Pilot-Scale Production, Properties and Application of Fe/Cu Catalytic-Ceramic-Filler for Nitrobenzene Compounds Wastewater Treatment
Abstract
:1. Introduction
2. Result and Discussion
2.1. Basic Properties of CCF
2.2. Result of the Sequence Wastewater Treatment Test
2.2.1. Effect of Iron Content and Copper Plating Rate for the Removal of TNCs
2.2.2. Effect of CFF and CF for the Biodegradability of Wastewater
2.3. Results of Backwash Frequency and the Volume Half-Life Test
2.3.1. Result of Backwash Frequency Test for CCF and CF
2.3.2. Result of Volume Half-Life Test for CCF and CF
2.4. Results of Field Pilot-Scale Test
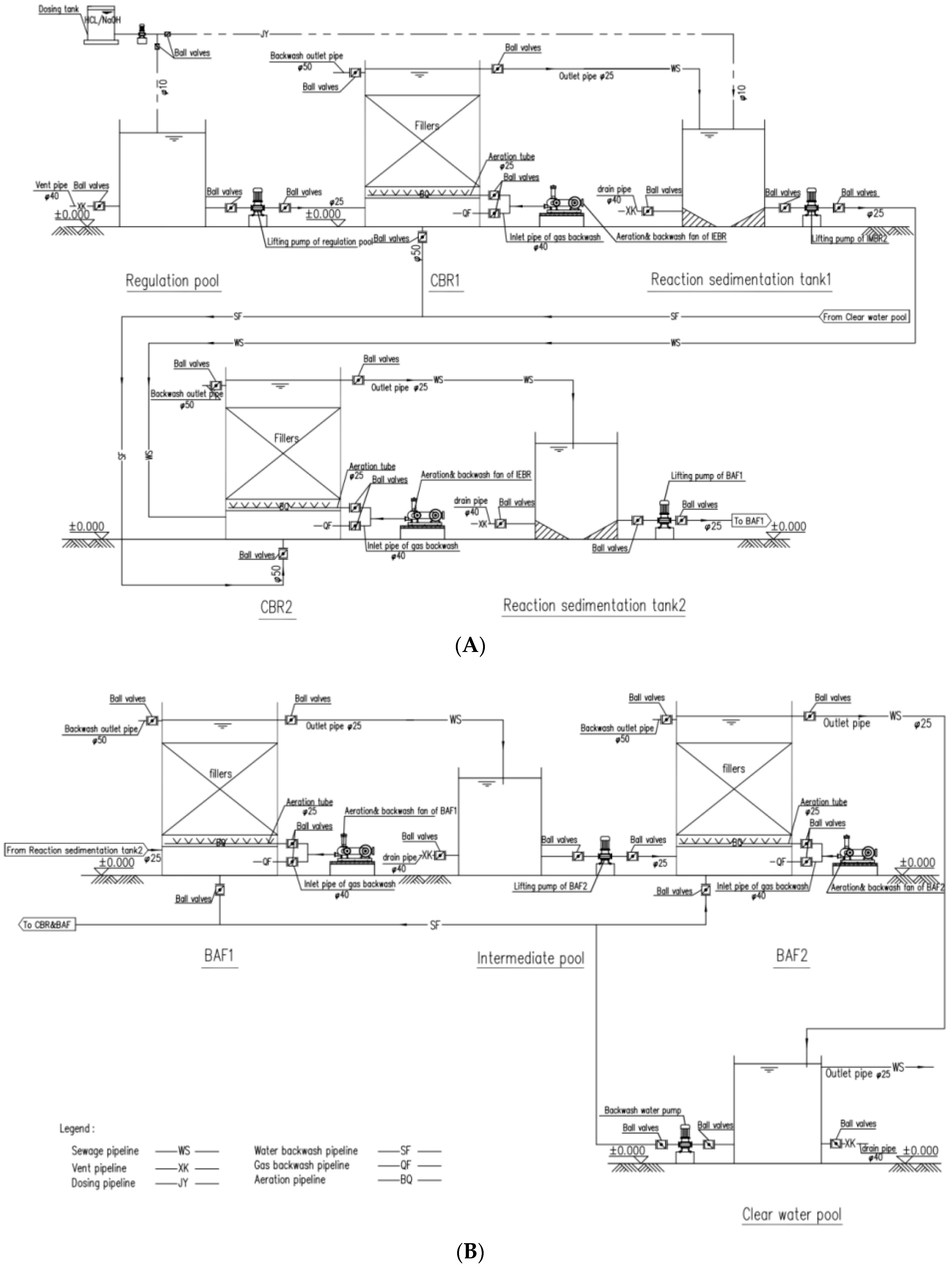
2.4.1. The Design and Operating Method of the Two-Stage Wastewater Treatment System
- Part 1:
- Preliminary treatment portion: the initial wastewater was stored at regulation pool (effective volume was 2 m3). And at the bottom of the pool, ball valves and vent pipe were designed for the discharge of sediment and sludge. HCl which stored in the dosing tank was added into the regulation pool by automatic dosing unit (adjust the pH of initial wastewater lower than 3.00).
- Part 2:
- The catalytic-biological treatment portion: the catalytic treatment process (stage1) and biological treatment process (stage2), which was considered as the core of the system, both shown in Figure 9. Wastewater was pump by lifting pump of regulation pool into the bottom of CBR-1, and the effluent of CBR-1 flow automatically through the outlet pipe and stored in Reaction sedimentation tank 1, then the lifting pump of CBR-2 pumped the wastewater into the bottom of CBR-2 and the effluent was stored at Reaction sedimentation tank 2. The same pathway was implemented in the BAF-1 and BAF-2 treatment process.
- Part 3:
- Backwash portion: both the CBRs and BAFs are performed the same backwash method which has been mentioned above. Each CBRs was separately backwash every 3 days. The backwash procedure for BAFs was not start until the system operated for 50 days, and from 52–90 day each BAFs was separately backwash every 7 days. In addition, the lifting pump was applied as backwash pump at backwash process.
- 1st day to 30th day, quantity of influent was 2 m3 per day (Q = 2 m3/d) and no other preliminary process operated except for regulation of pH.
- 31th day to 60th day, PAM was extra added into the regulation pool for the removal of the suspended solid (SS). And the quantity of influent was improved to 2.5 m3/d in order to verify the resistance of the system.
- 61th day to 90th day, the quantity of influent was 2 m3 per day (Q = 2 m3/d) in order to verify the resumption performance of the system. The scene images of effluent at 90th day were shown in Figure 10.
2.4.2. The TNCs and CODcr Removal by System
3. Materials and Methods
3.1. Pilot-Scale Production and Basic Property Test of Fe/Cu Catalytic-Ceramic-Filler
3.1.1. Raw Materials
3.1.2. Pilot-Scale Production Process Flow of Fe/Cu Catalytic-Ceramic-Filler
3.1.3. Basic Property Test of CCF/CF
3.2. Performance Test of CCF/CF
3.2.1. Half-Life and Effectiveness Evaluation Reactor and Wastewater
3.2.2. The Sequence Wastewater Treatment Test of CCF and CF
3.2.3. The Backwash Frequency and the Volume Half-Life Test of CCF and CF
3.3. The Field Pilot-Scale Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Q.; Ye, Z.; Wang, Z.; Zhang, M. Progress on the treatment of TNT waste water. Environ. Chem. 2010, 5, 796–801. [Google Scholar]
- Nguyen, T.D.; Le, T.V.; Show, P.L.; Nguyen, T.T.; Tran, M.H.; Tran, T.N.; Lee, S.Y. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent. Bioresour. Technol. 2019, 272, 34–39. [Google Scholar] [CrossRef] [PubMed]
- National Standard. Discharge Standard for Water Pollutions from Ordnance Industry Powder and Explosive. GB 14470.1-2002. 2002. Available online: http://www.gbstandards.org/China_standards/GB/GB%2014470.1-2002.htm (accessed on 18 November 2002).
- Ma, C.; Peng, Y. Treatment and Control for High-Concentration Recalcitrant Organic Wastewater, 2nd ed.; Chemical Industry Press: Beijing, China, 2010; pp. 266–288. [Google Scholar]
- Ryosuke, S.; Toshinari, M.; Yoichiro, H.; Nobuo, N.; Hiroaki, O. Biological treatment of harmful Nitrobenzene compounds wastewater containing a high concentration of nitrogen compounds by waste activated sludge. J. Biotechnol. 2010, 150, 226–227. [Google Scholar] [CrossRef]
- Shi, J.; Han, Y.; Xu, C.; Han, H. Biological coupling process for treatment of toxic and refractory compounds in coal gasification wastewater. Rev. Environ. Sci. Bio/Technol. 2018, 17, 765–790. [Google Scholar] [CrossRef]
- Maloney, S.W.; Adrian, N.R.; Hickey, R.F.; Heine, R.L. Anaerobic treatment of pinkwater in a fluidized bed reactor containing GAC. J. Hazard. Mater. 2002, 92, 77–88. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, Q.; Zhang, M. Acute toxicity evaluation of explosive wastewater by bacterial bioluminescence assays using a freshwater luminescent bacterium, Vibrio qinghaiensis sp. Nov. J. Hazard. Mater. 2011, 186, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bai, H. Progress in Biodegradation of Nitrobenzene compounds wastewater. Chem. Intem. 2011, 10, 10–12. [Google Scholar]
- Lin, H.; Lin, Y.; Wen, Y.; Gan, L. Degradation of TNT in Aqueous Solution by Uncultureed Soil Bacterium Clone UD3. Chin. J. Energ. Mater. 2009, 17, 630–634. [Google Scholar]
- Payan, A.; Fattahi, M.; Jorfi, S.; Roozbehani, B.; Payan, S. Synthesis and characterization of titanate nanotube/single-walled carbon nanotube (TNT/SWCNT) porous nanocomposite and its photocatalytic activity on 4-chlorophenol degradation under UV and solar irradiation. Appl. Surf. Sci. 2018, 434, 336–350. [Google Scholar] [CrossRef]
- Matta, R.; Hanna, K.; Kone, T.; Chiron, S. Oxidation of 2,4,6-trinitrotoluene in the presence of different iron-bearing minerals atneutral pH. Chem. Eng. J. 2008, 144, 453–458. [Google Scholar] [CrossRef]
- Yu, Y.; Guoji, D.; Yongqiang, Z.; Deyong, L.; Zheng, J. Study on treatment of the midcourse wastewater from alkali straw pulp by using a new biological film integrative reactor. Ind. Water Treat. 2010, 30, 42–45. [Google Scholar]
- Wu, Y.G.; Zhao, D.W. Experiment studies on the Degradation of TNT-containing Wastewater by Ozone Oxidization. Chin. J. Energy Mater. 2003, 11, 201–204. [Google Scholar]
- Wu, Y.; Zhao, C.; Wang, Q.; Ding, K. Integrated effects of selected ions on 2,4,6-trinitrotoluene removal by O3/H2O2. J. Hazard. Mater. 2006, 132, 232–236. [Google Scholar] [CrossRef]
- Diao, J.; Liu, Y.; Wang, H.; Li, P.; Kang, R. O3/H2O2 Oxidative treatment of TNT Red-Water in a Rotating Packed Bed. Chin. J. Energy Mater. 2007, 15, 281–284. [Google Scholar]
- Chang, S.; Liu, C. Treatment of Nitrobenzene compounds wastewater by supercritical water oxidation. Chin. J. Energy Mater. 2007, 25, 285–288. [Google Scholar]
- Li, G.X.; Huaug, Y.H.; Chen, T.C.; Shih, Y.J.; Zhang, H. Reduction and Immobilization of Potassium Permanganate on Iron Oxide Catalyst by Fluidized-Bed Crystallization Technology. Appl. Sci. 2012, 2, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, R.; Zappi, M.; Kuo, C.H. Chloride effect on TNT degradation by zerovalent iron or zinc duringwater treatment. Environ. Sci. Technol. 2004, 38, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Rodrigues, M.; Silva, F.T.; Paiva, T.C. Optimization of brazilian TNT industry wastewater treatment using combined zero-valent iron and fenton processes. J. Hazard. Mater. 2009, 168, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Ma, L. Catalytic Reduction Treatment for Wastewater—Mechanism and Application; Beijing Science Press: Beijing, China, 2008; Volume 169–179, pp. 260–279. [Google Scholar]
- Wang, G.; Zhang, J.; Liu, L.; Zhou, J.Z.; Liu, Q.; Qian, G.; Xu, Z.P.; Richards, R.M. Novel multi-metal containing MnCr catalyst made from manganese slag and chromium wastewater for effective selective catalytic reduction of nitric oxide at low temperature. J. Clean. Prod. 2018, 183, 917–924. [Google Scholar] [CrossRef]
- Li, G. Analysis and Test Method for Water and Wastewater; Chemical Industry Press: Beijing, China, 2012; Volume 65–70, pp. 201–230. [Google Scholar]
- China Association for Engineering Construction Standardization. Technical Specification for Biological Aerated Filter Engineering CECS 265-2009; China Planning Press: Beijing, China, 2009; pp. 15–16. [Google Scholar]
- National Standard. Lightweight Aggregates and Its Test Methods. GB/T 17431-1998. 1998. Available online: http://218.196.240.38/root/eWebEditor/uploadfile/20170503133740612.pdf (accessed on 15 July 1998).
- Lemos, B.R.; Teixeira, A.P.; Ardisson, J.D.; Macedo, W.A.; Fernandez-Outon, L.E.; Amorim, C.C.; Moura, F.C.; Lago, R.M. Magnetic Amphiphilic Composites Applied for the Treatment of Biodiesel Wastewaters. Appl. Sci. 2012, 2, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Barreto-Rodrigues, M.; Silva, F.T.; Paiva, T.C. Combined zero-valent iron and fenton processes for the treatment of brazilian TNT industry wastewater. J. Hazard. Mater. 2009, 165, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qi, Y.; He, S.; Fan, C.; Dai, B.; Zhou, W.; Gao, L.; Huang, J. Preparation and application of novel catalytic-ceramic-filler in a coupled system for TNT manufacturing wastewater treatment. Chem. Eng. J. 2015, 280, 417–425. [Google Scholar] [CrossRef]
- Qi, Y.; Dai, B.; He, S.; Wu, S.; Huang, J.; Xi, F.; Ma, Y.; Meng, M. Effect of chemical constituents of oxytetracycline mycelia residue and dredged sediments on characteristics of ultra-lightweight ceramsite. J. Taiwan Inst. Chem. E 2016, 65, 225–232. [Google Scholar] [CrossRef]
- Qi, Y.F.; He, S.B.; Wu, S.Q.; Dai, B.B.; Hu, C.H. Utilization of micro-electrolysis, up-flow anaerobic sludge bed, anoxic/oxic-activated sludge process, and biological aerated filter in penicillin G wastewater treatment. Desalin. Water Treat. 2015, 55, 1480–1487. [Google Scholar] [CrossRef]
- Qi, Y.; Yue, Q.; Han, S.; Yue, M.; Gao, B.; Yu, H.; Shao, T. Preparation and mechanism of ultra-lightweight ceramics produced from sewage sludge. J. Hazard. Mater. 2010, 176, 76–84. [Google Scholar] [CrossRef]
- López Peñalver, J.J.; Gómez Pacheco, C.V.; Sánchez Polo, M.; Rivera Utrilla, J. Degradation of tetracyclines in different water matrices by advanced oxidation/reduction processes based on gamma radiation. J. Chem. Technol. Biotechnol. 2013, 88, 1096–1108. [Google Scholar] [CrossRef]












| Degradation of CODcr | |||||
| t1COD | Y0(CODcr) | R 2 | KCOD 1 | ||
| CCF | 1964.4 mg/L | 192.44 min | 519.4 mg/L | 0.9973 | 6.45/min |
| CF | 1344.97 mg/L | 154.01 min | 1147.93 mg/L | 0.9777 | 5.52/min |
| Degradation of TNCs | |||||
| t1NC | Y0(NC) | R 2 | KNC 2 | ||
| CCF | 383.27 mg/L | 58.32 min | 45.13 mg/L | 0.9798 | 4.15/min |
| CF | 341.78 mg/L | 99.13 min | 89.11 mg/L | 0.9913 | 2.18/min |
| Materials | BD 4/kg m−3 | GD 5/kg m−3 | 24 WA 6/% | NTP 7/Mpa | ARSC 8/% | Diameter/mm | Iron/% | Carbon % |
|---|---|---|---|---|---|---|---|---|
| CF | 1085 | 1548 | 3.8 | 4.2 | 92.5% | 4–6 | 26.5% | 5.2% |
| LCF | 981 | 1238 | 3.2 | 8.6 | 100% | 3–6 | ~ | ~ |
| Materials | CODcr/mg·L−1 | BOD5/mg·L−1 | Biodegradability BOD5/CODcr | mono-NCs/di-NCs 1/mg·L−1 | tri-NCs 2/mg·L−1 | TNCs 3/mg·L−1 | pH |
|---|---|---|---|---|---|---|---|
| Wastewater | 2460 | 270 | 0.11 | 297 | 123 | 420 | 2.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Qi, Y.; Liu, R. Pilot-Scale Production, Properties and Application of Fe/Cu Catalytic-Ceramic-Filler for Nitrobenzene Compounds Wastewater Treatment. Catalysts 2019, 9, 11. https://doi.org/10.3390/catal9010011
Yang B, Qi Y, Liu R. Pilot-Scale Production, Properties and Application of Fe/Cu Catalytic-Ceramic-Filler for Nitrobenzene Compounds Wastewater Treatment. Catalysts. 2019; 9(1):11. https://doi.org/10.3390/catal9010011
Chicago/Turabian StyleYang, Bingchuan, Yuanfeng Qi, and Rutao Liu. 2019. "Pilot-Scale Production, Properties and Application of Fe/Cu Catalytic-Ceramic-Filler for Nitrobenzene Compounds Wastewater Treatment" Catalysts 9, no. 1: 11. https://doi.org/10.3390/catal9010011
APA StyleYang, B., Qi, Y., & Liu, R. (2019). Pilot-Scale Production, Properties and Application of Fe/Cu Catalytic-Ceramic-Filler for Nitrobenzene Compounds Wastewater Treatment. Catalysts, 9(1), 11. https://doi.org/10.3390/catal9010011





