MgAl-Layered Double Hydroxide Solid Base Catalysts for Henry Reaction: A Green Protocol
Abstract
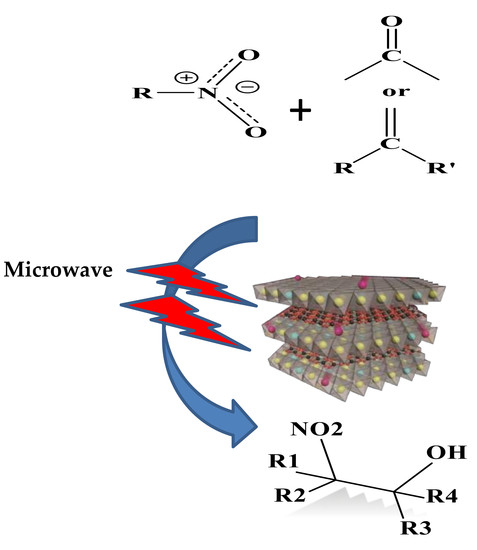
:1. Introduction
2. Results and Discussion
2.1. Elemental Chemical Analysis (ICP)
2.2. X-ray Diffraction (XRD)
2.3. Scanning Electron Microscopy (SEM)
2.4. N2 Physisorption
2.5. CO2 Temperature-Programmed Desorption (CO2-TPD)
2.6. Catalytic Activity Study
3. Experimental Section
3.1. Reagents
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
3.4. Characterization of Reaction Products
3.5. Typical Procedure for the Catalytic Test Reaction
3.5.1. Method A: Conventional Method
3.5.2. Method B: Microwave Irradiation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olah, G.A.; Krishnamurti, R.; Prakash, G.K.S. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon Press: Oxford, UK, 1991; Volume III, p. 293. [Google Scholar]
- Rosini, G. Comprehensive Organic Synthesis; Heathcock, C.H., Trost, B.M., Fleming, I., Eds.; Pergamon Press: Oxford, UK, 1991; Volume 2, p. 321. [Google Scholar]
- Ballini, R.; Bosica, G.; Forconi, P. Nitroaldol (Henry) reaction catalyzed by amberlyst A-21 as a far superior heterogeneous catalyst. Tetrahedron 1996, 52, 1677–1684. [Google Scholar] [CrossRef]
- Contantino, U.; Curini, M.; Marmottini, F.; Rosati, O.; Pisani, E. Potassium Exchanged Layered Zirconium Phosphate as Base Catalyst in the Synthesis of 2-Nitroalkanols. Chem. Lett. 1994, 23, 2215–2218. [Google Scholar] [CrossRef]
- Sheldon, R.A. Catalysis: The key to waste minimization. J. Chem. Technol. Biotechnol. 1997, 68, 381–388. [Google Scholar] [CrossRef]
- De Vries, A.H.M.; de Vries, J.G.; van Assema, F.B.J.; de Lange, B.; Mink, D.; Hyett, D.J. Asymmetric Synthesis of (S)-2-Indolinecarboxylic Acid by Combining Biocatalysis and Homogeneous Catalysis. ChemCatChem 2011, 3, 289–292. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Reddy, C.V.; Rao, K.K.; Figueras, F. Henry reactions catalysed by modified Mg–Al hydrotalcite: An efficient reusable solid base for selective synthesis of β-nitroalkanols. Green Chem. 1999, 1, 187–189. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Kavita, B. Synthesis of 2-nitroalkanols by Mg3Al2O-t-Bu hydrotalcite. J. Mol. Catal. A Chem. 2001, 169, 193–197. [Google Scholar] [CrossRef]
- Seebach, D.; Beck, A.K.; Mukhopdyay, T.; Thomas, E.H. Diastereoselective Synthesis of Nitroaldol Derivatives. Helv. Chim. Acta 1982, 65, 1101–1133. [Google Scholar] [CrossRef]
- Rosini, G.; Ballini, R.; Sorrenti, P. Synthesis of 2-Nitroalkanols on Alumina Surfaces without Solvent: A Simple, Mild and Convenient Method. Synthesis 1983, 1983, 1014–1016. [Google Scholar] [CrossRef]
- Melot, J.M.; Texier-Boullet, F.; Foucaud, A. Preparation and oxidation of α-nitro alcohols with supported reagents. Tetrahedron Lett. 1986, 27, 493–496. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G. Nitroaldol Reaction in Aqueous Media: An Important Improvement of the Henry Reaction. J. Org. Chem. 1997, 62, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Kloetstra, K.R.; van Bekkum, H. Base and acid catalysis by the alkali-containing MCM-41 mesoporous molecular sieve. J. Chem. Soc. Chem. Commun. 1995, 1005–1006. [Google Scholar] [CrossRef]
- Mokhtar, M.; Saleh, T.S.; Ahmed, N.S.; Al-Thabaiti, S.A.; Al-Shareef, R.A. An eco-friendly N-sulfonylation of amines using stable and reusable Zn–Al–hydrotalcite solid base catalyst under ultrasound irradiation. Ultrason. Sonochem. 2011, 18, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Saleh, T.S.; Basahel, S.N. Mg–Al hydrotalcites as efficient catalysts for Aza-Michael addition reaction: A green protocol. J. Mol. Catal. A Chem. 2012, 353–354, 122–131. [Google Scholar] [CrossRef]
- Saleh, T.S.; Narasimharao, K.; Ahmed, N.S.; Basahel, S.N.; Al-Thabaiti, S.A.; Mokhtar, M. Mg–Al hydrotalcite as an efficient catalyst for microwave assisted regioselective 1,3-dipolar cycloaddition of nitrilimines with the enaminone derivatives: A green protocol. J. Mol. Catal. A Chem. 2013, 367, 12–22. [Google Scholar] [CrossRef]
- Narasimharao, K.; Al-Sabban, E.; Saleh, T.; Gallastegu, A.G.; Sanfiz, A.C.; Basahel, S.; Al-Thabaiti, S.; Alyoubi, A.; Obaid, A.; Mokhtar, M. Microwave assisted efficient protocol for the classic Ullman homocoupling reaction using Cu-MG-Al hydrotalcite catalysts. J. Mol. Catal. A Chem. 2013, 379, 152–162. [Google Scholar] [CrossRef]
- Basahel, S.N.; Al-Thabaiti, S.A.; Narasimharao, K.; Ahmed, N.S.; Mokhtar, M. Nanostructured Mg–Al Hydrotalcite as Catalyst for Fine Chemical Synthesis. J. Nanosci. Nanotechnol. 2014, 14, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Sanfiz, A.C.; Vega, N.M.; de Marco, M.; Mokhtar, D.I.M.; Bawaked, S.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Shaffer, M.S.P. Self-condensation of acetone over Mg–Al layered double hydroxide supported on multi-walled carbon nanotube catalysts. J. Mol. Catal. A Chem. 2015, 398, 50–57. [Google Scholar] [CrossRef]
- Phukan, M.; Borah, K.J.; Borah, R. Henry reaction in environmentally benign methods using imidazole as catalyst. Green Chem. Lett. Rev. 2009, 2, 249–253. [Google Scholar] [CrossRef]
- Bulbule, V.J.; Deshpande, V.H.; Velu, S.; Sudalai, A.; Sivasankar, S.; Sathe, V.T. Heterogeneous Henry reaction of aldehydes: Diastereoselective synthesis of nitroalcohol derivatives over Mg–Al hydrotalcites. Tetrahedron 1999, 55, 9325–9332. [Google Scholar] [CrossRef]
- Mokhtar, M.; Inayat, A.; Ofili, J.; Schwieger, W. Thermal decomposition, gas phase hydration and liquid phase reconstruction in the system Mg/Al hydrotalcite/mixed oxide: A comparative study. Appl. Clay Sci. 2010, 50, 176–181. [Google Scholar] [CrossRef]
- Tichit, D.; Fajula, F. Layered double hydroxides as solid base catalysts and catalyst precursors. Stud. Surf. Sci. Catal. 1999, 125, 329–340. [Google Scholar]
- Scherrer, P. Göttinger Nachrichten Gesell; Springer: Berlin, Germany, 1918; Volume 2, p. 98. [Google Scholar]
- Reichle, W.T.; Kang, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. J. Catal. 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Tichit, D.; Pérez-Ramírez, J.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Aldol condensation of campholenic aldehyde and MEK over activated hydrotalcite. Appl. Catal. B Environ. 2007, 70, 577–584. [Google Scholar] [CrossRef]
- Chimentao, R.J.; Abello, S.; Medina, F.; Llorca, J.; Sueiras, J.E.; Cesteros, Y.; Salagre, P. Defect-induced strategies for the creation of highly active hydrotalcites in base-catalyzed reactions. J. Catal. 2007, 252, 249–257. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Durand, R.; Tichit, D. Investigation of Acid−Base Properties of Catalysts Obtained from Layered Double Hydroxides. J. Phys. Chem. B 2000, 104, 1117–11126. [Google Scholar] [CrossRef]
- Abello, S.; Medina, F.; Tichit, D.; Perez-Ramirez, J.; Rodriguez, X.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Study of alkaline-doping agents on the performance of reconstructed Mg–Al hydrotalcites in aldol condensations. Appl. Catal. A Gen. 2005, 281, 191–198. [Google Scholar] [CrossRef]
- Klein, T.A.; Schkeryantz, J.M. Tandem Hass-Bender/Henry reaction for the synthesis of dimethylnitro alcohols from benzylic halides. Tetrahedron Lett. 2005, 46, 4535–4538. [Google Scholar] [CrossRef]
- Soengas, R.G.; Silva, A.M.S. Indium-catalyzed Henry-type reaction of aldehydes with bromonitroalkanes. Synlett 2012, 23, 873–876. [Google Scholar] [CrossRef]
- Moustakim, M.; Clark, P.G.K.; Trulli, L.; de Arriba, A.L.F.; Ehebauer, M.T.; Chaikuad, A.; Murphy, E.J.; Mendez-Johnson, J.; Daniels, D.; Hou, C.-D.; et al. Discovery of a PCAF Bromodomain Chemical Probe. Angew. Chem. 2017, 56, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-X.; Zhao, P.-S.; Sun, X.-J.; An, L.-T.; Deng, Y.; Wu, J.-M. Direct synthesis and application of bridged diamino-functionalized periodic mesoporous organosilicas with high nitrogen contents. J. Solid State Chem. 2017, 255, 70–75. [Google Scholar] [CrossRef]
- Pagano, M.A.; Poletto, G.; di Maira, G.; Cozza, G.; Ruzzene, M.; Sarno, S.; Bain, J.; Elliott, M.; Moro, S.; Zagotto, G.; et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. ChemBioChem 2007, 8, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-C.; Hynes, J., Jr.; Pandit, C.R.; Zhao, R.; Skoumbourdis, A.P.; Wu, H.; Sundeen, J.E.; Leftheris, K. A general largescale synthesis of 2-alkyl-7-methoxyindoles. Heterocycles 2001, 55, 951–960. [Google Scholar] [CrossRef]
- Bandgarand, B.P.; Uppalla, L.S. Gel entrapped base catalyzed (GEBC) Henry reaction: Synthesis of conjugated nitroalkenes. Synth. Commun. 2000, 30, 2071–2075. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T. Base-catalyzed reactions enhanced by solid acids: Amine-catalyzed nitroaldol (Henry) reactions enhanced by silica gel or mesoporous silica SBA-15. Tetrahedron Lett. 2018, 59, 392–396. [Google Scholar] [CrossRef]
- Li, T.; Miras, H.N.; Song, Y. Polyoxometalate (POM)–Layred Double Hydroxides (LDH) Composite Materials: Design and Catalytic Applications. Catalysts 2017, 7, 260. [Google Scholar] [CrossRef]
- Nakhate, A.V.; Rasal, K.B.; Deshmukh, G.P.; Gupta, S.S.R.; Mannepalli, L.K. Synthesis of quinoxaline derivatives from terminal alkynes and o-phenylenediamines by using copper alumina catalyst. J. Chem. Sci. 2017, 129, 1761–1769. [Google Scholar] [CrossRef]







| Sample | SBET (m2/g) | Vp (cm3/g) | rp (Å) | C-Constant |
|---|---|---|---|---|
| MgAl-HT | 84 | 0.1642 | 39 | 177 |
| MgAlOx | 167 | 0.2645 | 34 | 86 |
| MgAl-HT-RH | 134 | 0.2014 | 30 | 567 |
| Catalyst | Conventional Method * | Microwave Method ** | Product Structure (4a) | ||
|---|---|---|---|---|---|
| Time (h) | Yield (%) | Time (min.) | Yield (%) | ||
| MgAl-HT | 8 | 61 | 20 | 90 |  |
| MgAlOx | 6 | 77 | 18 | 96 | |
| MgAl-HT-RH | 5 | 90 | 14 | 98 | |
| Compound | Reactants | Henry Product Structure | Current Work | Literature Data | |||
|---|---|---|---|---|---|---|---|
| Yield % | Time min | Yield % | Time h | Ref. | |||
| 3a | 1c,2a |  | 95 | 12 | 71 | 11 | [30] |
| 3b | 1c,2b |  | 99 | 8 | - | - | - |
| 3c | 1c,2c |  | 92 | 21 | - | - | - |
| 3d | 1c,2d |  | 96 | 10 | - | - | - |
| 3e | 1c,2e |  | 91 | 23 | 68 | 9 | [31] |
| 4a | 1a,2a |  | 98 | 14 | 78 | 6 | [32] |
| 4b | 1b,2a |  | 93 | 18 | 80 | 6 | [33] |
| 4c | 1a,2b |  | 98 | 8 | 75 | 6 | [34] |
| 4d | 1b,2b |  | 91 | 21 | - | - | - |
| 4e | 1a,2c |  | 97 | 6 | 76 | 6 | [35] |
| 4f | 1b,2c |  | 94 | 16 | 79 | 7 | [36] |
| 4g | 1a,2d |  | 96 | 8 | - | - | - |
| 4h | 1b,2d |  | 96 | 14 | - | - | - |
| 4i | 1a,2e |  | 94 | 16 | 68 | 8 | [32] |
| 4j | 1b,2e |  | 95 | 12 | 78 | 12 | [37] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellattif, M.H.; Mokhtar, M. MgAl-Layered Double Hydroxide Solid Base Catalysts for Henry Reaction: A Green Protocol. Catalysts 2018, 8, 133. https://doi.org/10.3390/catal8040133
Abdellattif MH, Mokhtar M. MgAl-Layered Double Hydroxide Solid Base Catalysts for Henry Reaction: A Green Protocol. Catalysts. 2018; 8(4):133. https://doi.org/10.3390/catal8040133
Chicago/Turabian StyleAbdellattif, Magda H., and Mohamed Mokhtar. 2018. "MgAl-Layered Double Hydroxide Solid Base Catalysts for Henry Reaction: A Green Protocol" Catalysts 8, no. 4: 133. https://doi.org/10.3390/catal8040133
APA StyleAbdellattif, M. H., & Mokhtar, M. (2018). MgAl-Layered Double Hydroxide Solid Base Catalysts for Henry Reaction: A Green Protocol. Catalysts, 8(4), 133. https://doi.org/10.3390/catal8040133