. 2020, 22, 24526–24534. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Z.; Tan, H.; Zheng, S.; Fan, B.; Zhang, Y. Suppression of the hydrogen evolution reaction of Iron–chromium flow batteries by organic compounds containing the imidazole group. J. Electroanal. Chem. 2024, 978, 118874. [Google Scholar] [CrossRef]


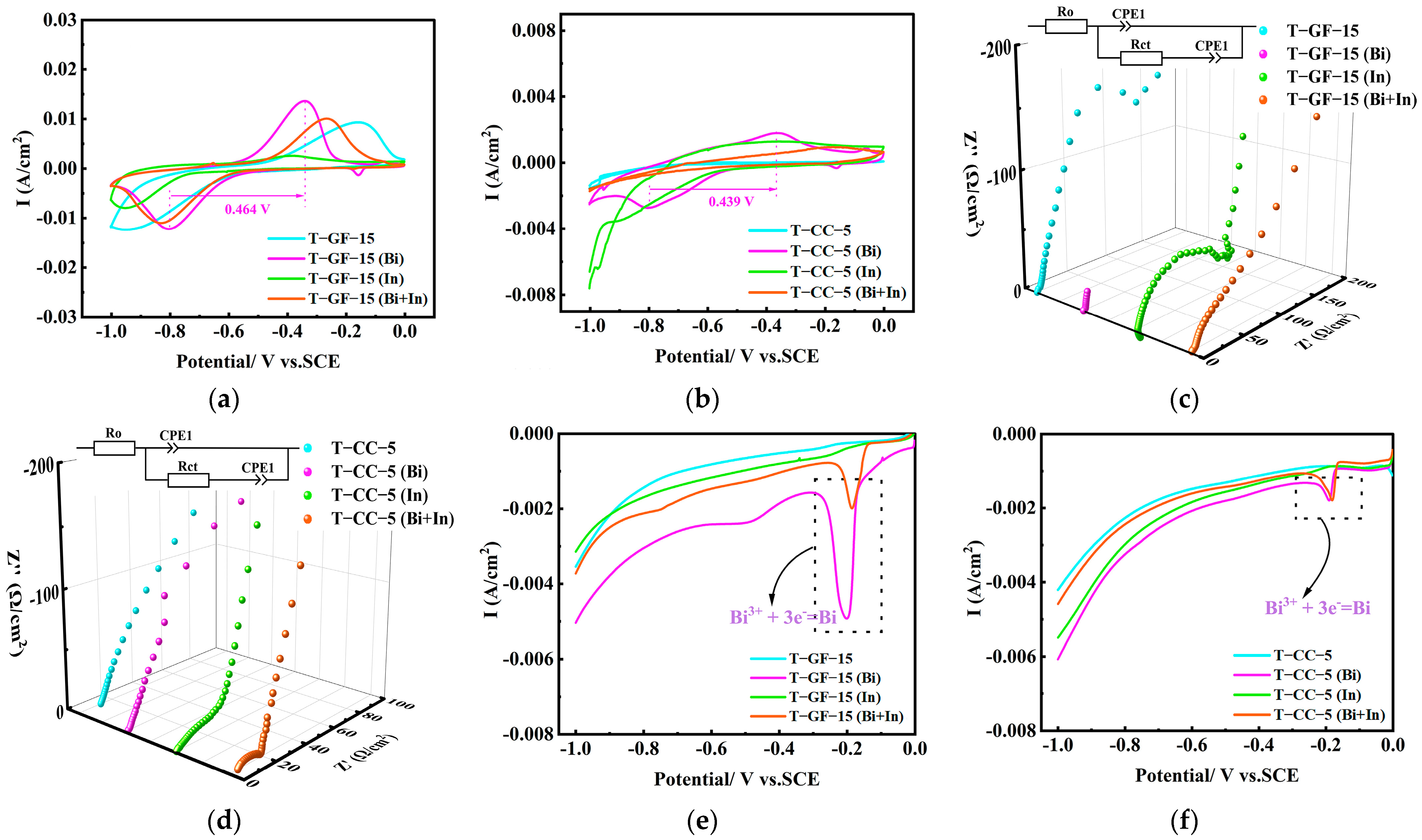

| Samples | Rct (Ω/cm2) | Samples | Rct (Ω/cm2) |
|---|---|---|---|
| T-GF-15 | 175.00 | T-CC-5 | 234.70 |
| T-GF-15 (Bi) | 0.76 | T-CC-5 (Bi) | 0.53 |
| T-GF-15 (In) | 102.27 | T-CC-5 (In) | 16.90 |
| T-GF-15 (Bi + In) | 1.27 | T-CC-5 (Bi + In) | 11.90 |
| Samples | Initial Cell Resistance (mΩ·cm2) | Electrolyte Flow Rate (mL·min) | Membrane | Catalyst |
|---|---|---|---|---|
| T-GF-15 | 123.55 (40 °C) | 40(+)/45(−) | Nafion 212 | - |
| T-CC-5 | 145.95 (60 °C) | 45(+)/45(−) | Nafion 212 | Bi (0.05 M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Sun, C.; Qu, B.; Zhang, H.; Liu, S.; Fei, M.; Yan, S. Effects of the Intrinsic Structures of Graphite Felt and Carbon Cloth on the Working Condition of Iron-Chromium Redox Flow Batteries. Catalysts 2025, 15, 399. https://doi.org/10.3390/catal15040399
Tian J, Sun C, Qu B, Zhang H, Liu S, Fei M, Yan S. Effects of the Intrinsic Structures of Graphite Felt and Carbon Cloth on the Working Condition of Iron-Chromium Redox Flow Batteries. Catalysts. 2025; 15(4):399. https://doi.org/10.3390/catal15040399
Chicago/Turabian StyleTian, Jun, Chuanyu Sun, Bowen Qu, Huan Zhang, Shuqi Liu, Meiqi Fei, and Shuang Yan. 2025. "Effects of the Intrinsic Structures of Graphite Felt and Carbon Cloth on the Working Condition of Iron-Chromium Redox Flow Batteries" Catalysts 15, no. 4: 399. https://doi.org/10.3390/catal15040399
APA StyleTian, J., Sun, C., Qu, B., Zhang, H., Liu, S., Fei, M., & Yan, S. (2025). Effects of the Intrinsic Structures of Graphite Felt and Carbon Cloth on the Working Condition of Iron-Chromium Redox Flow Batteries. Catalysts, 15(4), 399. https://doi.org/10.3390/catal15040399







