Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Phase
2.2. Morphology and the Amount of TiO2
2.3. Hydrophilicity and Water Permeation Flux
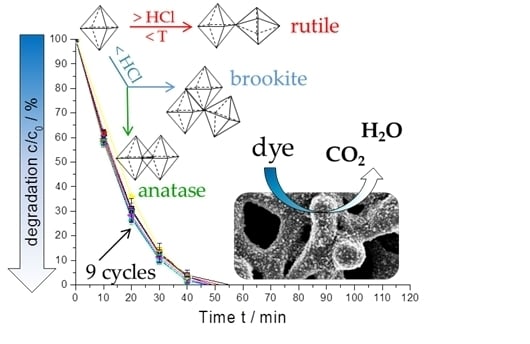
2.4. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Nanoparticle Synthesis on the Membrane
3.3. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Kimura, S.Y. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2016, 88, 546–582. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Bansal, A. Photocatalysis by nanoparticles of titanium dioxide for drinking water purification: A conceptual and state-of-art review. Mater. Sci. Forum 2013, 764, 130–150. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Loddo, V.; Palmisano, L. Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catal. Today 2006, 118, 205–213. [Google Scholar] [CrossRef]
- Sarasidis, V.C.; Plakas, K.V.; Patsios, S.I.; Karabelas, A.J. Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chem. Eng. J. 2014, 239, 299–311. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Dedication. In Nanomaterial and Polymer Membranes; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Wang, Q.; Lian, J.; Bai, Y.; Hui, J.; Zhong, J.; Li, J.; An, N.; Yu, J.; Wang, F. Photocatalytic activity of hydrogen production from water over TiO2 with different crystal structures. Mater. Sci. Semicond. Process. 2015, 40, 418–423. [Google Scholar] [CrossRef]
- Henderson, M. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Liao, Y.; Que, W. Preparation and photocatalytic activity of TiO2 nanotube powders derived by a rapid anodization process. J. Alloys Compd. 2010, 505, 243–248. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Li, Z.; Cong, S.; Xu, Y. Brookite vs anatase TiO2 in the photocatalytic activity for organic degradation in water. ACS Catal. 2014, 4, 3273–3280. [Google Scholar] [CrossRef]
- Vequizo, J.J.M.; Matsunaga, H.; Ishiku, T.; Kamimura, S.; Ohno, T.; Yamakata, A. Trapping-induced enhancement of photocatalytic activity on brookite TiO2 powders: Comparison with anatase and rutile TiO2 powders. ACS Catal. 2017, 7, 2644–2651. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Kosslick, H.; Ibad, M.F.; Fischer, C.; Bentrup, U.; Vuong, T.H.; Nguyen, L.Q.; Schulz, A. Photocatalytic performance of highly active brookite in the degradation of hazardous organic compounds compared to anatase and rutile. Appl. Catal. B Environ. 2017, 200, 647–658. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B. Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl. Catal. B Environ. 2010, 94, 295–302. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, E.G.; Park, S.D.; Jeon, C.J.; Cho, Y.H.; Rhee, C.K.; Kim, W.W. Photocatalytic effects of rutile phase TiO2 ultrafine powder with high specific surface area obtained by a homogeneous precipitation process at low temperatures. J. Sol-Gel Sci. Technol. 2001, 22, 63–74. [Google Scholar] [CrossRef]
- Ozawa, T.; Iwasaki, M.; Tada, H.; Akita, T.; Tanaka, K.; Ito, S. Low-temperature synthesis of anatase–brookite composite nanocrystals: The junction effect on photocatalytic activity. J. Colloid Interface Sci. 2005, 281, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, X.; Bian, Z.; Fuhr, A.; Zhang, D.; Zhu, J. Highly photocatalytic activity of brookite/rutile TiO2 nanocrystals with semi-embedded structure. Appl. Catal. B Environ. 2016, 180, 551–558. [Google Scholar] [CrossRef]
- Liu, C.; Yu, T.; Tan, X. Characterization and photocatalytic activity of mixed nanocrystalline TiO2 powders prepared by xerogel-hydrothermal method in different acid solutions. Trans. Tianjin Univ. 2016, 22, 473–479. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V.; Lim, M.; Amal, R. Titania nanocomposite polyethersulfone ultrafiltration membranes fabricated using a low temperature hydrothermal coating process. J. Membr. Sci. 2011, 380, 98–113. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Braeken, L.; Luis, P.; Wang, X.-L.; Van der Bruggen, B. Novel binding procedure of TiO2 nanoparticles to thin film composite membranes via self-polymerized polydopamine. J. Membr. Sci. 2013, 437, 179–188. [Google Scholar] [CrossRef]
- Petrochenko, P.; Scarel, G.; Hyde, G.K.; Parsons, G.; Skoog, S.; Zhang, Q.; Goering, P.; Narayan, R. Prevention of ultraviolet (UV)-induced surface damage and cytotoxicity of polyethersulfone using atomic layer deposition (ALD) titanium dioxide. JOM 2013, 65, 550–556. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, X.; Boo, C.; Zhu, S.; Lu, Y.; Fan, W.; Huo, M.; Elimelech, M.; Yang, X. Self-cleaning anti-fouling hybrid ultrafiltration membranes via side chain grafting of poly(aryl ether sulfone) and titanium dioxide. J. Membr. Sci. 2017, 529, 1–10. [Google Scholar] [CrossRef]
- Cruz, N.K.O.; Semblante, G.U.; Senoro, D.B.; You, S.-J.; Lu, S.-C. Dye degradation and antifouling properties of polyvinylidene fluoride/titanium oxide membrane prepared by sol–gel method. J. Taiwan Inst. Chem. Eng. 2014, 45, 192–201. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Teli, S.B.; Molina, S.; Sotto, A.; Calvo, E.G.; Abajob, J.D. Fouling resistant polysulfone–PANI/TiO2 ultrafiltration nanocomposite membranes. Ind. Eng. Chem. Res. 2013, 52, 9470–9479. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Membr. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Wang, P.; Ma, J.; Shi, F.; Ma, Y.; Wang, Z.; Zhao, X. Behaviors and effects of differing dimensional nanomaterials in water filtration membranes through the classical phase inversion process: A review. Ind. Eng. Chem. Res. 2013, 52, 10355–10363. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with ionic liquid modified nano-TiO2 particles. J. Membr. Sci. 2013, 427, 259–269. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Mao, L.; Jiang, C.; Ang, J.; Lu, X. Doping polysulfone ultrafiltration membrane with TiO2-PDA nanohybrid for simultaneous self-cleaning and self-protection. J. Membr. Sci. 2017, 532, 20–29. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, L.; Yang, Z.; Zhang, R.; Liu, Y.-N.; He, M.; Su, Y.; Jiang, Z. Loose nanofiltration membrane for dye/salt separation through interfacial polymerization with in-situ generated TiO2 nanoparticles. Appl. Surf. Sci. 2017, 410, 494–504. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Cheng, B. Effect of crystallization methods on morphology and photocatalytic activity of anodized TiO2 nanotube array films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- Krengvirat, W.; Sreekantan, S.; Mohd Noor, A.-F.; Negishi, N.; Kawamura, G.; Muto, H.; Matsuda, A. Low-temperature crystallization of TiO2 nanotube arrays via hot water treatment and their photocatalytic properties under visible-light irradiation. Mater. Chem. Phys. 2013, 137, 991–998. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Zhang, F.; Tao, K.; Pippel, E.; Domen, K. Spontaneous phase and morphology transformations of anodized titania nanotubes induced by water at room temperature. Nano Lett. 2011, 11, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Que, W.; Zhong, P.; Zhang, J.; He, Y. A facile method to crystallize amorphous anodized TiO2 nanotubes at low temperature. ACS Appl. Mater. Interfaces 2011, 3, 2800–2804. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Hasegawa, H.; Maeda, D.; Ishitsuka, M.; Sato, T. Synthesis of visible-light-active nanosize rutile titania photocatalyst by low temperature dissolution–reprecipitation process. J. Photochem. Photobiol. A Chem. 2004, 163, 1–8. [Google Scholar] [CrossRef]
- Yin, S.; Li, R.; He, Q.; Sato, T. Low temperature synthesis of nanosize rutile titania crystal in liquid media. Mater. Chem. Phys. 2002, 75, 76–80. [Google Scholar] [CrossRef]
- Wang, W.; Gu, B.; Liang, L.; Hamilton, W.A.; Wesolowski, D.J. Synthesis of rutile (α-TiO2) nanocrystals with controlled size and shape by low-temperature hydrolysis: Effects of solvent composition. J. Phys. Chem. B 2004, 108, 14789–14792. [Google Scholar] [CrossRef]
- Karim, M.R.; Bhuiyan, M.T.I.; Dar, M.A.; Seikh, A.H.; Ali Shar, M.; Zaman, M.B.; Lee, C.J.; Kim, H.J.; Lee, M.S. Synthesis and characterization of highly organized crystalline rutile nanoparticles by low-temperature dissolution-reprecipitation process. J. Mater. Res. 2015, 30, 1887–1893. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, J.; Zhao, Z.; Qi, L. Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 1995, 7, 663–671. [Google Scholar] [CrossRef]
- Wu, M.; Lin, G.; Chen, D.; Wang, G.; He, D.; Feng, S.; Xu, R. Sol-hydrothermal synthesis and hydrothermally structural evolution of nanocrystal titanium dioxide. Chem. Mater. 2002, 14, 1974–1980. [Google Scholar] [CrossRef]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Ovenstone, J. Crystallization of anatase from amorphous titania using the hydrothermal technique: Effects of starting material and temperature. J. Phys. Chem. B 1999, 103, 7781–7787. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Chen, Y. A novel method for preparation of nanocrystalline rutile TiO2 powders by liquid hydrolysis of TiCl4. J. Mater. Chem. 2002, 12, 1387–1390. [Google Scholar] [CrossRef]
- Bignold, G.J.; Brewer, A.D.; Hearn, B. Specific conductivity and ionic product of water between 50 and 271 °C. Trans. Faraday Soc. 1971, 67, 2419–2430. [Google Scholar] [CrossRef]
- Yanqing, Z.; Erwei, S.; Zhizhan, C.; Wenjun, L.; Xingfang, H. Influence of solution concentration on the hydrothermal preparation of titania crystallites. J. Mater. Chem. 2001, 11, 1547–1551. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Li, G.; Qi, L.; Liu, P.; Ding, Y.; Zhang, S.; Yang, M. Low temperature synthesis and mechanism of finely dispersed nanorod rutile titanium dioxide. RSC Adv. 2015, 5, 62160–62166. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Kumar, R. Superhydrophilic TiO2 thin film by nanometer scale surface roughness and dangling bonds. Appl. Surf. Sci. 2016, 364, 51–60. [Google Scholar] [CrossRef]
- Bormashenko, E.Y. Wetting of Real Surfaces; De Gruyter: Berlin, Germany, 2013. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Guo, H.; Yang, H.; Zhang, G.; Ji, S.; Zeng, T. Low-temperature synthesis of anatase TiO2 nanoparticles with tunable surface charges for enhancing photocatalytic activity. PLoS ONE 2014, 9, e114638. [Google Scholar] [CrossRef] [PubMed]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett. 2010, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Fu, H.; Jing, L.; Tian, C. Synthesis and photocatalytic activity of stable nanocrystalline TiO2 with high crystallinity and large surface area. J. Hazard. Mater. 2009, 161, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Graphene nanomesh by zno nanorod photocatalysts. ACS Nano 2010, 4, 4174–4180. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Abdolahad, M.; Esfandiar, A.; Mohatashamifar, M. Photodegradation of graphene oxide sheets by TiO2 nanoparticles after a photocatalytic reduction. J. Phys. Chem. C 2010, 114, 12955–12959. [Google Scholar] [CrossRef]
- Yuwono, A.H.; Sofyan, N.; Kartini, I.; Ferdiansyah, A.; Pujianto, T.H. Nanocrystallinity enhancement of TiO2 nanotubes by post-hydrothermal treatment. Adv. Mater. Res. 2011, 277, 90–99. [Google Scholar] [CrossRef]
- Fischer, K.; Gläser, R.; Schulze, A. Nanoneedle and nanotubular titanium dioxide–PES mixed matrix membrane for photocatalysis. Appl. Catal. B Environ. 2014, 160–161, 456–464. [Google Scholar] [CrossRef]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, K.; Gawel, A.; Rosen, D.; Krause, M.; Abdul Latif, A.; Griebel, J.; Prager, A.; Schulze, A. Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis. Catalysts 2017, 7, 209. https://doi.org/10.3390/catal7070209
Fischer K, Gawel A, Rosen D, Krause M, Abdul Latif A, Griebel J, Prager A, Schulze A. Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis. Catalysts. 2017; 7(7):209. https://doi.org/10.3390/catal7070209
Chicago/Turabian StyleFischer, Kristina, Alina Gawel, David Rosen, Maria Krause, Amira Abdul Latif, Jan Griebel, Andrea Prager, and Agnes Schulze. 2017. "Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis" Catalysts 7, no. 7: 209. https://doi.org/10.3390/catal7070209
APA StyleFischer, K., Gawel, A., Rosen, D., Krause, M., Abdul Latif, A., Griebel, J., Prager, A., & Schulze, A. (2017). Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis. Catalysts, 7(7), 209. https://doi.org/10.3390/catal7070209