Prickly Pear-Like Three-Dimensional Porous MoS2: Synthesis, Characterization and Advanced Hydrogen Evolution Reaction
Abstract
:1. Introduction
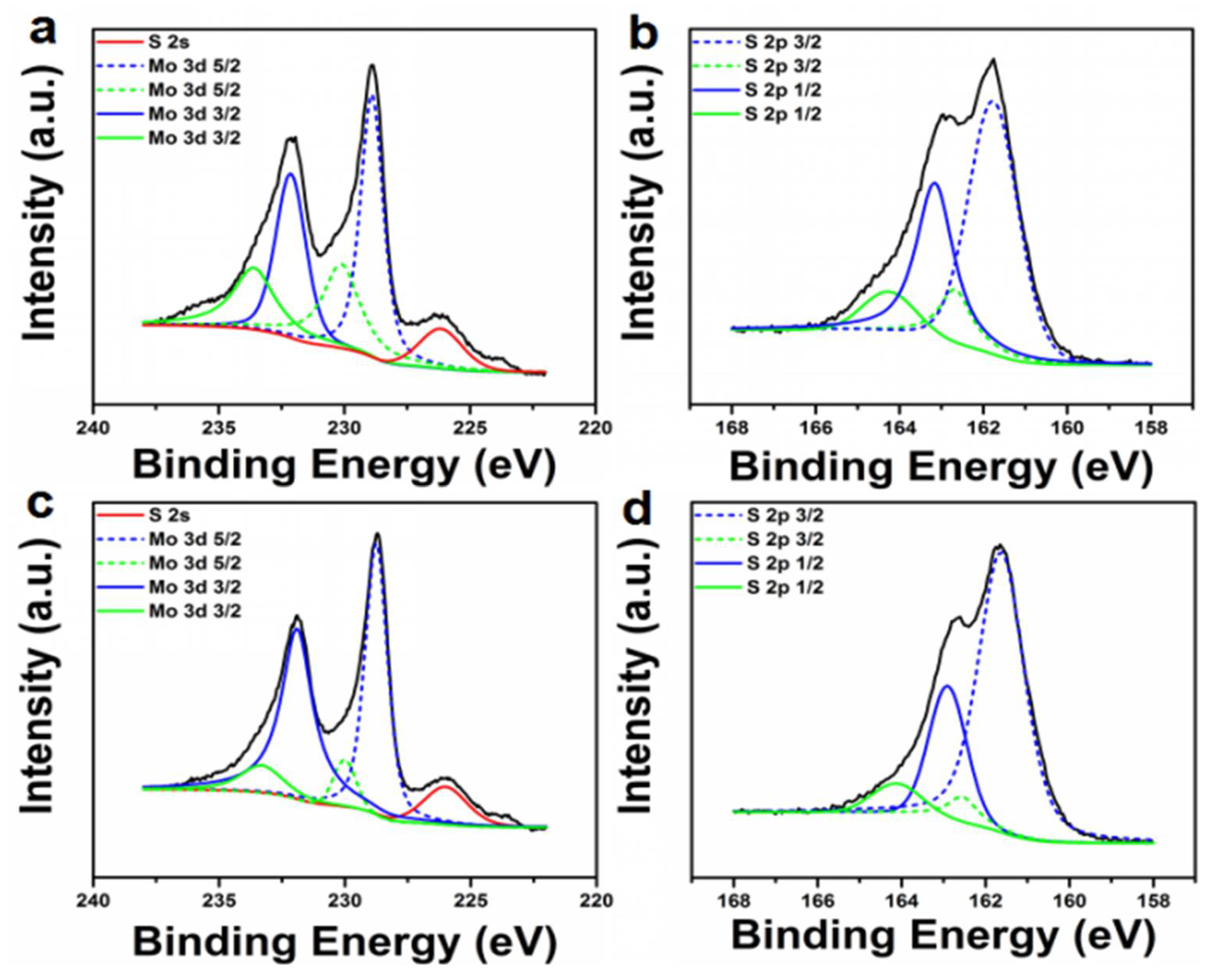
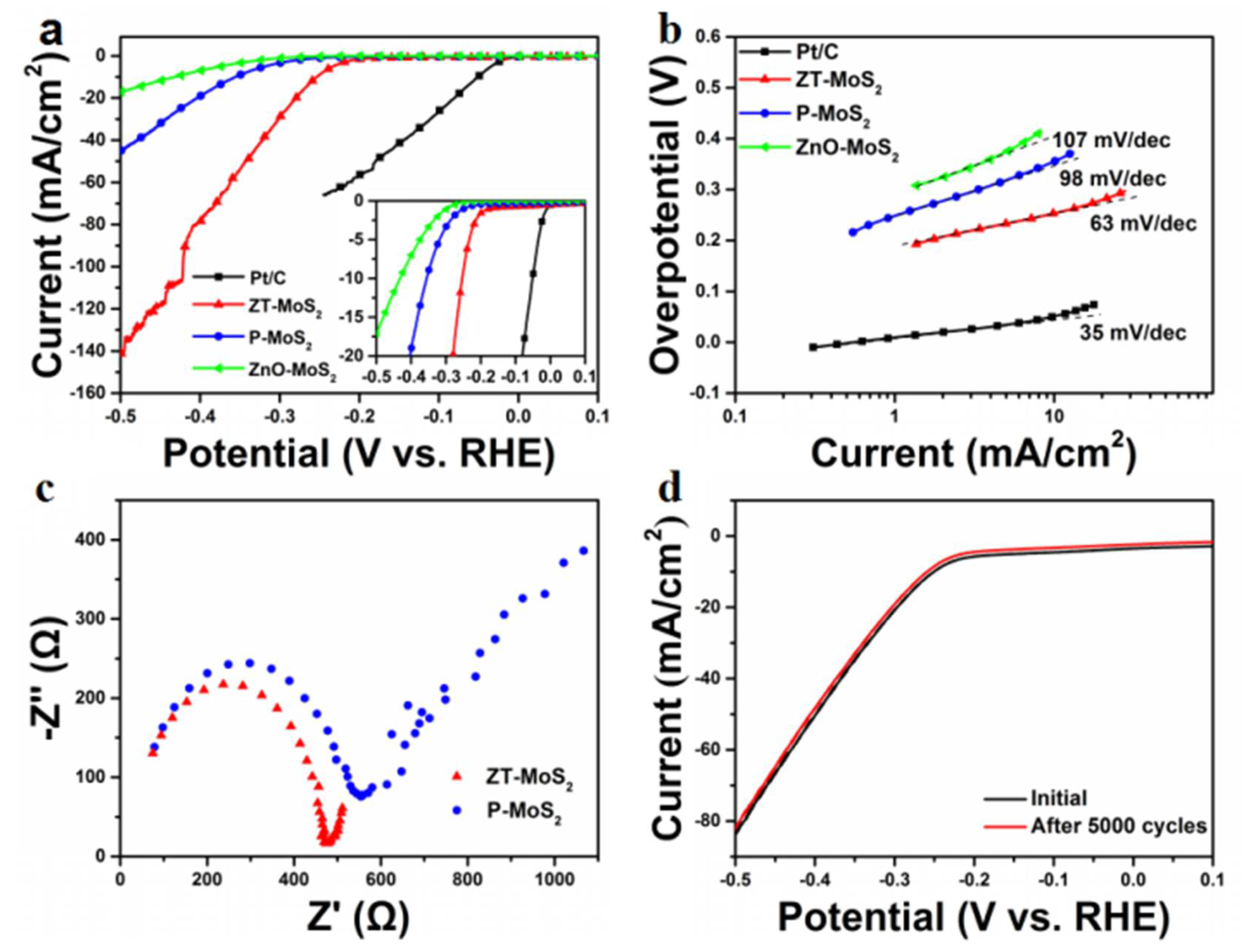
2. Results and Discussion
3. Materials and Methods
3.1. Reagent
3.2. Preparation of ZnO Nanorods (NRs)
3.3. Fabrication of ZT-MoS2 and P-MoS2
3.4. Characterization
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Fox, M.A. Artificial photosynthesis: Solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.X.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Norskov, J.K.; Christensen, C.H. Chemistry—Toward efficient hydrogen production at surfaces. Science 2006, 312, 1322–1323. [Google Scholar] [CrossRef] [PubMed]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.B.; Norskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.D.; Laursen, A.B.; Zhang, J.S.; Zhang, G.G.; Zhu, Y.S.; Wang, X.C.; Dahl, S.; Chorkendorff, I. Layered nanojunctions for hydrogen-evolution catalysis. Angew. Chem. Int. Ed. 2013, 52, 3621–3625. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Hasin, P.; Wu, Y.Y. NixCO3-XO4 nanowire arrays for electrocatalytic oxygen evolution. Adv. Mater. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Casado-Rivera, E.; Volpe, D.J.; Alden, L.; Lind, C.; Downie, C.; Vazquez-Alvarez, T.; Angelo, A.C.D.; DiSalvo, F.J.; Abruna, H.D. Electrocatalytic activity of ordered intermetallic phases for fuel cell applications. J. Am. Chem. Soc. 2004, 126, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Yan, C.; Handoko, A.D.; Seh, Z.W.; Li, H.; Liu, Z. Ultrathin two-dimensional materials for photo- and electrocatalytic hydrogen evolution. Mater. Today 2018. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Karunadasa, H.I.; Montalvo, E.; Sun, Y.J.; Majda, M.; Long, J.R.; Chang, C.J. A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 2012, 335, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.B.; Kegnaes, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides-efficient and viable materials for electro- and photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Wang, T.Y.; Liu, L.; Zhu, Z.W.; Papakonstantinou, P.; Hu, J.B.; Liu, H.Y.; Li, M.X. Enhanced electrocatalytic activity for hydrogen evolution reaction from self-assembled monodispersed molybdenum sulfide nanoparticles on an Au electrode. Energy Environ. Sci. 2013, 6, 625–633. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.B.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xia, B.Y.; Ge, X.M.; Liu, Z.L.; Wang, J.Y.; Wang, X. Ultrathin MoS2 nanoplates with rich active sites as highly efficient catalyst for hydrogen evolution. ACS Appl. Mater. Interfaces 2013, 5, 12794–12798. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.F.; Liu, C.H.; Ye, H.T.; Hu, L.P.; Fugetsu, B.S.; Dai, W.H.; Cao, Y.; Qi, X.Q.; Lu, H.T.; Zhang, X.J. Three-dimensional nitrogen-doped graphene supported molybdenum disulfide nanoparticles as an advanced catalyst for hydrogen evolution reaction. Sci. Rep. 2015, 5, 17542. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Li, D.; Wu, P.Y. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhou, K.; Hou, D.M.; Liu, X.J.; Li, G.Q.; Sang, Y.H.; Liu, H.; Li, L.G.; Chen, S.W. Three-dimensional hierarchical frameworks based on MoS2 nanosheets self-assembled on graphene oxide for efficient electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2014, 6, 21534–21540. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.P.; Ma, Q.; Fan, H.B.; Pang, L.Q.; Zhang, Y.X.; Yao, Y.; Ren, X.D.; Liu, S.Z. A Se-doped MoS2 nanosheet for improved hydrogen evolution reaction. Chem. Commun. 2015, 51, 15997–16000. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, J.; Wang, C.; Zhai, T.T.; Bao, W.J.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Hot electron of Au nanorods activates the electrocatalysis of hydrogen evolution on MoS2 nanosheets. J. Am. Chem. Soc. 2015, 137, 7365–7370. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Z.; Shen, J.X.; Nie, H.G.; Cai, Q.R.; Li, L.H.; Ge, M.Z.; Gu, C.C.; Chen, X.; Yang, K.Q.; et al. Subnanometer molybdenum sulfide on carbon nanotubes as a highly active and stable electrocatalyst for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Wang, H.L.; Xie, L.M.; Liang, Y.Y.; Hong, G.S.; Dai, H.J. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.W.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.S.; Vivekanandan, G.; Ravinder, D.; Eswaramoorthy, M. ZnO: A versatile template to obtain unusual morphologies of silica, gold and carbon nanostructures. Chem. Commun. 2010, 46, 2989–2991. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.B.; Cai, W.P.; Liu, P.S.; Xu, X.X.; Zhou, H.J.; Klingshirn, C.; Kalt, H. ZnO-based hollow nanoparticles by selective etching: Elimination and reconstruction of metal-semiconductor interface, improvement of blue emission and photocatalysis. ACS Nano 2008, 2, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Chen, W.S.; He, J.P.; Tong, Y.; Liu, C.; Su, L.; Gao, B.W.; Yang, H.K.; Zhang, Y.; Zhang, X.J. Substrate-independent and large-area synthesis of carbon nanotube thin films using ZnO nanorods as template and dopamine as carbon precursor. Carbon 2015, 83, 275–281. [Google Scholar] [CrossRef]
- Liu, J.P.; Jiang, J.; Bosman, M.; Fan, H.J. Three-dimensional tubular arrays of MnO2-NiO nanoflakes with high areal pseudocapacitance. J. Mater. Chem. 2012, 22, 2419–2426. [Google Scholar] [CrossRef]
- Zhang, K.N.; Zhang, Y.; Zhang, T.N.; Dong, W.J.; Wei, T.X.; Sun, Y.; Chen, X.; Shen, G.Z.; Dai, N. Vertically coupled ZnO nanorods on MoS2 monolayers with enhanced Raman and photoluminescence emission. Nano Res. 2015, 8, 743–750. [Google Scholar] [CrossRef]
- Kong, D.S.; Wang, H.T.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Matte, H.; Maitra, U. Graphene analogues of inorganic layered materials. Angew. Chem. Int. Ed. 2013, 52, 13162–13185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Lu, Z.Y.; Kong, D.S.; Sun, J.; Hymel, T.M.; Cui, Y. Electrochemical tuning of MoS2 nanoparticles on three-dimensional substrate for efficient hydrogen evolution. ACS Nano 2014, 8, 4940–4947. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jorgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Norskov, J.K. Biornimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.F.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.M.; Wang, Y.; Wong, J.I.; Tan, A.Y.S.; Hsu, C.L.; Li, L.J.; Lu, Y.C.; Yang, H.Y. Self-assembly of hierarchical MoSx/CNT nanocomposites (2 < x < 3): Towards high performance anode materials for lithium ion batteries. Sci. Rep. 2013, 3, 2169. [Google Scholar] [CrossRef] [PubMed]
- Pingli, Q.; Guojia, F.; Weijun, K.; Fei, C.; Qiao, Z.; Jiawei, W.; Hongwei, L.; Xingzhong, Z. In situ growth of double-layer MoO3/MoS2 film from MoS2 for hole-transport layers in organic solar cell. J. Mater. Chem. A 2014, 2, 2742–2756. [Google Scholar] [CrossRef]
- Dai, W.H.; Dong, H.F.; Fugetsu, B.; Cao, Y.; Lu, H.T.; Ma, X.L.; Zhang, X.J. Tunable fabrication of molybdenum disulfide quantum dots for intracellular microRNA detection and multiphoton bioimaging. Small 2015, 11, 4158–4164. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.S.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.B.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core-shell MoO3-MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef] [PubMed]
- Merki, D.; Hu, X.L. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef]
- Behranginia, A.; Asadi, M.; Liu, C.; Yasaei, P.; Kumar, B.; Phillips, P.; Foroozan, T.; Waranius, J.C.; Kim, K.; Abiade, J.; et al. Highly efficient hydrogen evolution reaction using crystalline layered three-dimensional molybdenum disulfides grown on graphene film. Chem. Mater. 2016, 28, 549–555. [Google Scholar] [CrossRef]
- Handoko, A.D.; Liew, L.-L.; Lin, M.; Sankar, G.; Du, Y.; Su, H.; Dong, Z.; Goh, G.K.L. Elucidation of thermally induced internal porosity in zinc oxide nanorods. Nano Res. 2018, 11, 2412–2423. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Chen, X.; Dai, W.; Zhang, K.; Liu, C.; Dong, H. Prickly Pear-Like Three-Dimensional Porous MoS2: Synthesis, Characterization and Advanced Hydrogen Evolution Reaction. Catalysts 2018, 8, 235. https://doi.org/10.3390/catal8060235
Lu H, Chen X, Dai W, Zhang K, Liu C, Dong H. Prickly Pear-Like Three-Dimensional Porous MoS2: Synthesis, Characterization and Advanced Hydrogen Evolution Reaction. Catalysts. 2018; 8(6):235. https://doi.org/10.3390/catal8060235
Chicago/Turabian StyleLu, Huiting, Xin Chen, Wenhao Dai, Kai Zhang, Conghui Liu, and Haifeng Dong. 2018. "Prickly Pear-Like Three-Dimensional Porous MoS2: Synthesis, Characterization and Advanced Hydrogen Evolution Reaction" Catalysts 8, no. 6: 235. https://doi.org/10.3390/catal8060235
APA StyleLu, H., Chen, X., Dai, W., Zhang, K., Liu, C., & Dong, H. (2018). Prickly Pear-Like Three-Dimensional Porous MoS2: Synthesis, Characterization and Advanced Hydrogen Evolution Reaction. Catalysts, 8(6), 235. https://doi.org/10.3390/catal8060235





