Facile Synthesis of Binuclear Imidazole-Based Poly(ionic liquid) via Monomer Self-Polymerization: Unlocking High-Efficiency CO2 Conversion to Cyclic Carbonate
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.2. Evaluation of Catalytic Performance
2.2.1. Catalyst Preparation Condition Optimization
2.2.2. Effects of Reaction Conditions on CO2 Cycloaddition Performance
2.2.3. Recyclability Investigation
2.2.4. Substrate Universality Investigation
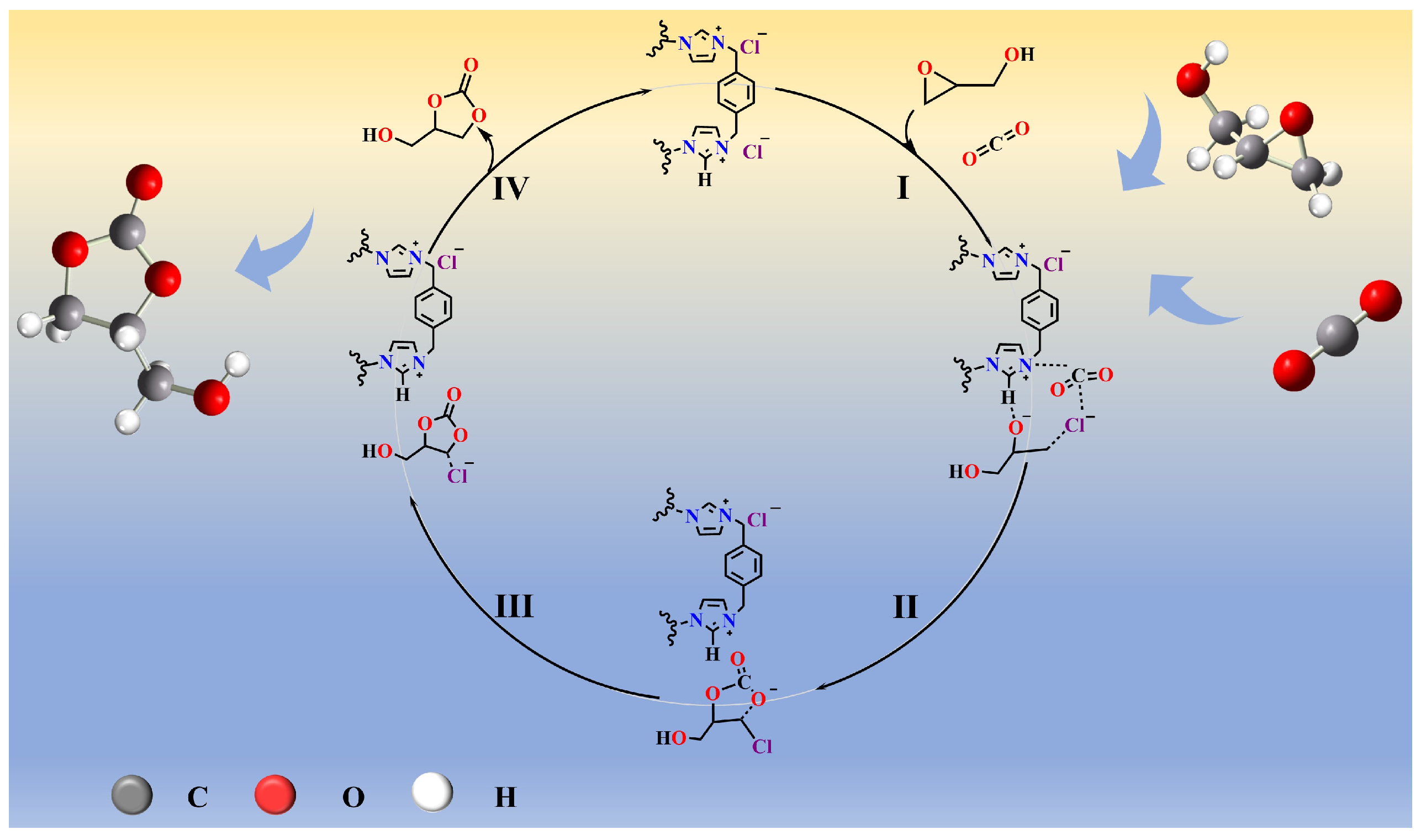
2.3. Mechanistic Study of CO2 Cycloaddition
2.3.1. Active Site Identification
2.3.2. Reaction Pathway Analysis
3. Experimental Section
3.1. Materials
3.2. Characterization
3.3. Catalyst Preparation
3.3.1. Preparation of Binuclear Ionic Liquid Monomer
3.3.2. Synthesis of Binuclear Imidazole-Based Poly(ionic liquid)
3.4. CO2 Cycloaddition Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Han, Y.; Lin, H.; Wu, N.; Li, Q.; Jiang, J.; Zhu, J. Cobalt-Salen-Based Porous Ionic Polymer: The Role of Valence on Cooperative Conversion of CO2 to Cyclic Carbonate. ACS Appl. Mater. Interfaces 2020, 12, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, H.; Dong, A.; Zhang, H.; Zhou, Y.; Dong, H.; Tang, B.; Liu, Y.; Zhang, L.; Liu, X.; et al. Strong Electronic Metal-support Interaction Between Iridium Single Atoms and WO3 Support Promotes Highly Efficient and Robust CO2 Cycloaddition. Adv. Mater. 2022, 34, e2206991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Bian, Y.; Lv, H.; Qiu, B.; Zhang, Y.; Qin, R.; Zhu, D.; Zhang, S.; Li, D.; et al. A Novel Conjugated Microporous Polymer Microspheres Comprising Cobalt Porphyrins for Efficient Catalytic CO2 Cycloaddition under Ambient Conditions. J. CO2 Util. 2022, 58, 101924. [Google Scholar] [CrossRef]
- Liu, X.; Ding, M.; Ma, P.; Duan, C.; Yao, J. Rational Fabrication of ZIF-8 Forests via Metal Template-Guided Growth for Promoting CO2 Chemical Transformation. Sep. Purif. Technol. 2022, 295, 121336. [Google Scholar] [CrossRef]
- Wang, P.; Lv, Q.; Tao, Y.; Cheng, L.; Li, R.; Jiao, Y.; Fang, C.; Li, H.; Geng, C.; Sun, C.; et al. One-Pot Efficient Fixation of Low-Concentration CO2 into Cyclic Carbonate by Mesoporous Pyridine-Functionalized Binuclear Poly(ionic liquid)s. Mol. Catal. 2023, 544, 113157. [Google Scholar] [CrossRef]
- Roshan, K.R.; Palissery, R.A.; Kathalikkattil, A.C.; Babu, R.; Mathai, G.; Lee, H.-S.; Park, D.-W. A Computational Study of the Mechanistic Insights into Base Catalysed Synthesis of Cyclic Carbonates from CO2: Bicarbonate Anion as an Active Species. Catal. Sci. Technol. 2016, 6, 192–199. [Google Scholar] [CrossRef]
- Wang, L.; Kodama, K.; Hirose, T. DBU/Benzyl Bromide: An Efficient Catalytic System for the Chemical Fixation of CO2 into Cyclic Carbonates under Metal- and Solvent-Free Conditions. Catal. Sci. Technol. 2016, 6, 3872–3877. [Google Scholar] [CrossRef]
- Kihara, N.; Hara, N. Catalytic Activity of Various Salts in the Reaction of 2,3-Epoxypropyl Phenyl Ether and Carbon Dioxide under Atmospheric Pressure. J. Org. Chem. 1993, 58, 6198. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, X.; Wang, J.; Mu, T. Bifunctional Boron Phosphate as an Efficient Catalyst for Epoxide Activation to Synthesize Cyclic Carbonates with CO2. Chem. Asian J. 2017, 12, 2271–2277. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Liu, H.; Qian, Q.; Xie, C.; Han, B. Dual-Ionic Liquid System: An Efficient Catalyst for Chemical Fixation of CO2 to Cyclic Carbonates under Mild Conditions. Green Chem. 2018, 20, 2990–2994. [Google Scholar] [CrossRef]
- Lei, Y.; Gunaratne, H.Q.N.; Jin, L. Design and Synthesis of Pyridinamide Functionalized Ionic Liquids for Efficient Conversion of Carbon Dioxide into Cyclic Carbonates. J. CO2 Util. 2022, 58, 101930. [Google Scholar] [CrossRef]
- Kulal, N.; Vasista, V.; Shanbhag, G.V. Identification and Tuning of Active Sites in Selected Mixed Metal Oxide Catalysts for Cyclic Carbonate Synthesis from Epoxides and CO2. J. CO2 Util. 2019, 33, 434–444. [Google Scholar] [CrossRef]
- Qaroush, A.K.; Alsoubani, F.A.; Al-Khateeb, A.M.; Nabih, E.; Al-Ramahi, E.; Khanfar, M.F.; Assaf, K.I.; Eftaiha, A.F. An Efficient Atom-Economical Chemoselective CO2 Cycloaddition Using Lanthanum Oxide/Tetrabutyl Ammonium Bromide. Sustain. Energ. Fuels 2018, 2, 1342–1349. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, P.; Cheng, L.; Wei, Y.; Wang, Y.; Lin, J.; Zhang, Z.; Fang, C.; Li, H.; Ding, J.; et al. Triazole-Based COF Tightly Hugging Ionic Liquids through Interactions of Hydrogen Bonds for Enhanced Atmospheric CO2 Conversion. Sep. Purif. Technol. 2025, 352, 128175. [Google Scholar] [CrossRef]
- Sun, J.; Chen, W.; Shen, H.; Mu, M.; Yin, X. Rational Engineering of Multifunctional Ionic Covalent Organic Frameworks for Metal-Free and Efficient Chemical Fixation of CO2 under Mild Conditions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131554. [Google Scholar] [CrossRef]
- Qu, Q.; Cheng, L.; Wang, P.; Fang, C.; Li, H.; Ding, J.; Wan, H.; Guan, G. Guanidine-Functionalized Basic Binuclear Poly(ionic liquid)s for Low Partial Pressure CO2 Fixation into Cyclic Carbonate. Sep. Purif. Technol. 2024, 339, 126682. [Google Scholar] [CrossRef]
- Hui, W.; He, X.; Xu, X.; Chen, Y.; Zhou, Y.; Li, Z.; Zhang, L.; Tao, D. Highly Efficient Cycloaddition of Diluted and Waste CO2 into Cyclic Carbonates Catalyzed by Porous Ionic Copolymers. J. CO2 Util. 2020, 36, 169–176. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, Q.; Shi, Y.; Li, J.; Yang, X.; Hou, W.; Zhou, Y.; Wang, J. Tethering Dual Hydroxyls into Mesoporous Poly(ionic liquid)s for Chemical Fixation of CO2 at Ambient Conditions: A Combined Experimental and Theoretical Study. ACS Catal. 2017, 7, 6770–6780. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Feng, N.; Cheng, L.; Chen, C.; Wan, H.; Guan, G. Synchronous Activation for Boosting CO2 Cycloaddition over the DABCO-Derived Ionic Liquid Confined in MIL-101(Cr) Nanocages. J. Environ. Chem. Eng. 2024, 12, 112137. [Google Scholar] [CrossRef]
- Helal, A.; Usman, M.; Arafat, M.E.; Abdelnaby, M.M. Allyl Functionalized UiO-66 Metal-Organic Framework as a Catalyst for the Synthesis of Cyclic Carbonates by CO2 Cycloaddition. J. Ind. Eng. Chem. 2020, 89, 104–110. [Google Scholar] [CrossRef]
- McNutt, M. Time’s Up, CO2. Science 2019, 365, 411. [Google Scholar] [CrossRef]
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable Fossil Fuels in a 1.5 °C World. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using Carbon Dioxide as a Building Block in Organic Synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, J.; Fu, Y.; Lin, J.; Wang, H.; Tu, S.; Li, J. Poly(ionic liquid)s with High Density of Nucleophile/Electrophile for CO2 Fixation to Cyclic Carbonates at Mild Conditions. J. CO2 Util. 2019, 32, 281–289. [Google Scholar] [CrossRef]
- Yi, Q.; Liu, T.; Wang, X.; Shan, Y.; Li, X.; Ding, M.; Shi, L.; Zeng, H.; Wu, Y. One-Step Multiple-Site Integration Strategy for CO2 Capture and Conversion into Cyclic Carbonates under Atmospheric and Cocatalyst/Metal/Solvent-Free Conditions. Appl. Catal. B-Environ. 2021, 283, 119620. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Qu, Y.; Zhang, J.; Sun, J. Phase-Controllable Polymerized Ionic Liquids for CO₂ Fixation into Cyclic Carbonates. Sustain. Energ. Fuels 2021, 5, 1026–1033. [Google Scholar] [CrossRef]
- Zou, Y.; Ge, Y.; Zhang, Q.; Liu, W.; Li, X.; Cheng, G.; Ke, H. Polyamine-Functionalized Imidazolyl Poly(ionic liquid)s for the Efficient Conversion of CO₂ into Cyclic Carbonates. Catal. Sci. Technol. 2022, 12, 273–281. [Google Scholar] [CrossRef]
- Zou, Y.; Amuti, Q.; Zou, Z.; Xu, Y.; Yan, C.; Cheng, G.; Ke, H. Diamide-Linked Imidazolyl Poly(Dicationic Ionic Liquid)s for the Conversion of CO₂ to Cyclic Carbonates under Ambient Pressure. J. Colloid Interf. Sci. 2024, 656, 47–57. [Google Scholar] [CrossRef]
- Yang, J.; Chen, P.; Pan, Y.; Liang, Y. Fixation of Carbon Dioxide with Functionalized Ionic Liquids. Asian J. Org. Chem. 2022, 11, 174–188. [Google Scholar] [CrossRef]
- Luo, R.; Liu, X.; Chen, M.; Liu, B.; Fang, Y. Recent Advances on Imidazolium-Functionalized Organic Cationic Polymers for CO₂ Adsorption and Simultaneous Conversion into Cyclic Carbonates. ChemSusChem 2020, 13, 3945–3966. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, D.; Guo, Z.; Liu, Y.; Lyu, Y.; Zhou, Y.; Wang, J. Imidazolinium Based Porous Hypercrosslinked Ionic Polymers for Efficient CO2 Capture and Fixation with Epoxides. Green Chem. 2017, 19, 2675–2686. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, X.; Xie, J.; Wang, X.; Zhou, Y.; Wang, J. Hydroxyl-Exchanged Nanoporous Ionic Copolymer toward Low-Temperature Cycloaddition of Atmospheric Carbon Dioxide into Carbonates. ACS Appl. Mater. Interfaces 2016, 8, 12812–12821. [Google Scholar] [CrossRef]
- Feng, X.; Gao, C.; Guo, Z.; Zhou, Y.; Wang, J. Pore Structure Controllable Synthesis of Mesoporous Poly(ionic liquid)s by Copolymerization of Alkylvinylimidazolium Salts and Divinylbenzene. RSC Adv. 2014, 4, 23389–23395. [Google Scholar] [CrossRef]
- Liao, X.; Wang, Z.; Li, Z.; Kong, L.; Tang, W.; Qin, Z.; Lin, J. Tailoring Hypercrosslinked Ionic Polymers with High Ionic Density for Rapid Conversion of CO2 into Cyclic Carbonates at Low Pressure. Chem. Eng. J. 2023, 471, 144455. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Ravi, S.; Yu, K.; Ahn, W.S. CO2 Adsorption and Conversion into Cyclic Carbonates over a Porous ZnBr2-Grafted N-Heterocyclic Carbene-Based Aromatic Polymer. Appl. Catal. B Environ. 2019, 251, 195–205. [Google Scholar] [CrossRef]
- Long, J.; Dai, W.; Zou, M.; Li, B.; Zhang, S.; Yang, L.; Mao, J.; Mao, P.; Luo, S.; Luo, X. Chemical Conversion of CO2 into Cyclic Carbonates Using a Versatile and Efficient All-in-One Catalyst Integrated with DABCO Ionic Liquid and MIL-101(Cr). Micropor. Mesopor. Mater. 2021, 318, 111027. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, Y.; Qu, Q.; Yang, J.; Cheng, L.; Fang, C.; Li, H.; Ding, J.; Wan, H.; Guan, G. The Embedding of 1,8-Diazabicyclo [5.4.0]undec-7-ene into Hyper-Crosslinked Ionic Polymers for Efficient CO2 Conversion at Atmospheric Pressure. Sep. Purif. Technol. 2025, 357, 130148. [Google Scholar] [CrossRef]
- Zhao, Q.; Yao, X.; Su, Q.; Deng, L.; Chen, J.; Li, Y.; Dong, L.; Yang, Z.; Cheng, W. High Density Poly(ionic liquid)s with Spatial Structure Regulation for Efficient Carbon Dioxide Cycloaddition. ChemCatChem 2023, 15, e202300754. [Google Scholar] [CrossRef]
- Dai, W.; Mao, P.; Liu, Y.; Zhang, S.; Li, B.; Yang, L.; Luo, X.; Zou, J. Quaternary Phosphonium Salt-Functionalized Cr-MIL-101: A Bifunctional and Efficient Catalyst for CO2 Cycloaddition with Epoxides. J. CO2 Util. 2020, 36, 295–305. [Google Scholar] [CrossRef]
- Zou, M.; Dai, W.; Mao, P.; Li, B.; Mao, J.; Zhang, S.; Yang, L.; Luo, S.; Luo, X.; Zou, J. Integration of Multifunctionalities on Ionic Liquid-Anchored MIL-101(Cr): A Robust and Efficient Heterogeneous Catalyst for Conversion of CO2 into Cyclic Carbonates. Micropor. Mesopor. Mater. 2020, 312, 110750. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Song, Q.; Liu, X.F.; Ma, R.; He, L. Cooperative Calcium-Based Catalysis with 1,8-Diazabicyclo[5.4.0]-Undec-7-ene for the Cycloaddition of Epoxides with CO2 at Atmospheric Pressure. Green Chem. 2016, 18, 2871–2876. [Google Scholar] [CrossRef]
- D’Elia, V.; Dong, H.; Rossini, A.J.; Widdifield, C.M.; Vummaleti, S.V.C.; Minenkov, Y.; Poater, A.; Abou-Hamad, E.; Pelletier, J.D.A.; Cavallo, L.; et al. Cooperative Effect of Monopodal Silica-Supported Niobium Complex Pairs Enhancing Catalytic Cyclic Carbonate Production. J. Am. Chem. Soc. 2015, 137, 7728–7739. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Didas, S.A.; Zhu, R.; Brunelli, N.A.; Sholl, D.S.; Jones, C.W. Thermal, Oxidative, and CO2 Induced Degradation of Primary Amines Used for CO2 Capture: Effect of Alkyl Linker on Stability. J. Phys. Chem. C 2014, 118, 12302–12311. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Bulat, F.A. Average Local Ionization Energy: A Review. J. Mol. Model. 2010, 16, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T. Efficient Evaluation of Electrostatic Potential with Computerized Optimized Code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbé, A. New Dual Descriptor for Chemical Reactivity. J. Phys. Chem. A 2004, 109, 205–212. [Google Scholar] [CrossRef]
- Yuan, K.; Feng, K.; Liao, S.; Liao, X.; Zou, Y.; Hou, D.; Nie, X.; Xiong, W. Synthesis, Characterization, and Electrochemical Properties of Phenyl-Coupled Diimidazolium Hexafluorophosphate Ionic Liquids. J. Mol. Liq. 2023, 392 Pt 1, 123515. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Lu, C.; Wang, F.; Ma, C.; Chen, Z.; Yang, G. Imidazolium-Based Polymeric Ionic Liquids for Heterogeneous Catalytic Conversion of CO2 into Cyclic Carbonates. Micropor. Mesopor. Mater. 2020, 292, 109751. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Lyu, Y.; Chen, X.; Li, C.; Zhang, Y.; Song, X.; Ding, Y. Highly Recyclable Polymer Supported Ionic Liquids as Efficient Heterogeneous Catalysts for Batch and Flow Conversion of CO2 to Cyclic Carbonates. RSC Adv. 2017, 7, 2836–2841. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Xiao, M.; Liu, L.; Liu, Y.; Liu, X.; Gai, H. Design of Novel Poly(ionic liquids) for the Conversion of CO₂ to Cyclic Carbonates under Mild Conditions without Solvent. ACS Sustain. Chem. Eng. 2019, 7, 9489–9497. [Google Scholar] [CrossRef]
- Du, H.; Ye, Y.; Xu, P.; Sun, J. Experimental and Theoretical Study on Dicationic Imidazolium-Derived Poly(ionic liquid)s for Catalytic Cycloaddition of CO2-Epoxide. J. CO₂ Util 2023, 67 (Suppl. C), 102325. [Google Scholar] [CrossRef]



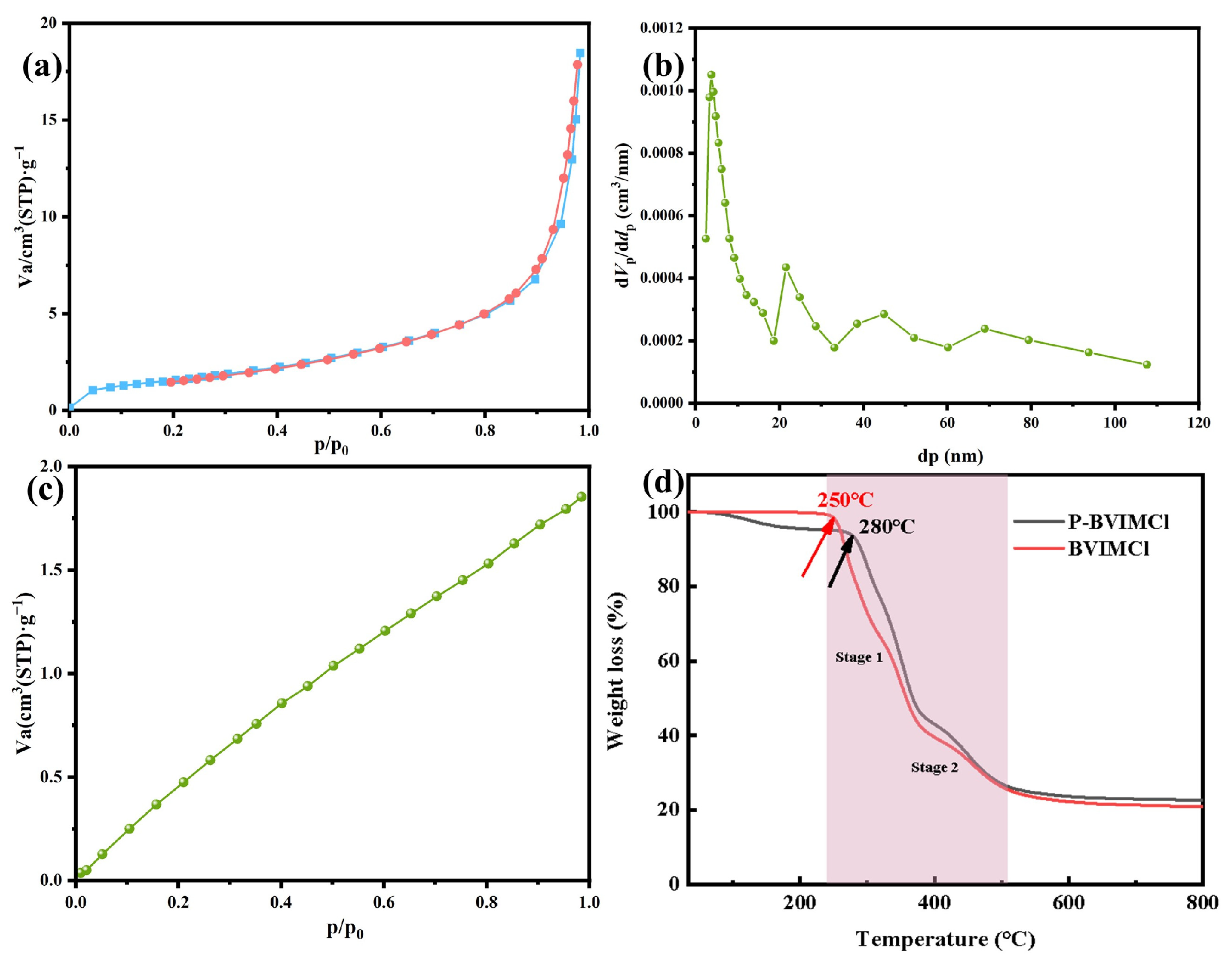
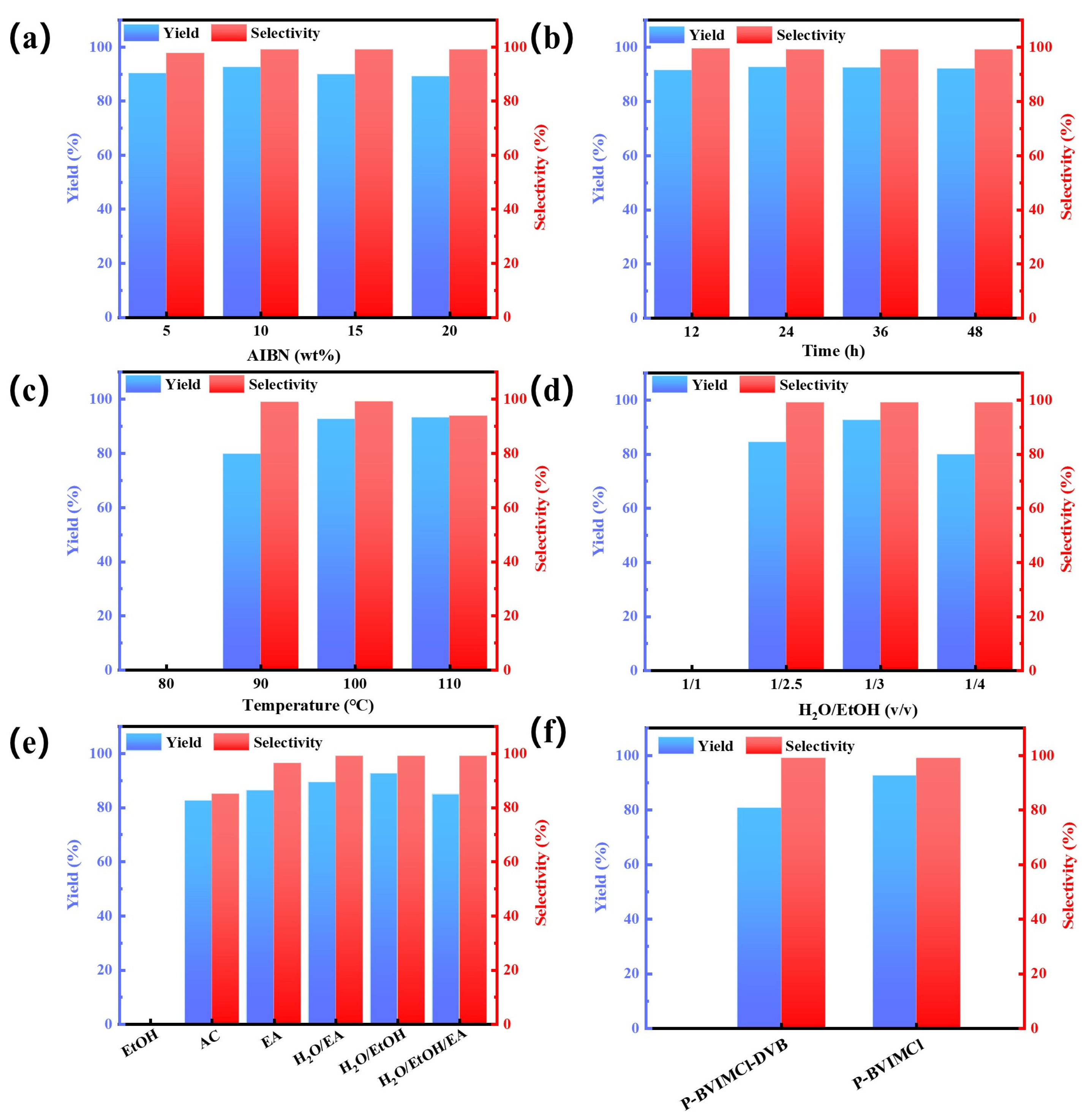

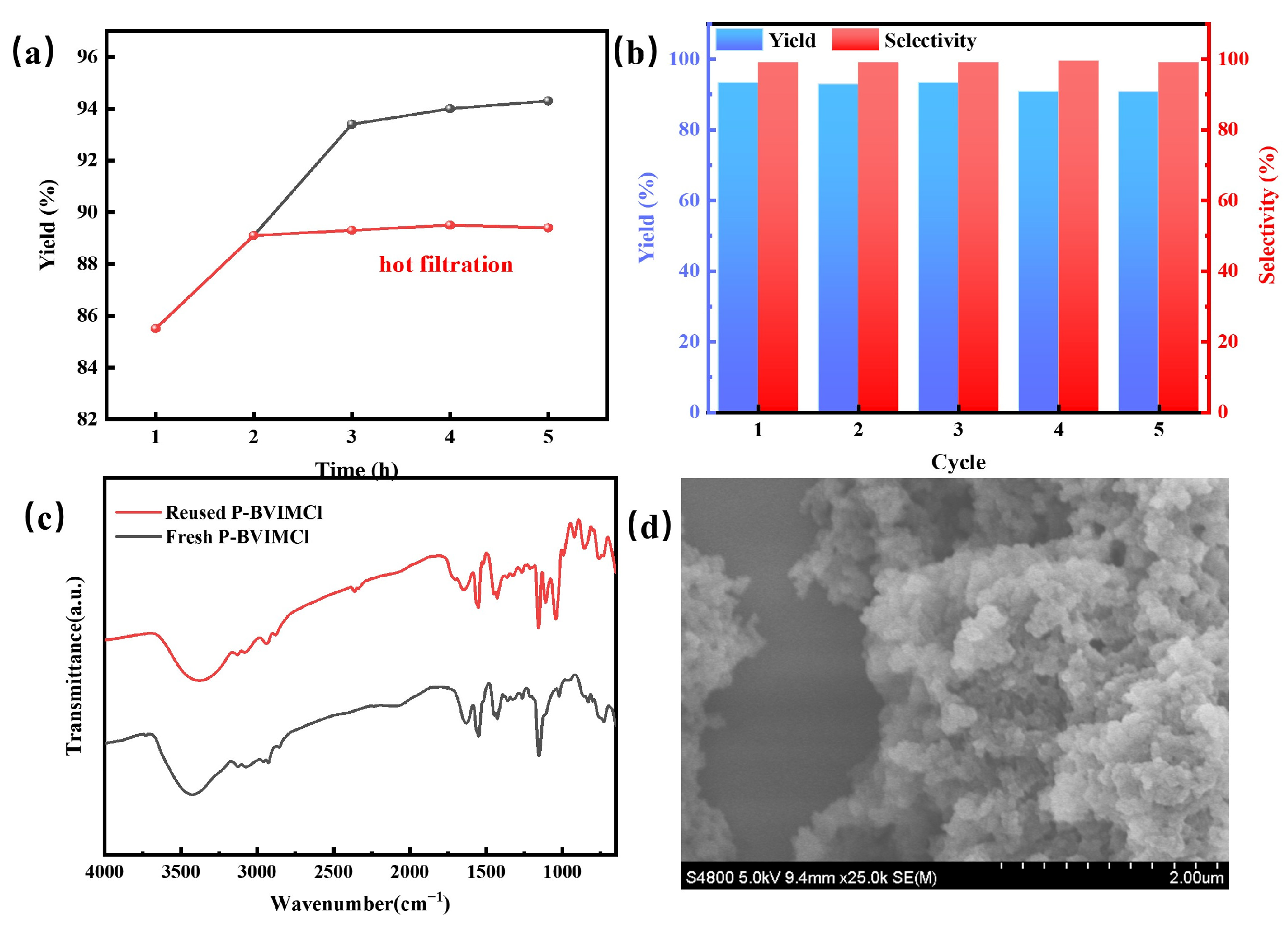


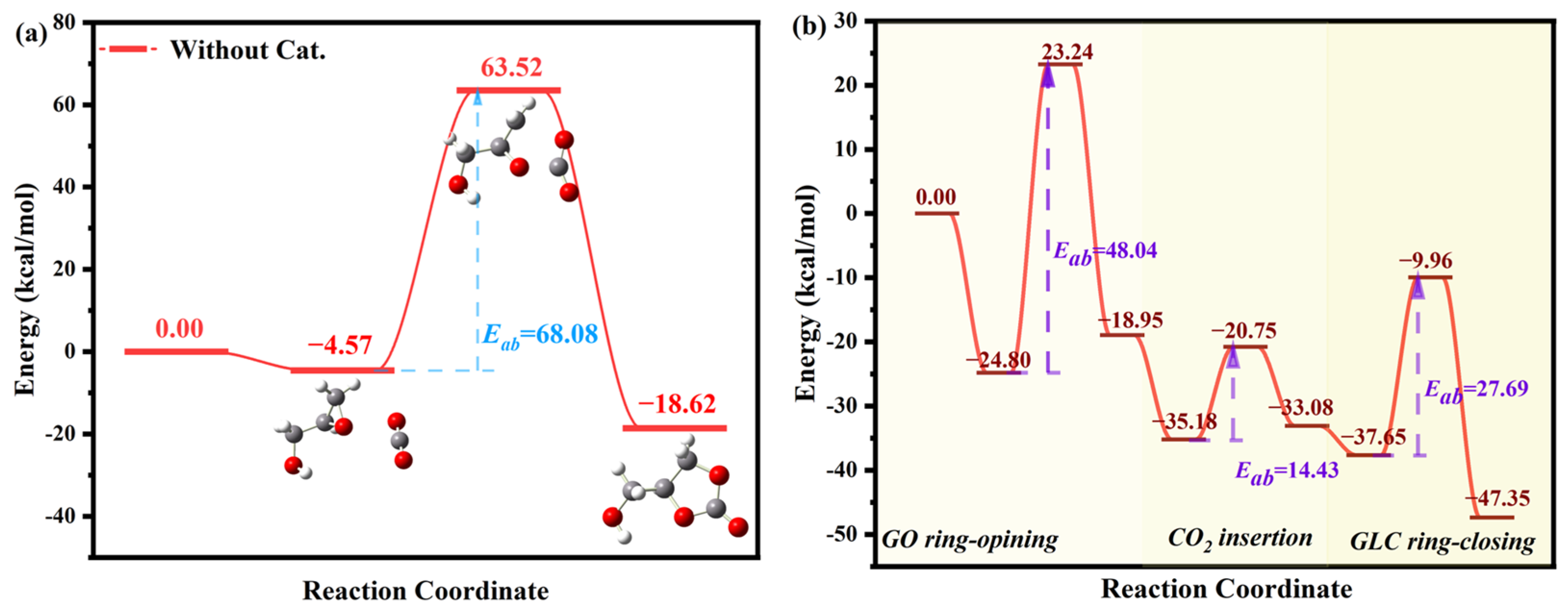


| Catalyst | SBET (m2·g−1) | VP (cm3·g−1) | Dave (nm) | CO2 Adsorption (cm3·g−1) |
|---|---|---|---|---|
| P-BVIMCl | 6.0164 | 0.02855 | 3.72 | 1.853 |
| Entry | Substrate | Product | Temperature (°C) | Time (h) | Y (%) | S (%) |
|---|---|---|---|---|---|---|
| 1 |  | 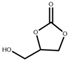 | 100 | 3 | 93.4 | 99.6 |
| 2 b | 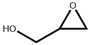 | 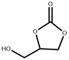 | 100 | 12 | 91.2 | 95.8 |
| 3 |  | 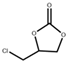 | 120 | 4 | 93.7 | 98.9 |
| 4 | 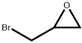 | 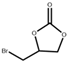 | 120 | 4 | 94.0 | 95.0 |
| 5 | 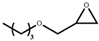 | 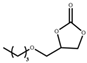 | 120 | 10 | 84.7 | 98.1 |
| 6 |  |  | 100 | 10 | 92.4 | 98.4 |
| 7 |  | 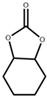 | 120 | 10 | 17.9 | 93.0 |
| Catalyst | C (wt%) a | N (wt%) a | H (wt%) a | Cl (mmol·g−1) b |
|---|---|---|---|---|
| P-BVIMCl | 54.52 | 14.00 | 5.41 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Jiang, Y.; Cheng, L.; Fang, C.; Li, H.; Ding, J.; Wan, H.; Guan, G. Facile Synthesis of Binuclear Imidazole-Based Poly(ionic liquid) via Monomer Self-Polymerization: Unlocking High-Efficiency CO2 Conversion to Cyclic Carbonate. Catalysts 2025, 15, 406. https://doi.org/10.3390/catal15050406
Li R, Jiang Y, Cheng L, Fang C, Li H, Ding J, Wan H, Guan G. Facile Synthesis of Binuclear Imidazole-Based Poly(ionic liquid) via Monomer Self-Polymerization: Unlocking High-Efficiency CO2 Conversion to Cyclic Carbonate. Catalysts. 2025; 15(5):406. https://doi.org/10.3390/catal15050406
Chicago/Turabian StyleLi, Ranran, Yuqiao Jiang, Linyan Cheng, Cheng Fang, Hongping Li, Jing Ding, Hui Wan, and Guofeng Guan. 2025. "Facile Synthesis of Binuclear Imidazole-Based Poly(ionic liquid) via Monomer Self-Polymerization: Unlocking High-Efficiency CO2 Conversion to Cyclic Carbonate" Catalysts 15, no. 5: 406. https://doi.org/10.3390/catal15050406
APA StyleLi, R., Jiang, Y., Cheng, L., Fang, C., Li, H., Ding, J., Wan, H., & Guan, G. (2025). Facile Synthesis of Binuclear Imidazole-Based Poly(ionic liquid) via Monomer Self-Polymerization: Unlocking High-Efficiency CO2 Conversion to Cyclic Carbonate. Catalysts, 15(5), 406. https://doi.org/10.3390/catal15050406









