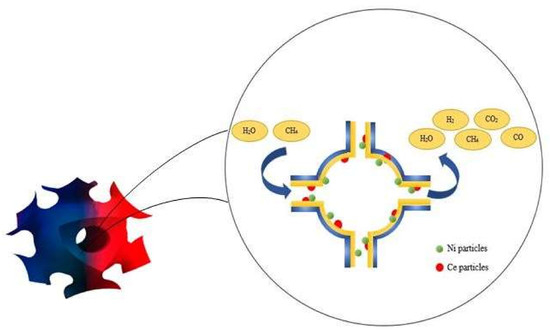
Synthesis and Application of Cerium-Incorporated SBA-16 Supported Ni-Based Oxygen Carrier in Cyclic Chemical Looping Steam Methane Reforming
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Characterization
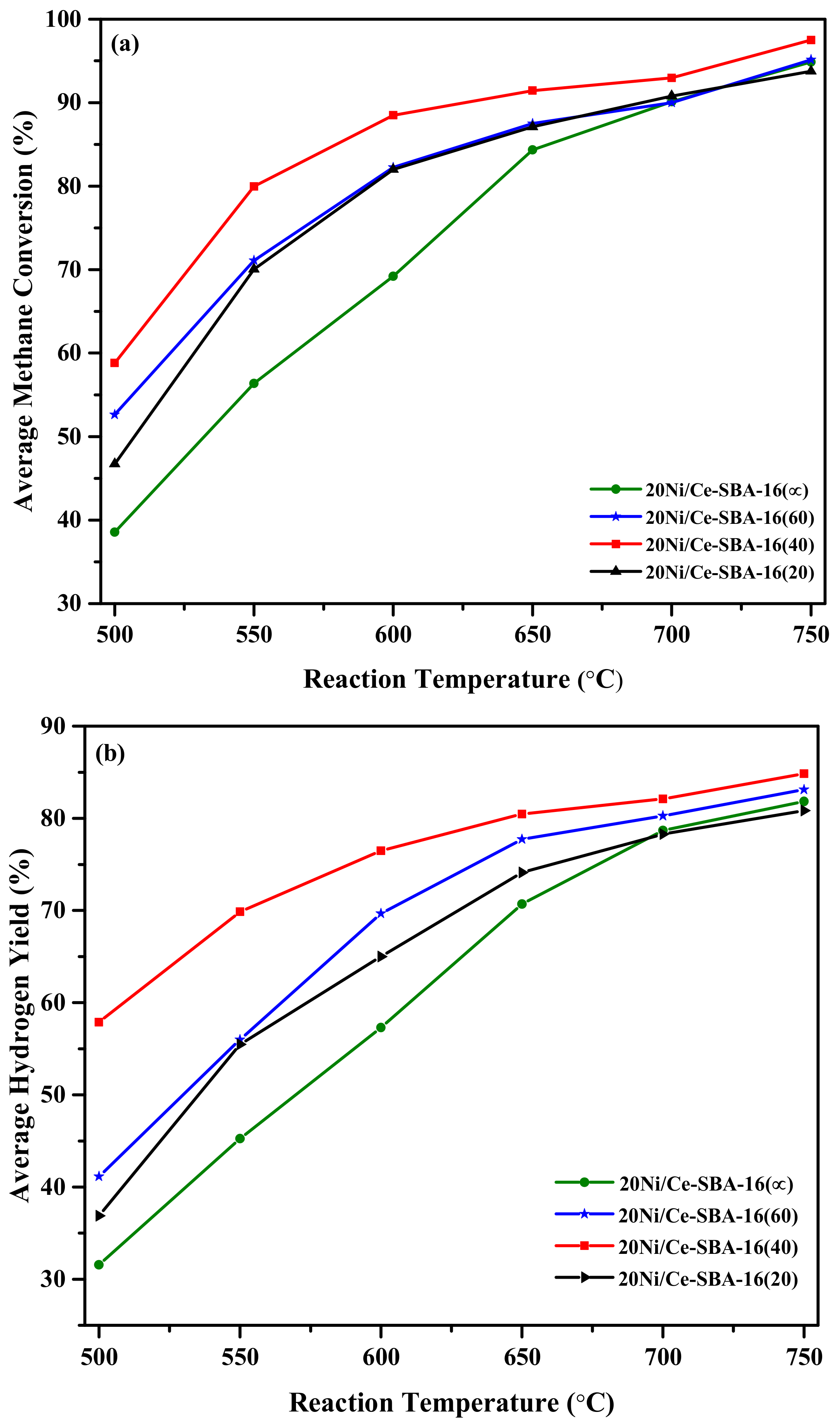
2.2. Effect od Si to Ce Molar Ratios on the Activity
2.3. Effect of Ni Loading Percentage on the Performance of xNi/Ce-SBA-16(40)
2.4. Time-on-Stream Behavior of Oxygen Carrier
3. Experimental Methods
3.1. Oxygen Carrier Preparation
3.2. Oxygen Carrier Characterization
3.3. Process Activity
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Udomsirichakorn, J.; Salam, P.A. Review of hydrogen-enriched gas production from steam gasification of biomass: The prospect of CaO-based chemical looping gasification. Renew. Sustain. Energy Rev. 2014, 30, 565–579. [Google Scholar] [CrossRef]
- Neal, L.; Shafiefarhood, A.; Li, F. Effect of core and shell compositions on MeOx@LaySr1−yFeO3 core–shell redox catalysts for chemical looping reforming of methane. Appl. Energy 2015, 157, 391–398. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Liang, T.; Yang, Z.; Yang, J.; Liu, S. Hydrogen generation from catalytic steam reforming of acetic acid by Ni/attapulgite catalysts. Catalysts 2016, 6, 172. [Google Scholar] [CrossRef]
- Neal, L.; Shafiefarhood, A.; Li, F. Significantly improved catalytic performance of Ni-based MgO catalyst in steam reforming of phenol by inducing mesostructure. Catalysis 2015, 5, 1721–1736. [Google Scholar] [CrossRef]
- Ryden, M.; Ramos, P. H2 production with CO2 capture by sorption enhanced chemical-looping reforming using NiO as oxygen carrier and CaO as CO2 sorbent. Fuel Process. Technol. 2012, 96, 27–36. [Google Scholar] [CrossRef]
- Irani, M.; Alizadehdakhel, A.; Pour, A.N.; Hoseini, N.; Adinehnia, M. CFD modeling of hydrogen production using steam reforming of methane in monolith reactors: Surface or volume-base reaction model? Int. J. Hydrogen Energy 2011, 36, 15602–15610. [Google Scholar] [CrossRef]
- Bernareggi, M.; Dozzi, M.V.; Bettini, L.G.; Ferretti, A.M.; Chiarello, G.L.; Selli, E. Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production. Catalysts 2017, 7, 301. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Wei, Y.; Wang, H.; Sun, L. Chemical-looping steam methane reforming over a CeO2–Fe2O3 oxygen carrier: Evolution of its structure and reducibility. Energy Fuels 2014, 28, 754–760. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, H. Thermodynamic analysis of chemical-looping hydrogen generation. Appl. Energy 2013, 112, 800–807. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process. Int. J. Hydrogen Energy 2015, 40, 16159–16168. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Hydrogen production by chemical looping steam reforming of methane over Mg promoted iron oxygen carrier: Optimization using design of experiments. J. Taiwan Inst. Chem. Eng. 2016, 62, 140–149. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, P.; Li, W. Supported Ni catalyst on a natural halloysite derived silica-alumina composite oxide with unexpected coke-resistant stability for steam-CO2 dual reforming of methane. RSC Adv. 2016, 6, 49487–49496. [Google Scholar] [CrossRef]
- Mishra, A.; Galinsky, N.; He, F.; Santiso, E.E.; Li, F. Perovskite-structured AMnxB1−xO3 (A = Ca or Ba; B = Fe or Ni) redox catalysts for partial oxidation of methane. Catal. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Song, Y.; Zhang, C.; Du, B.; Chen, H.; Wang, C.; Xu, Y. Hydrogen production from chemical looping steam reforming of glycerol by Ni-based oxygen carrier in a fixed-bed reactor. Chem. Eng. J. 2015, 280, 459–467. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Hydrogen production via chemical looping steam methane reforming process: Effect of cerium and calcium promoters on the performance of Fe2O3/Al2O3 oxygen carrier. Appl. Energy 2015, 165, 685–694. [Google Scholar] [CrossRef]
- Forutan, H.R.; Karimi, E.; Hafizi, A.; Rahimpour, M.R.; Keshavarz, P. Expert representation chemical looping reforming: A comparative study of Fe, Mn, Co and Cu as oxygen carriers supported on Al2O3. J. Ind. Eng. Chem. 2015, 21, 900–911. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Saeidia, S.; Fazlollahi, F.; Najarid, S.; Iranshahi, D.; Klemeše, J.J.; Baxter, L.L. Hydrogen production: Perspectives, separation with special emphasis on kinetics of WGS reaction: A state-of-the-art review. J. Ind. Eng. Chem. 2017, 49, 1–25. [Google Scholar] [CrossRef]
- Ortiz, M.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Gayán, P.; Adánez, J. Catalytic Activity of Ni-Based Oxygen-Carriers for Steam Methane Reforming in Chemical-Looping Processes. Energy Fuels 2012, 26, 791–800. [Google Scholar] [CrossRef]
- Daneshmand-Jahromi, S.; Rahimpour, M.R.; Meshksar, M.; Hafizi, A. Hydrogen production from cyclic chemical looping steam methane reforming over yttrium promoted Ni/SBA-16 oxygen carrier. Catalysts 2017, 7, 286. [Google Scholar] [CrossRef]
- Zafar, Q.; Mattisson, T.; Gevert, B. Redox investigation of some oxides of transition-state metals Ni, Cu, Fe, and Mn supported on SiO2 and MgAl2O4. Energy Fuels 2006, 20, 34–44. [Google Scholar] [CrossRef]
- De Diego, L.F.; Ortiz, M.; Garcia-Labiano, F.; Adanez, J.; Abad, A.; Gayan, P. Synthesis gas generation by chemical-looping reforming using a Nibased oxygen carrier. Energy Procedia 2009, 1, 3–10. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.-Y.; Wah, H.H.A.; Zeng, G.; Li, Y.; Yang, Y. Carbon dioxide reforming of methane over nickel-grafted SBA-15 and MCM-41 catalysts. Catal. Today 2009, 148, 243–250. [Google Scholar] [CrossRef]
- Luo, J.; Yu, Z.; Ng, C.; Au, C. CO2/CH4 reforming over Ni–La2O3/5A: An investigation on carbon deposition and reaction steps. J. Catal. 2000, 194, 198–210. [Google Scholar] [CrossRef]
- Qin, L.; Sakamoto, Y.; Anderson, M.W. Controlling the window size in mesoporous SBA-16. Phys. Chem. Chem. Phys. 2014, 16, 15640–15645. [Google Scholar] [CrossRef] [PubMed]
- Lindo, M.; Vizcaíno, A.; Calles, J.; Carrero, A. Ethanol steam reforming on Ni/Al-SBA-15 catalysts: Effect of the aluminium content. Int. J. Hydrogen Energy 2011, 35, 5895–5901. [Google Scholar] [CrossRef]
- Qian, L.; Ma, Z.; Ren, Y.; Shi, H.; Yue, B.; Feng, S.; Shen, J.; Xie, S. Investigation of La promotion mechanism on Ni/SBA-15 catalysts in CH4 reforming with CO2. Fuel 2014, 122, 47–53. [Google Scholar] [CrossRef]
- Wang, N.; Chu, W.; Zhang, T.; Zhao, X.S. Synthesis, characterization and catalytic performances of Ce-SBA-15 supported nickel catalysts for methane dry reforming to hydrogen and syngas. Int. J. Hydrogen Energy 2012, 37, 19–30. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. High purity hydrogen production via sorption enhanced chemical looping reforming: Application of 22Fe2O3/MgAl2O4 and 22Fe2O3/Al2O3 as oxygen carriers and cerium promoted CaO as CO2 sorbent. Appl. Energy 2016, 169, 629–641. [Google Scholar] [CrossRef]
- Wang, K.; Dou, B.; Jiang, B.; Song, Y.; Zhang, C.; Zhang, Q.; Chen, H.; Xu, Y. Renewable hydrogen production from chemical looping steam reforming of ethanol using xCeNi/SBA-15 oxygen carriers in a fixed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 12899–12909. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-Doped Ni/SBA-16 Catalysts for Dry Reforming of Methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B Environ. 2015, 176, 532–541. [Google Scholar] [CrossRef]
- Kong, L.; Li, J.; Zhao, Z.; Liu, Q.; Sun, Q.; Liu, J.; Wei, Y. Oxidative dehydrogenation of ethane to ethylene over Mo-incorporated mesoporous SBA-16 catalysts: The effect of MoOx dispersion. Appl. Catal. A Gen. 2016, 510, 84–97. [Google Scholar] [CrossRef]
- Ramanathan, A.; Zhu, H.; Maheswari, R.; Thapa, P.S.; Subramaniam, B. Comparative study of Nb-incorporated cubic mesoporous silicates as epoxidation catalysts. Ind. Eng. Chem. Res. 2015, 54, 4236–4242. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, Y.M.; Liang, P. An Investigation on Properties of Sn-SBA-16 Synthesized without Mineral Acid Added. Adv. Mater. Res. Trans. Tech. Publ. 2013, 707–710. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Y.; Wei, Y.; Wu, Z.; Yan, J.; Pan, L.; Liu, Y. The hydroxyalkylation of phenol with formaldehyde over mesoporous M(Al, Zr, Al–Zr)-SBA-15 catalysts: The effect on the isomer distribution of bisphenol F. Catal. Commun. 2015, 67, 21–25. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Pawelec, B.; Loricera, C.; Rivera-Muñoz, E.; Nava, R.; Torres, B.; Fierro, J. Comparison of the morphology and HDS activity of ternary Ni (Co)-Mo-W catalysts supported on Al-HMS and Al-SBA-16 substrates. Appl. Catal. B Environ. 2012, 125, 473–485. [Google Scholar] [CrossRef]
- Dong, Y.; Zhan, X.; Niu, X.; Li, J.; Yuan, F.; Zhu, Y.; Fu, H. Facile synthesis of Co-SBA-16 mesoporous molecular sieves with EISA method and their applications for hydroxylation of benzene. Microporous Mesoporous Mater. 2014, 185, 97–106. [Google Scholar] [CrossRef]
- Rodríguez-Castellón, E.; Díaz, L.; Braos-García, P.; Mérida-Robles, J.; Maireles-Torres, P.; Jiménez-López, A.; Vaccari, A. Nickel-impregnated zirconium-doped mesoporous molecular sieves as catalysts for the hydrogenation and ring-opening of tetralin. Appl. Catal. A Gen. 2003, 240, 83–94. [Google Scholar] [CrossRef]
- Akbari-Emadabadi, S.; Rahimpour, M.R.; Hafizi, A.; Keshavarz, P. Production of hydrogen-rich syngas using Zr modified Ca-Co bifunctional catalyst-sorbent in chemical looping steam methane reforming. Appl. Energy 2017, 206, 51–62. [Google Scholar] [CrossRef]
- Meshksar, M.; Daneshmand-Jahromi, S.; Rahimpour, M.R. Synthesis and characterization of cerium promoted Ni/SBA-16 oxygen carrier in cyclic chemical looping steam methane reforming. J. Taiwan Inst. Chem. Eng. 2017, 76, 73–82. [Google Scholar] [CrossRef]
- Akbari-Emadabadi, S.; Rahimpour, M.R.; Hafizi, A.; Keshavarz, P. Promotion of Ca-Co bifunctional catalyst/sorbent with yttrium hydrogen production in modified chemical looping steam methane reforming process. Catalysts 2017, 7, 270. [Google Scholar] [CrossRef]
- Zhu, X.; Wei, Y.; Wang, H.; Li, K. Ce–Fe oxygen carriers for chemical-looping steam methane reforming. Int. J. Hydrogen Energy 2013, 38, 4492–4501. [Google Scholar] [CrossRef]
- Zheng, Y.E.; Zhu, X.; Wang, H.; Li, K.Z.; Wang, Y.H.; Wei, Y.G. Characteristic of macroporous CeO2-ZrO2 oxygen carrier for chemical-looping steam methane reforming. J. Rare Earths 2014, 32, 842–848. [Google Scholar] [CrossRef]

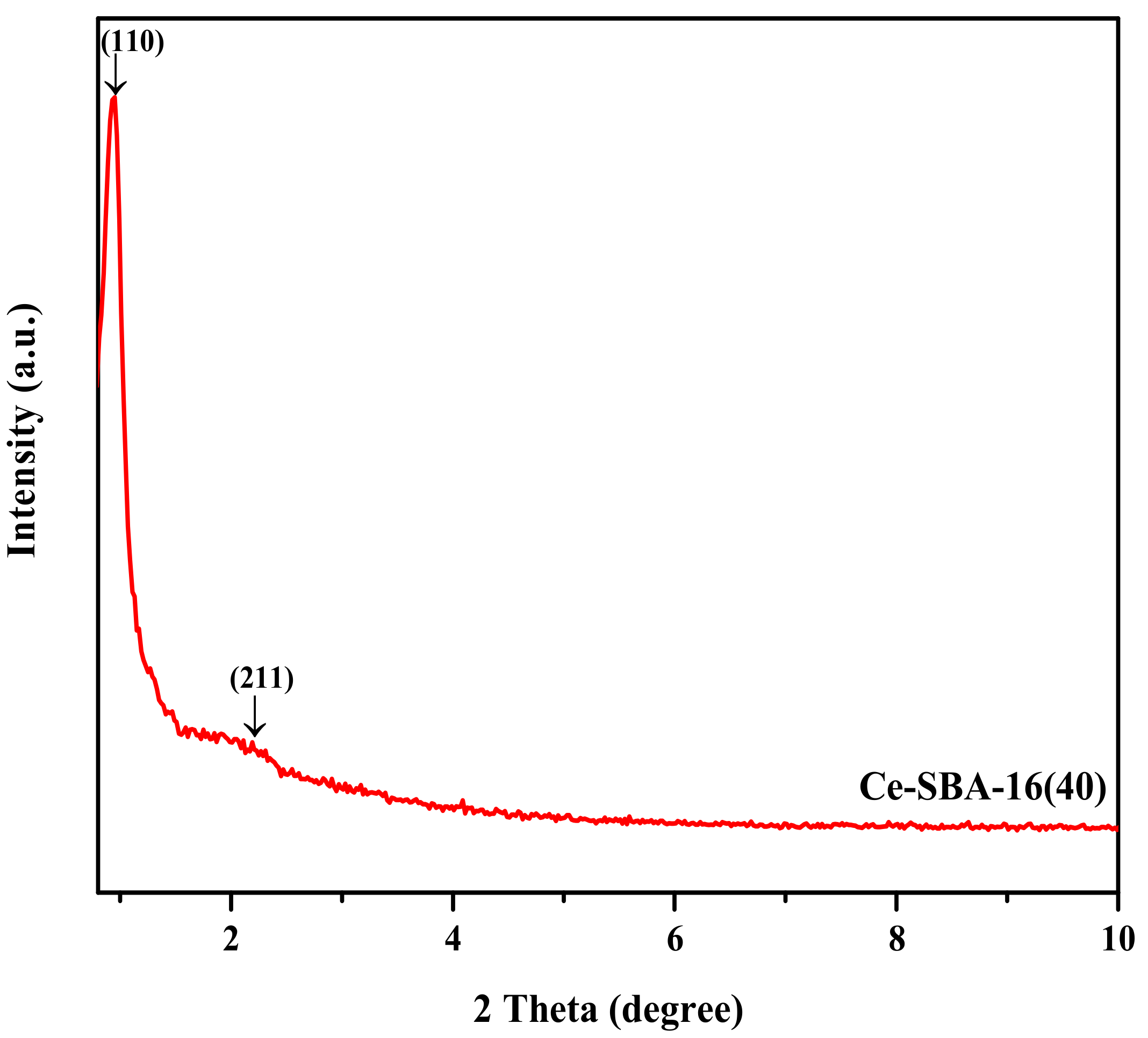










| Sample | BET Surface Area (m2/g) | Pore Diameter (nm) b | Pore Volume (cm3/g) c |
|---|---|---|---|
| Ce-SBA-16(∞ a) | 538.19 | 3.4 | 0.27 |
| Ce-SBA-16(60) | 700.55 | 3.41 | 0.28 |
| Ce-SBA-16(40) | 801.02 | 3.42 | 0.33 |
| Ce-SBA-16(20) | 540.47 | 3.38 | 0.23 |
| 15Ni/Ce-SBA-16(40) | 207.15 | 4.57 | 0.25 |
| Oxygen Carrier | BET Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| 15Ni/Ce-SBA-16(40) | 112.01 | 3.98 | 0.12 |
| Oxygen Carrier | Carbon (wt %) |
|---|---|
| 15Ni/Ce-SBA-16(40) | 0.23 |
| 15Ni/Ce-SBA-16(∞) | 3.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshksar, M.; Rahimpour, M.R.; Daneshmand-Jahromi, S.; Hafizi, A. Synthesis and Application of Cerium-Incorporated SBA-16 Supported Ni-Based Oxygen Carrier in Cyclic Chemical Looping Steam Methane Reforming. Catalysts 2018, 8, 18. https://doi.org/10.3390/catal8010018
Meshksar M, Rahimpour MR, Daneshmand-Jahromi S, Hafizi A. Synthesis and Application of Cerium-Incorporated SBA-16 Supported Ni-Based Oxygen Carrier in Cyclic Chemical Looping Steam Methane Reforming. Catalysts. 2018; 8(1):18. https://doi.org/10.3390/catal8010018
Chicago/Turabian StyleMeshksar, Maryam, Mohammad Reza Rahimpour, Sanaz Daneshmand-Jahromi, and Ali Hafizi. 2018. "Synthesis and Application of Cerium-Incorporated SBA-16 Supported Ni-Based Oxygen Carrier in Cyclic Chemical Looping Steam Methane Reforming" Catalysts 8, no. 1: 18. https://doi.org/10.3390/catal8010018
APA StyleMeshksar, M., Rahimpour, M. R., Daneshmand-Jahromi, S., & Hafizi, A. (2018). Synthesis and Application of Cerium-Incorporated SBA-16 Supported Ni-Based Oxygen Carrier in Cyclic Chemical Looping Steam Methane Reforming. Catalysts, 8(1), 18. https://doi.org/10.3390/catal8010018