Designing Multifunctionality into Single Phase and Multiphase Metal-Oxide-Selective Propylene Ammoxidation Catalysts
Abstract
:1. Introduction

2. Models of Multifunctionality in Heterogeneous Selective Oxidation Catalysts
- H-abstraction from an activated C-H bond—allylic (e.g., propylene, butene), aromatic (e.g., toluene, methylpyridine) or from an alkane C-H bond (e.g., butane, propane)
- O or N insertion into the surface reaction intermediate
- O2 reduction to O2− (lattice oxygen)
- Lattice oxygen transfer (from the O2 reduction site to the H-abstraction site)
- Reoxidation (i.e., regeneration) of the H-abstraction site
- “Support effects”
- “Remote control mechanism”
- “Interfacial effect” or “structural epitaxy”
3. Chemical and Structural Multifunctionality
3.1. Multifunctionality within a Single-Phase, Metal-Oxide Catalyst
3.1.1. Bismuth Molybdate
3.1.2. Bismuth–Iron Molybdate
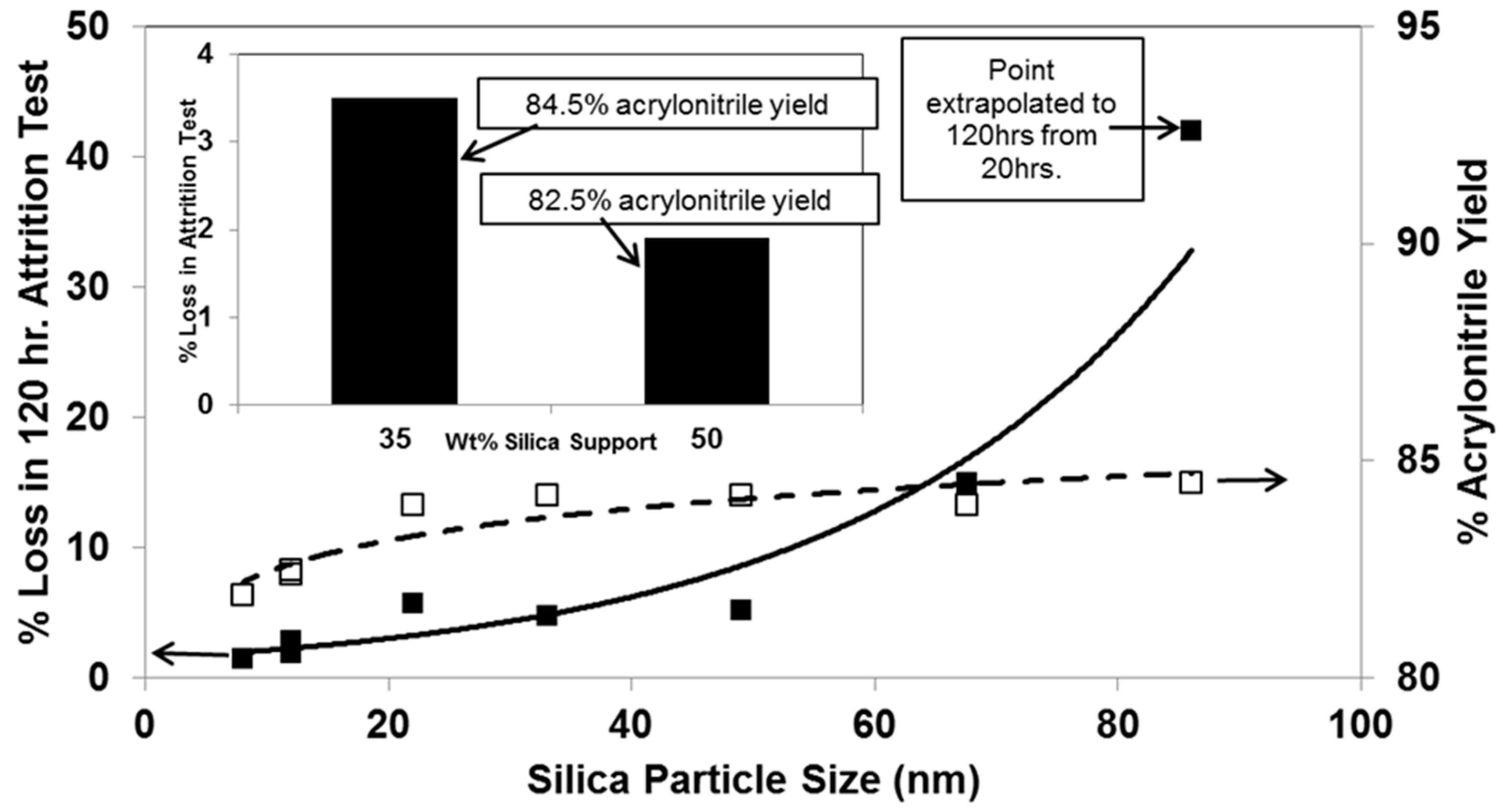
- Imparting the requisite physical strength to withstand the attritional environment of a fluid bed reactor; and
- Achieving the highest selectivity to the desired partial oxidation product, acrylonitrile.
3.1.3. Bismuth–Cerium Molybdate
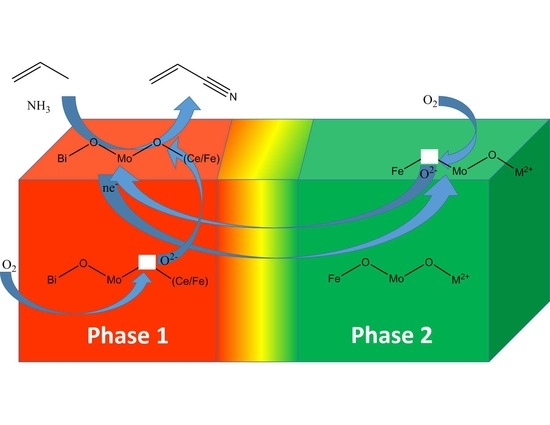
3.2. Multifunctionality in a Multiphase Metal-Oxide Catalyst
- Coherent interfaces in which the lattice planes of the two phases in contact are continuous. This is typically described as an epitaxial interface and usually occurs through structurally-directed intergrowth at elevated synthesis temperatures.
- Incoherent interfaces where no lattice plane continuity exists across two phases.
- Semi-coherent interfaces in which a portion of the contacting lattice planes are continuous but are interspersed with regions of discontinuity.
4. Physical Multifunctionality
- Surface area, which encompasses both the available geometric and chemical areas
- Porosity, including pore size, pore volume and pore structure
- Strength, including crush, ablative and attrition resistance
- Compositional integrity, which generally translate to a loss of catalyst components over time during operation
- Ensuring an adequate or surplus level of molybdenum in the catalyst formulation such that the stoichiometry for the formation of molybdate compounds with the cationic elemental component of the catalyst is satisfied [68].
- Adding to the reactor a supplementary catalyst containing an elevated level of molybdenum in its formulation [69].
5. Future Directions and Opportunities
Supplementary Materials
Conflicts of Interest
References
- Grasselli, R.K. Fundamental principles of selective heterogeneous oxidation catalysis. Top. Catal. 2002, 21, 79–88. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous artial (amm)oxidation and oxidative dehydrogenation catalysis on mixed metal oxides. Catalysts 2016, 6, 22. [Google Scholar] [CrossRef]
- Védrine, J.C.; Fechete, I. Heterogeneous partial oxidation catalysis on metal oxides. C. R. Chim. 2016, 19, 1203–1225. [Google Scholar] [CrossRef]
- Brazdil, J.F. Selective oxidation in industry: Applications of metal oxides in the petrochemical industry. In Metal Oxides in Heterogeneous Catalysis; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 455–502. ISBN 9780128116326. [Google Scholar]
- Grasselli, R.K. Site isolation and phase cooperation: Two important concepts in selective oxidation catalysis: A retrospective. Catal. Today 2014, 238, 10–27. [Google Scholar] [CrossRef]
- Schlögl, R. Selective oxidation: From a still immature technology to the roots of catalysis science. Top. Catal. 2016, 59, 1461–1476. [Google Scholar] [CrossRef]
- Callahan, J.L.; Grasselli, R.K. A selectivity factor in vapor-phase hydrocarbon oxidation catalysis. AIChE J. 1963, 9, 755–760. [Google Scholar] [CrossRef]
- Callahan, J.L.; Grasselli, R.K.; Milberger, E.C.; Strecker, H.A. Oxidation and ammoxidation of propylene over bismuth molybdate catalyst. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 134–142. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Toft, M.A. Ammoxidation. In Encyclopedia of Catalysis; Horváth, I.T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 9780471227618. [Google Scholar]
- Burrington, J.D.; Kartisek, C.T.; Grasselli, R.K. Surface intermediates in selective propylene oxidation and ammoxidation over heterogeneous molybdate and antimonate catalysts. J. Catal. 1984, 87, 363–380. [Google Scholar] [CrossRef]
- Licht, R.B.; Vogt, D.; Bell, A.T. The mechanism and kinetics of propene ammoxidation over a-bismuth molybdate. J. Catal. 2016, 339, 228–241. [Google Scholar] [CrossRef]
- Ponceblanc, H.; Millet, J.M.M.; Coudurier, G.; Herrmann, J.M.; Védrine, J.C. Study of multiphasic molybdate-based catalysts: I. Electrical conductivity study of valence states and solubility limits in mixed iron and cobalt molybdates. J. Catal. 1993, 142, 373–380. [Google Scholar] [CrossRef]
- Millet, J.M.M.; Ponceblanc, H.; Coudurier, G.; Herrmann, J.M.; Védrine, J.C. Study of multiphasic molybdate-based catalysts: II. Synergy effect between bismuth molybdates and mixed iron and cobalt molybdates in mild oxidation of propene. J. Catal. 1993, 142, 381–391. [Google Scholar] [CrossRef]
- Petkov, V.; Wanjala, B.N.; Loukrakpam, R.; Luo, J.; Yang, L.; Zhong, C.; Shastri, S. Pt–Au alloying at the nanoscale. Nano Lett. 2012, 12, 4289–4299. [Google Scholar] [CrossRef] [PubMed]
- Moro-oka, Y.; He, D.-H.; Ueda, W. Studies in Surface Science and Catalysis; Grasselli, R.K., Sleight, A.W., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1991; Volume 67, pp. 57–66. ISBN 978-0-444-88942-3. [Google Scholar]
- Wolfs, M.W.J.; Batist, P.H.A. The selective oxidation of 1-butene over a multicomponent molybdate catalyst. Influences of various elements on structure and activity. J. Catal. 1974, 32, 25–36. [Google Scholar] [CrossRef]
- Weng, L.T.; Sham, E.; Doumain, B.; Ruiz, P.; Delmon, B. Studies in Surface Science and Catalysis; Centi, G., Trifiro, F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1990; Volume 55, pp. 757–765. ISBN 9780080879185. [Google Scholar]
- Weng, L.T.; Patrono, P.; Sham, E.; Ruiz, P.; Delmon, B. Studies in Surface Science and Catalysis; Centi, G., Trifiro, F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1990; Volume 55, pp. 797–806. ISBN 9780080879185. [Google Scholar]
- Cadus, L.E.; Xiong, Y.L.; Gotor, F.J.; Acosta, D.; Naud, J.; Ruiz, P.; Delmon, B. Studies in Surface Science and Catalysis; Centi, G., Trifiro, F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1990; Volume 82, pp. 41–54. ISBN 9780080879901. [Google Scholar]
- Véjux, A.; Courtine, P. Interfacial reactions between V2O5 and TiO2 (anatase): Role of the structural properties. J. Solid State Chem. 1978, 23, 93–103. [Google Scholar] [CrossRef]
- Courtine, P. Thermodynamic and structural aspects of interfacial effects in mild oxidation catalysts. In Solid State Chemistry in Catalysis; Grasselli, R.K., Brazdil, J.F., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; Volume 279, pp. 57–74. ISBN 9780841211100. [Google Scholar]
- Courtine, P.; Bordes, E. Studies in Surface Science and Catalysis; Grasselli, R.K., Oyama, S.T., Gaffney, A.M., Lyons, J.E., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1997; Volume 110, pp. 177–184. ISBN 9780080544717. [Google Scholar]
- Bordes, E. Synergistic effects in selective oxidation catalysis: Does phase cooperation result in site isolation? Top. Catal. 2001, 15, 131–137. [Google Scholar] [CrossRef]
- Aykan, K. Reduction of Bi2O3 MoO3 catalyst during the ammoxidation of propylene in the absence of gaseous oxygen. J. Catal. 1968, 12, 281–290. [Google Scholar] [CrossRef]
- Van den Elzen, A.F.; Riech, G.D. An outline of the crystal-structure of Bi2Mo2O9. Mater. Res. Bull. 1975, 10, 1163–1168. [Google Scholar] [CrossRef]
- Teller, R.G.; Brazdil, J.F.; Grasselli, R.K.; Jorgensen, J.D. The structure of γ-bismuth molybdate, Bi2MoO6, by powder neutron diffraction. Acta Crystallogr. Sect. C 1984, 40, 2001–2005. [Google Scholar] [CrossRef]
- Sleight, A.W.; Jeitschko, W. Bi3(FeO4)(MoO4)2 and Bi3(GaO4)(MoO4)2—New compounds with scheelite related structures. Mater. Res. Bull. 1974, 9, 951–954. [Google Scholar] [CrossRef]
- Jeitschko, W.; Sleight, A.W.; McClellan, W.R.; Weiher, J.F. A comprehensive study of disordered and ordered scheelite-related Bi3(FeO4)(MoO4)2. Acta Crystallogr. Sect. B 1976, 32, 1163–1170. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Yoshida, J.; Yamaguchi, T. Oxide Catalyst and Method for Producing the Same, and Methods for Producing Unsaturated Aldehyde, Diolefin, and Unsaturated Nitrile. U.S. Patent 9,364,817, 14 June 2016. [Google Scholar]
- Brazdil, J.F.; Grasselli, R.K. Relationship between solid state structure and catalytic activity of rare earth and bismuth-containing molybdate ammoxidation catalysts. J. Catal. 1983, 79, 104–117. [Google Scholar] [CrossRef]
- Giordano, N.; Bart, J.C.J. On the mechanism of ammoxidation of propene over cerium-doped bismuth molybdate catalysts. Recl. Trav. Chim. Pays-Bas 1975, 94, 28–30. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Glaeser, L.C.; Grasselli, R.K. Role of interfacial phenomena in the catalytic behavior of multiphase selective ammoxidation catalysts. J. Phys. Chem. 1983, 87, 5485–5491. [Google Scholar] [CrossRef]
- Suresh, D.D.; Friedrich, M.S.; Seely, M.J. Catalyst for Process for Manufacture of Acrylonitrile and Methacrylonitrile. U.S. Patent 5,093,299, 3 March 1992. [Google Scholar]
- Suresh, D.D.; Friedrich, M.S.; Seely, M.J. Catalyst for the Manufacture of Acrylonitrile and Methacrylonitrile. U.S. Patent 5,212,137, 18 May 1993. [Google Scholar]
- Midorikawa, H.; Someya, K.; Aoki, K.; Nagano, O. Ammoxidation Catalyst Composition and Process for Producing Acrylonitrile or Methacrylonitrile Using the Same. U.S. Patent 5,658,842, 19 August 1997. [Google Scholar]
- Paparizos, C.; Jevne, S.C.; Seely, M.J. Catalyst for the Manufacture of Acrylonitrile. U.S. Patent 7,071,140, 4 July 2006. [Google Scholar]
- Paparizos, C.; Jevne, S.C.; Seely, M.J. Catalyst for the Manufacture of Acrylonitrile. U.S. Patent 7,348,291, 25 March 2008. [Google Scholar]
- Jeitschko, W. Crystal structure of La2(MoO4)3, a new ordered defect Scheelite type. Acta Crystallogr. 1973, B29, 2074–2081. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, J.; Li, W. Preparation of tetragonal defect scheelite-type RE2(MoO4)3 (RE = La to Ho) by precipitation method. Solid State Ionics 1989, 32/33, 244–249. [Google Scholar] [CrossRef]
- Brixner, L.H.; Sleight, A.W.; Licis, M.S. Cell Dimensions of the Molybdates La2(MoO4)3 Ce2(MoO4)3, Pr2(MoO4)3 and Nd2(MoO4)3. J. Solid State Chem. 1972, 5, 247–249. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Toft, M.A.; McKenna, S.T. Mixed Metal Oxide Catalysts. U.S. Patent 9,433,929, 6 September 2016. [Google Scholar]
- Brazdil, J.F.; Toft, M.A.; McKenna, S.T. Mixed Metal Oxide Catalysts. U.S. Patent 9,550,729, 24 January 2017. [Google Scholar]
- Woo, J.; Sanghavi, U.; Vonderheide, A.; Guliants, V. A study of M1/M2 phase synergy in the MoVTe(Nb,Ta)O catalysts for propane ammoxidation to acrylonitrile. Appl. Catal. A Gen. 2016, 515, 179–189. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Toft, M.A.; Lin, S.Y.; McKenna, S.T.; Zajac, G.; Kaduk, J.A.; Golab, J.T. Characterization of bismuth-cerium-molybdate selective propylene ammoxidation catalysts. Appl. Catal. A Gen. 2015, 495, 115–123. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Lin, S.Y. Ammoxidation Catalysts Containing Samarium. U.S. Patent 2017/0114007, 27 April 2017. [Google Scholar]
- Lankhorst, M.H.R.; Bouwmeester, H.J.M.; Verweij, H. Thermodynamics and transport of ionic and electronic defects in crystalline oxides. J. Am. Ceram. Soc. 1997, 80, 2175–2198. [Google Scholar] [CrossRef]
- Chen, D.; He, D.; Lu, J.; Zhong, L.; Liu, F.; Liu, J.; Yu, J.; Wan, G.; He, S.; Luo, Y. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designedH2-TPR characterization. Appl. Catal. B Environ. 2017, 218, 249–259. [Google Scholar] [CrossRef]
- Van Der Merwe, J.H. The role of lattice misfit in epitaxy. Crit. Rev. Solid State Mater. Sci. 1978, 7, 209–231. [Google Scholar] [CrossRef]
- De Schutter, B.; De Keyser, K.; Detavernier, C. Visualization and classification of epitaxial alignment at hetero-phase boundaries. Solid Films 2016, 599, 104–112. [Google Scholar] [CrossRef]
- Ponceblanc, H.; Millet, J.M.M.; Coudurier, G.; Legendre, O.; Védrine, J.C. Solid-solid phase equilibria in the binary system CoMoO4-FeMoO4 and effect of FeIII on the phase equilibria. J. Phys. Chem. 1992, 96, 9462–9465. [Google Scholar] [CrossRef]
- Brazdil, J.F.; Mehici, M.; Glaeser, L.C.; Hazle, M.A.S.; Grasselli, R.K. Correlation between spectroscopic measurements and catalytic behavior of selective oxidation catalysts. In Catalyst Characterization Science; Deviney, M.L., Gland, J.L., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; Volume 288, pp. 26–36. ISBN 9780841211209. [Google Scholar]
- Bordes, E. Comparative study of structure-sensitive oxidation of n-butane and 1-butene in maleic anhydride on two kinds of catalysts. In Studies in Surface Science and Catalysis; Centi, G., Trifiro, F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1990; Volume 55, pp. 585–592. ISBN 9780080879185. [Google Scholar]
- Brazdil, J.F. A critical perspective on the design and development of metal oxide catalysts for selective propylene ammoxidation and oxidation. Appl. Catal. A Gen. 2017, 543, 225–233. [Google Scholar] [CrossRef]
- Volkov, S.; Vonk, V.; Khorshidi, N.; Franz, D.; Kubicek, M.; Kilic, V.; Felici, R.; Huber, T.M.; Navickas, E.; Rupp, G.M.; et al. Operando X-ray Investigation of Electrode/Electrolyte Interfaces in Model Solid Oxide Fuel Cells. Chem. Mater. 2016, 28, 3727–3733. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, E.; Gregori, G.; Wang, Y.; Sigle, W.; van Aken, P.A.; Maier, J. Interface Effects on the Ion Transport of Epitaxial Y2Zr2O7 Films. ACS Appl. Mater. Interfaces 2017, 9, 27257–27265. [Google Scholar] [CrossRef] [PubMed]
- Besecker, C.J.; Brazdil, J.F.; Toft, M.A.; Seely, M.J.; Gustaferro, R.A. Process for Preparing Improved Mixed Metal Oxide Ammoxidation Catalysts. U.S. Patent 8,258,073, 4 September 2012. [Google Scholar]
- Lugmair, C.G. Metal Oxide Catalyst Material and Process for Making and Using Same. U.S. Patent 9,815,045, 14 November 2017. [Google Scholar]
- Yanagi, H.; Midorikawa, H.; Ueda, T. Particulate Porous Ammoxidation Catalyst. U.S. Patent 7,473,666, 6 January 2009. [Google Scholar]
- Brazdil, J.F.; Toft, M.A.; Seely, M.J.; Besescker, C.J.; Gustaferro, R.A. Attrition Resistant Mixed Metal Oxide Ammoxidation Catalysts. U.S. Patent 8,455,388, 4 June 2013. [Google Scholar]
- Callahan, J.L.; Shaw, W.G.; Miller, A.F. Reactivation of Molybdenum Containing Oxidation Catalysts in Fluid Bed Reactors. U.S. Patent 3,882,159, 6 May 1975. [Google Scholar]
- Sasaki, Y.; Kiyomiya, Y.; Nakamura, T. Process for Improving the Activity of Tellurium Containing Metal Oxide Catalysts. U.S. Patent 4,709,071, 24 November 1987. [Google Scholar]
- Valente, J.S.; Armendáriz-Herrera, H.; Quintana-Solórzano, R.; del Ángel, P.; Nava, N.; Massó, A.; López Nieto, J.M. Chemical, structural, and morphological changes of a MoVTeNb catalyst during oxidative dehydrogenation of ethane. ACS Catal. 2014, 4, 1292–1301. [Google Scholar] [CrossRef]
- Lwin, S.; Diao, W.; Baroi, C.; Gaffney, A.M.; Fushimi, R.R. Characterization of MoVTeNbOx catalysts during oxidation reactions using in situ/operando techniques: A review. Catalysts 2017, 7, 109. [Google Scholar] [CrossRef]
- Popova, G.Y.; Andrushkevich, T.V.; Chesalov, Y.A.; Plyasova, L.M.; Dovlitova, L.S.; Ischenko, E.V.; Aleshina, G.I.; Khramov, M.I. Formation of active phases in MoVTeNb oxide catalysts for ammoxidation of propane. Catal. Today 2009, 144, 312–317. [Google Scholar] [CrossRef]
- Popova, G.Y.; Andrushkevich, T.V.; Dovlitova, L.S.; Aleshina, G.A.; Chesalov, Y.A.; Ishenko, A.V.; Ishenko, E.V.; Plyasova, L.M.; Malakhov, V.V.; Khramov, M.I. The investigation of chemical and phase composition of solid precursor of MoVTeNb oxide catalyst and its transformation during the thermal treatment. Appl. Catal. A Gen. 2009, 353, 249–257. [Google Scholar] [CrossRef]
- Kameo, H.; Kajitani, H.; Iwakai, K.; Takeo, H.; Orita, S.; Takeuchi, T. Method for Producing Conjugated Diene. U.S. Patent 9,340,472, 17 May 2016. [Google Scholar]
- Watanabe, H.; Yanagita, M.; Miyaki, K. Catalyst for Producing Acrylonitrile and Process for Producing Acrylonitrile. U.S. Patent 8,034,737, 11 October 2011. [Google Scholar]
- Chen, X.; Wu, L. Supplementary Catalyst for Ammoxidation Catalysts. U.S. Patent 5,177,048, 5 January 1993. [Google Scholar]
- Elischer, S.; Lienhard, K.; Tautz, H. Process for Reactivating Molybdenum-Containing Catalysts. DE 3,311,521 A1, 18 August 1984. [Google Scholar]
- Watanabe, H.; Yanagita, M.; Karasuda, T.; Nishida, K.; Miyaki, K. Method for Producing Acrylonitrile. U.S. Patent 9,334,233, 1 May 2016. [Google Scholar]
- Watanabe, H.; Yanagita, M.; Karasuda, T.; Nishida, K.; Miyaki, K. Method for Producing Acrylonitrile. U.S. Patent 9,181,178, 10 November 2015. [Google Scholar]
- Karasuda, T.; Watanabe, H.; Yanagita, M.; Nishida, K. Method for Producing Acrylonitrile. U.S. Patent 9,328,063, 3 May 2016. [Google Scholar]
- Deniau, B.; Nguyen, T.T.; Delichere, P.; Safonova, O.; Millet, J.M. Redox state dynamics at the surface of MoVTe(Sb)NbO M1 phase in selective oxidation of light alkanes. Top. Catal. 2013, 56, 1952–1962. [Google Scholar] [CrossRef]
- Yushuke, I.; Kazushi, O. Oxide Catalyst, Production Method Therefor, and Unsaturated Nitrile Production Method. WO2015133510A1, 4 March 2015. [Google Scholar]
- Tateno, E.; Ichihara, T.; Kato, T. Oxide Catalyst, Process for Producing Oxide Catalyst, Process for Producing Unsaturated Acid and Process for Producing Unsaturated Nitrile. U.S. Patent 9,427,727, 30 August 2016. [Google Scholar]
- Ushikubo, T.; Sawaki, I.; Oshima, K.; Inumaru, K.; Kobayakawa, S.; Kiyono, K. Process for Preparing a Catalyst Useful for Producing a Nitrile. U.S. Patent 5,422,328, 6 June 1995. [Google Scholar]
- Akiyoshi, F.; Masatoshi, K. Fluid bed Ammoxidation Reaction Catalyst, and Acrylonitrile Production Method. WO2017130909A1, 3 August 2017. [Google Scholar]
- Brazdil, J.F. Selective oxidation in industry: Applications of metal oxides in the petrochemical industry, Chapter 8–2. In Heterogeneous Catalysis by Metal Oxides; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Brazdil, J.F. Scheelite: A versatile structural template for selective alkene oxidation catalysts. Catal. Sci. Technol. 2015, 5, 3452–3458. [Google Scholar] [CrossRef]
- Joly, Y.; Matteo, S.D.; Bunău, O. Resonant X-ray diffraction: Basic theoretical principles. Eur. Phys. J. Spec. Top. 2012, 208, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Bond, G.E. The electronic structure of platinum-gold alloy particles. Platinum Metals Rev. 2007, 51, 63–68. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ruiz, J.A.; Cooper, A.R.; Li, G.; Albrecht, K.O. Enhanced hydrothermal stability and catalytic activity of LaxZryOz mixed oxides for ketonization of acetic acid in the aqueous condensed phase. ACS Catal. 2017, 7, 6400–6412. [Google Scholar] [CrossRef]
- DuBois, J. Method for the Synthesis of Acrylonitrile from Glycerol. U.S. Patent 8,829,223, 9 September 2014. [Google Scholar]
- Devaux, J.; DuBois, J. Process for Manufacturing Acrolein/Acrylonitrile. U.S. Patent 9,296,676, 29 March 2016. [Google Scholar]
- Goyal, A. Compositions and Methods Related to the Production of Acrylonitrile. U.S. Patent 2016/0368861, 22 December 2016. [Google Scholar]
- Karp, E.M.; Eaton, T.R.; Sànchez I Nogué, V.; Vorotnikov, V.; Biddy, M.J.; Tan, E.C.D.; Brandner, D.G.; Cywar, R.M.; Liu, R.; Manker, L.P.; et al. Renewable acrylonitrile production. Science 2017, 358, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Graselli, R.K.; Trifirò, F. Acrylonitrile from biomass: Still far from being a sustainable process. Top. Catal. 2016, 59. [Google Scholar] [CrossRef]
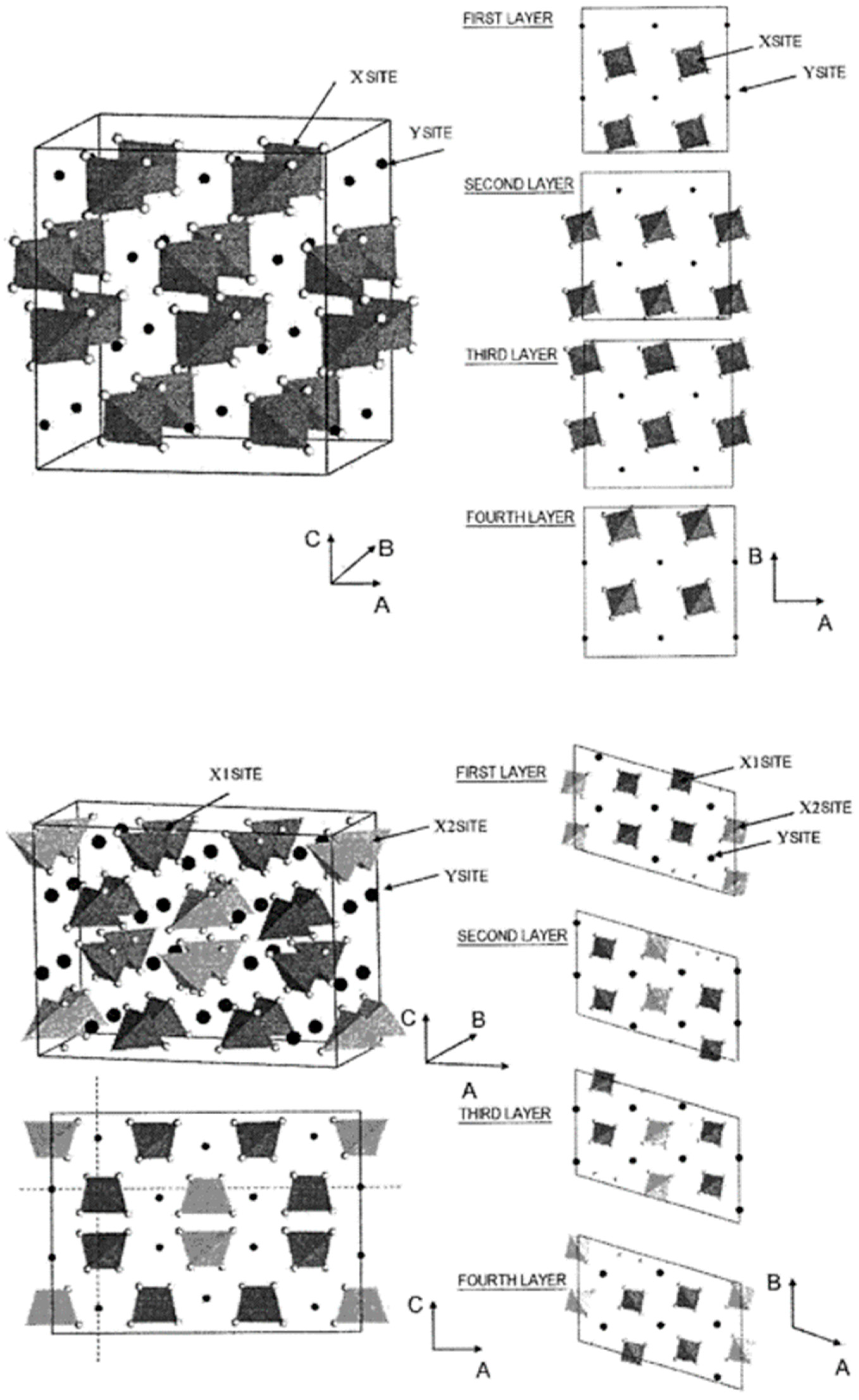
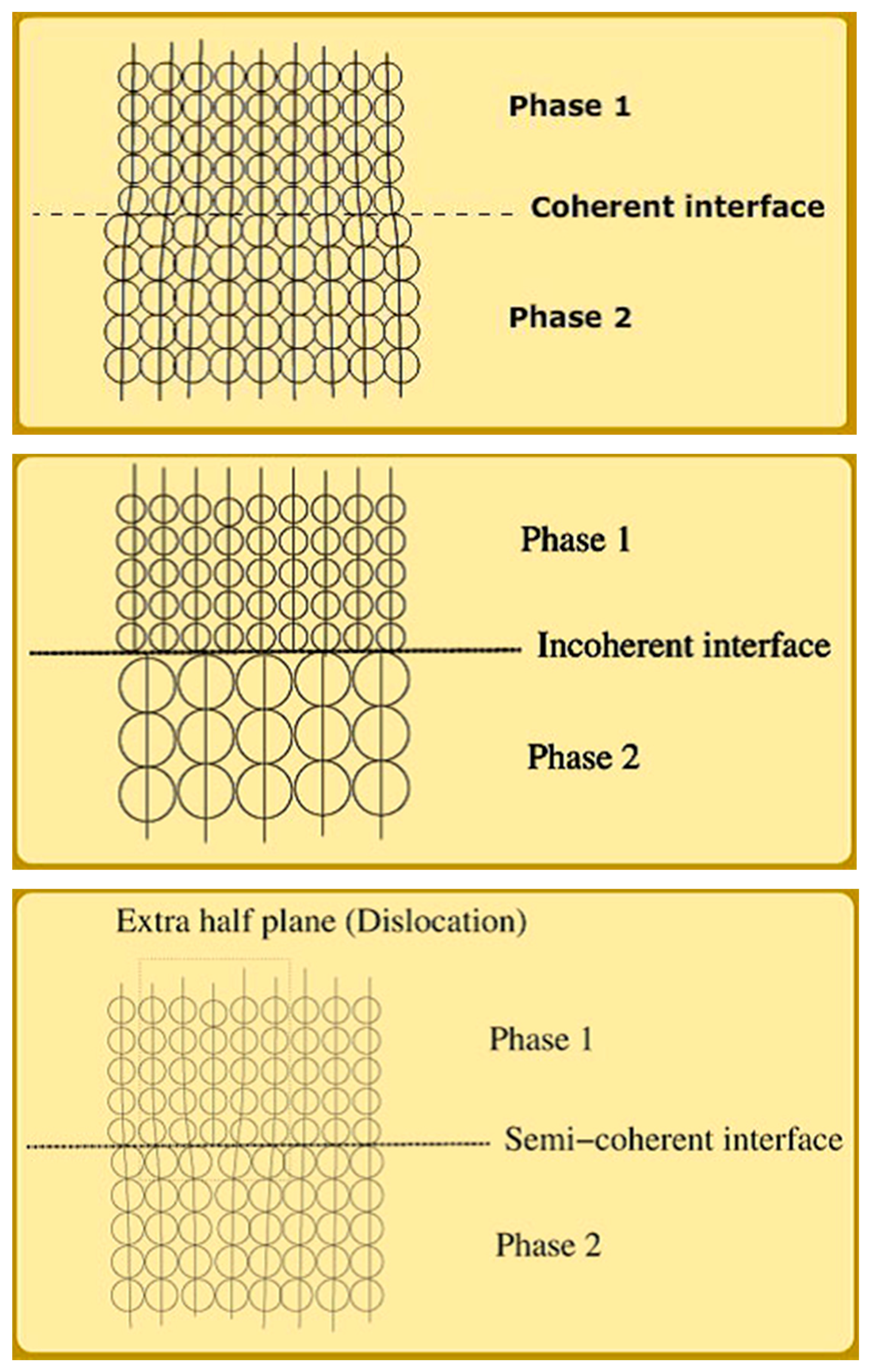
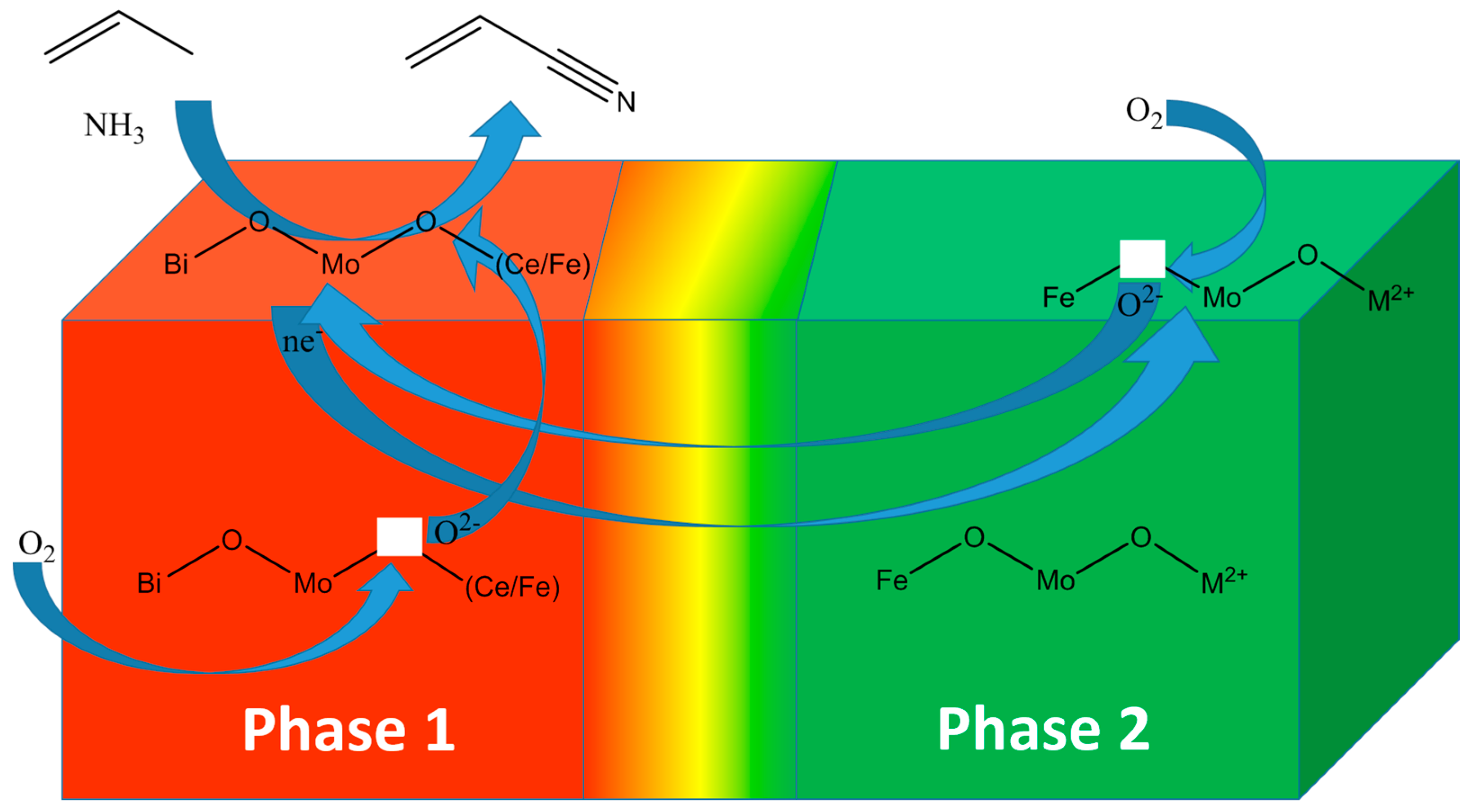
| Chemical Transformation | Required Chemical Functionality | Prototypical Catalyst Components |
|---|---|---|
| Hydrogenation |
| Pt, Pd, other Group 8,9,10 single metals |
| Hydrogenolysis |
| Pt, Pd, other Group 8,9,10 single and multiple metals |
| Reforming |
| Group 8,9,10 bimetallic and multimetallic supported on zeolites |
| Ammonia synthesis |
| Single and multimetallic |
| Selective oxidation |
| Multimetal oxides |
| Selective ammoxidation |
| Multimetal oxides |
| % of Bismuth–Cerium Molybdate in Monoclinic Scheelite Structure | % of Bismuth–Cerium Molybdate in Tetragonal Scheelite Structure | % Acrylonitrile Yield |
|---|---|---|
| 38 | 62 | 79.8 |
| 44 | 56 | 81.4 |
| 48 | 52 | 82.5 |
| 56 | 44 | 83.3 |
| 59 | 41 | 84.2 |
| Phase 1 | Phase 2 | Reference | Possible Epitaxial Contact Planes Phase1/Phase2 | Interplanar Spacing dPhase1/dPhase2 (nm) | Disregistry δ (%) |
|---|---|---|---|---|---|
| α-Bi2Mo3O12 | β-(Fe,Co)MoO4 | [51,52] | 100/001 | 7.68/7.07 | 8 |
| (VO)2P2O7 | β-CoMoO4 | [53] | 100/110 | 3.85/3.61 | 6 |
| Bi1.8Ce0.2MoO12 | Bi0.875Ce1.125MoO12 | [54] | 010/010 | 11.55/11.82 | 2 |
| Catalyst Description | % C3H6 Conv. | % C3H3N Yield | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cs | Co | Fe | Bi | Mo Oxide | SiO2 (12 and 44 nm particle size) | 99.1 | 76.3 | [30] | |
| Cs | Co | Fe | Bi | Ce | Mo Oxide | SiO2 (12 and 44 nm particle size) | 99.2 | 86.4 | [30] |
| Rb | CoNiMg | Fe | Bi | Ce | Mo Oxide | SiO2 (12 and 44 nm particle size) | 99.3 | 86.0 | [30] |
| Cs | Co | Fe | Bi | Pr | Mo Oxide | SiO2 (12 and 44 nm particle size) | 99.3 | 85.7 | [30] |
| Rb | CoNiMg | Fe | Bi | Ce | Mo Oxide | SiO2 (12 and 41 nm particle size) | 99.2 | 85.1 | [78] |
| Rb | NiMg | FeCr | Bi | CeSm | Mo Oxide | SiO2 (38.2 nm particle size) | 98.8 | 85.8 | [46] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazdil, J.F. Designing Multifunctionality into Single Phase and Multiphase Metal-Oxide-Selective Propylene Ammoxidation Catalysts. Catalysts 2018, 8, 103. https://doi.org/10.3390/catal8030103
Brazdil JF. Designing Multifunctionality into Single Phase and Multiphase Metal-Oxide-Selective Propylene Ammoxidation Catalysts. Catalysts. 2018; 8(3):103. https://doi.org/10.3390/catal8030103
Chicago/Turabian StyleBrazdil, James F. 2018. "Designing Multifunctionality into Single Phase and Multiphase Metal-Oxide-Selective Propylene Ammoxidation Catalysts" Catalysts 8, no. 3: 103. https://doi.org/10.3390/catal8030103
APA StyleBrazdil, J. F. (2018). Designing Multifunctionality into Single Phase and Multiphase Metal-Oxide-Selective Propylene Ammoxidation Catalysts. Catalysts, 8(3), 103. https://doi.org/10.3390/catal8030103