Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway
Abstract
:1. Introduction
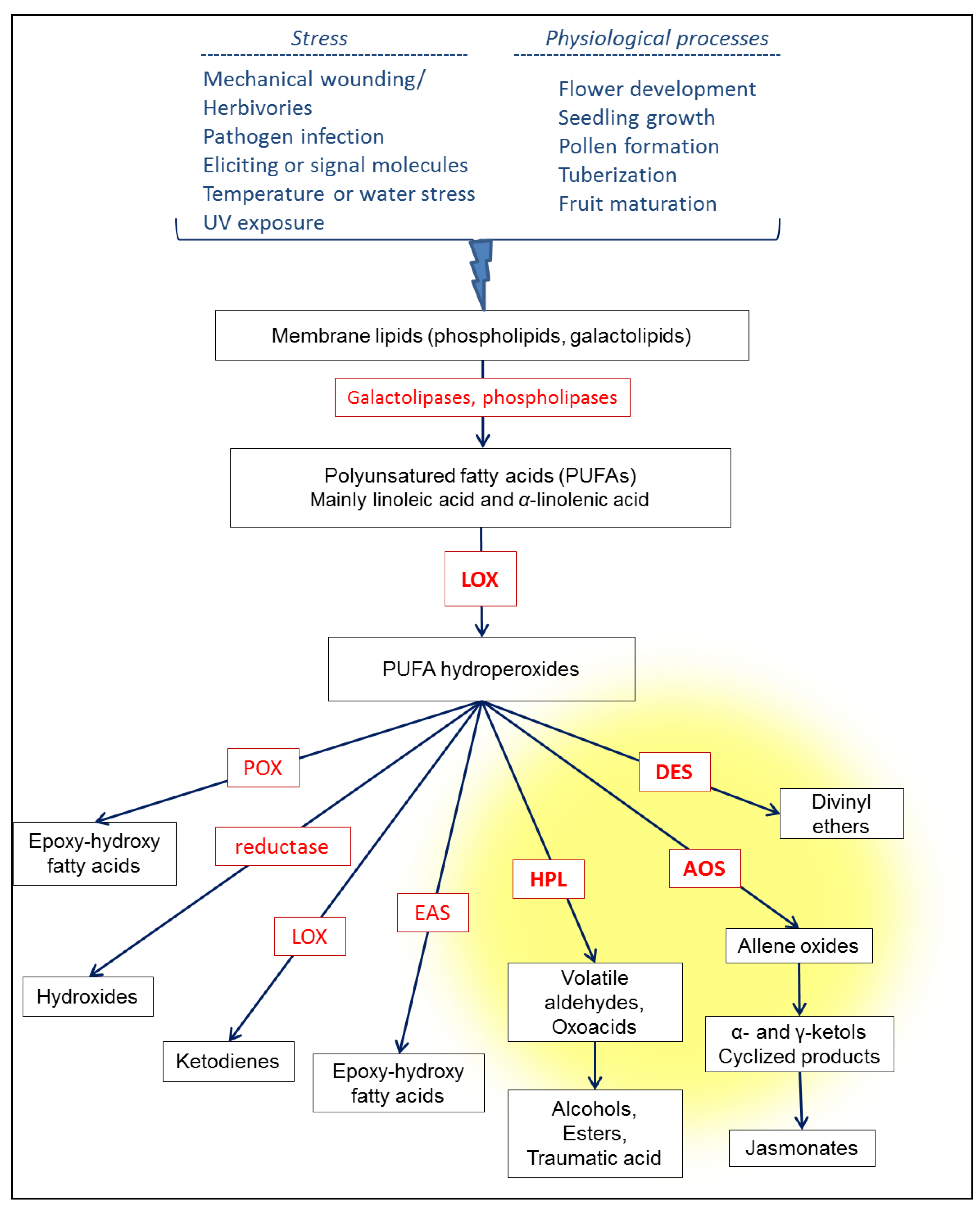
2. The Lipoxygenase Pathway in Higher Plants: Activation and Description of the Enzymatic Cascade
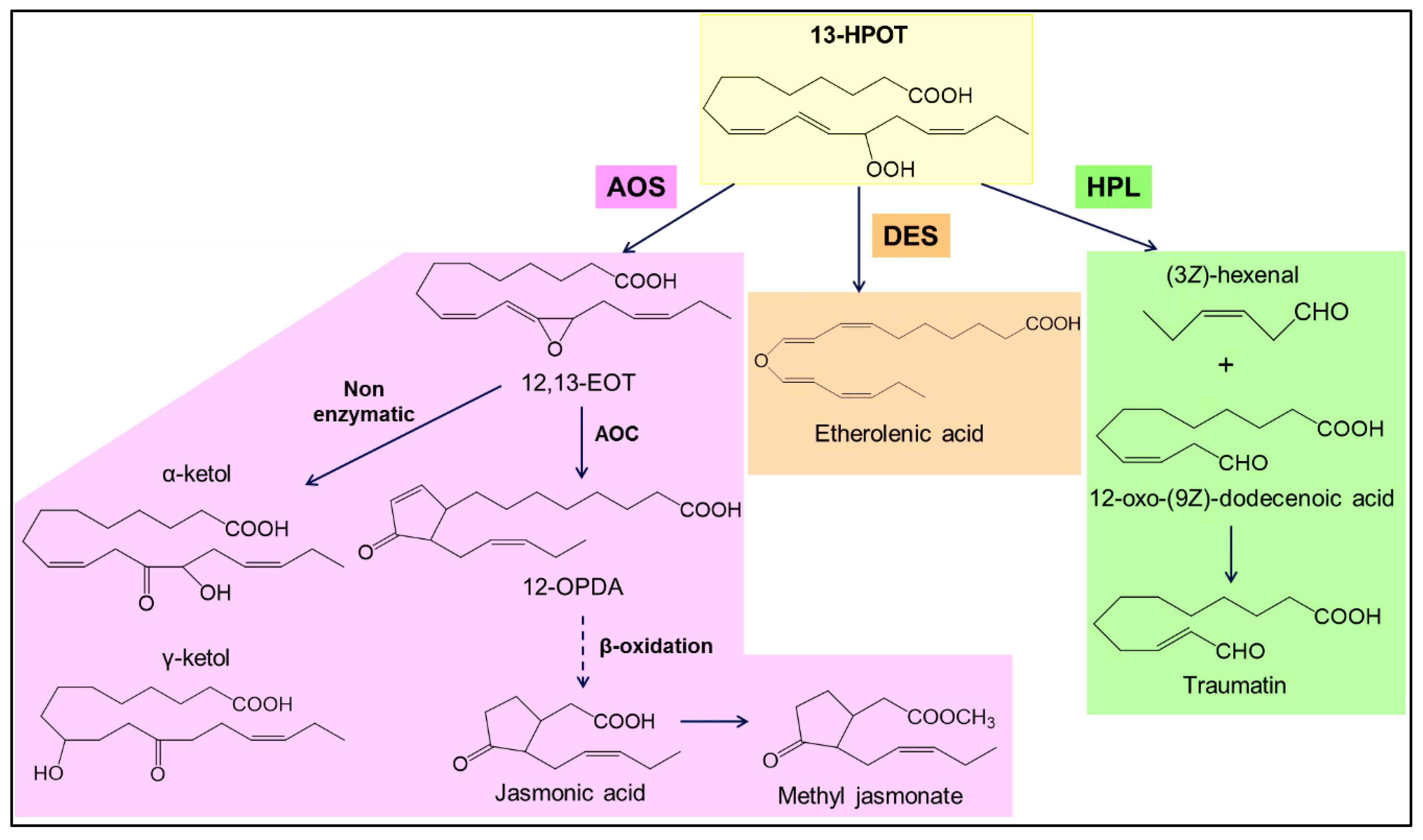
3. Biosynthesis of GLVs: The HPL Branch of the LOX Pathway
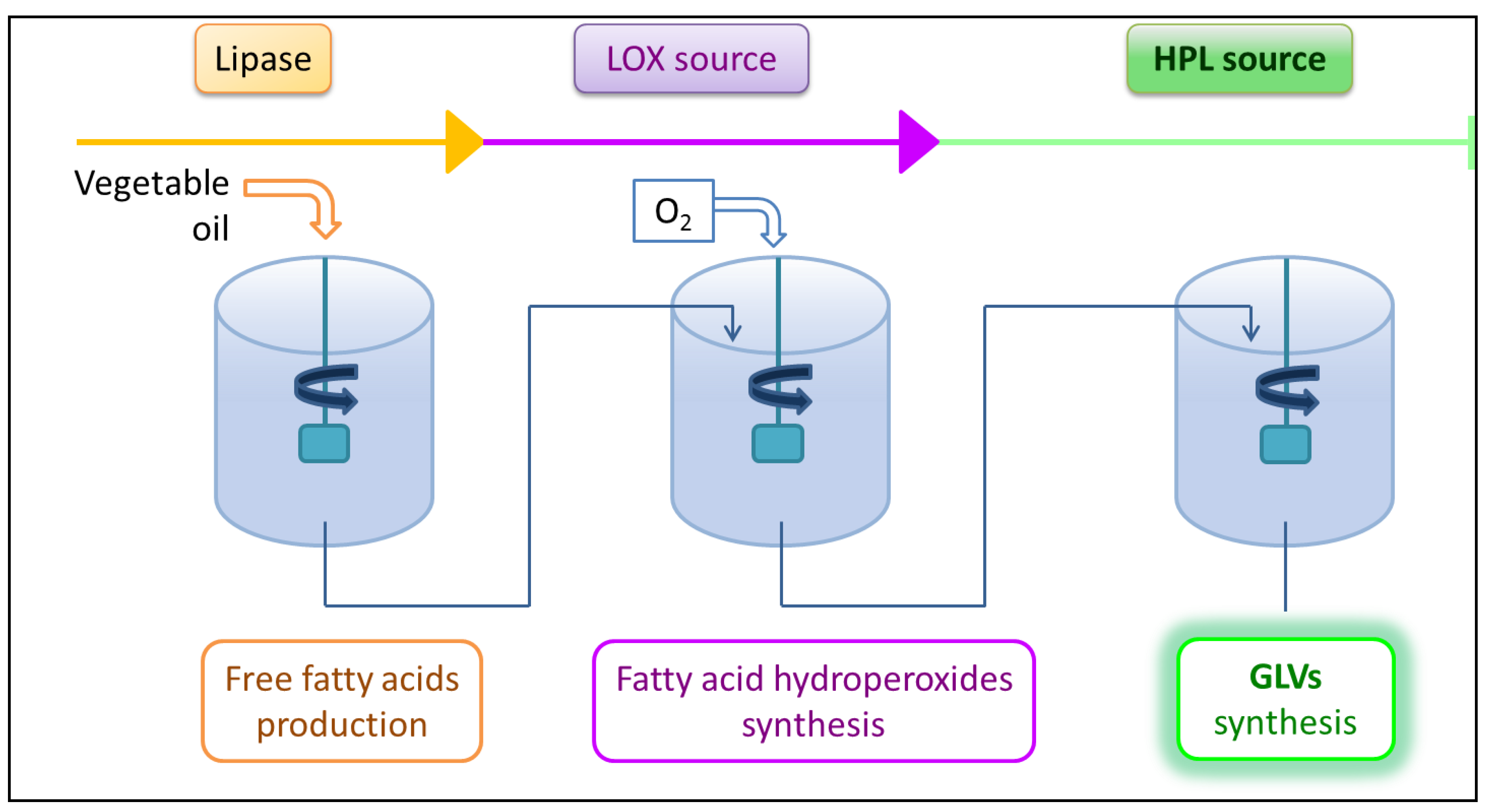
3.1. Lipase Activity
3.2. LOX Activity
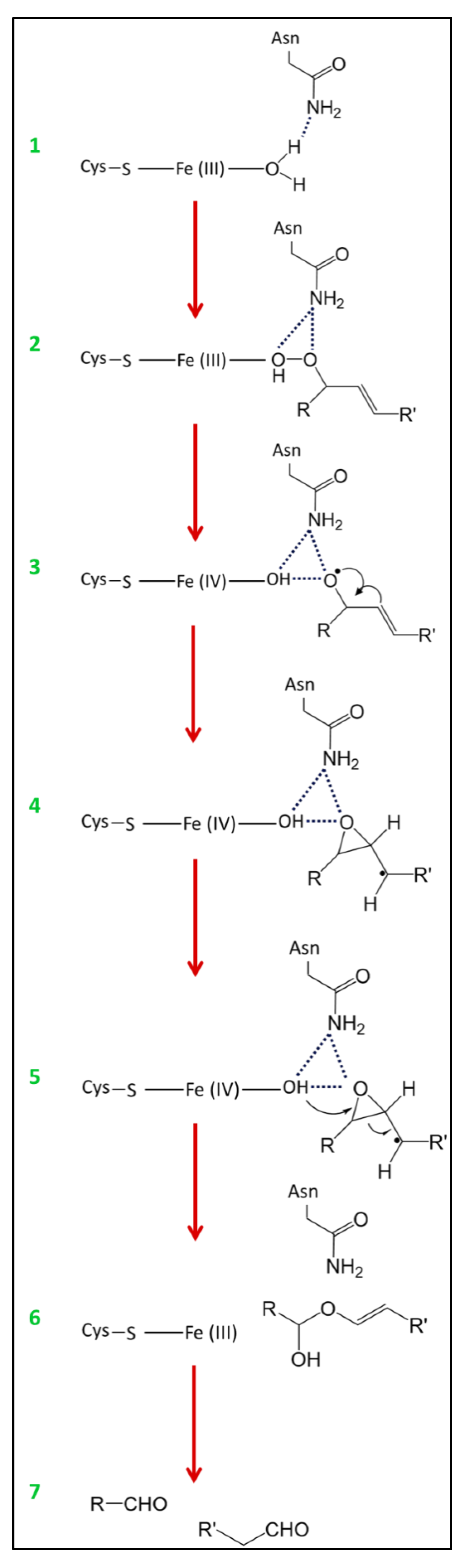
3.3. HPL Activity
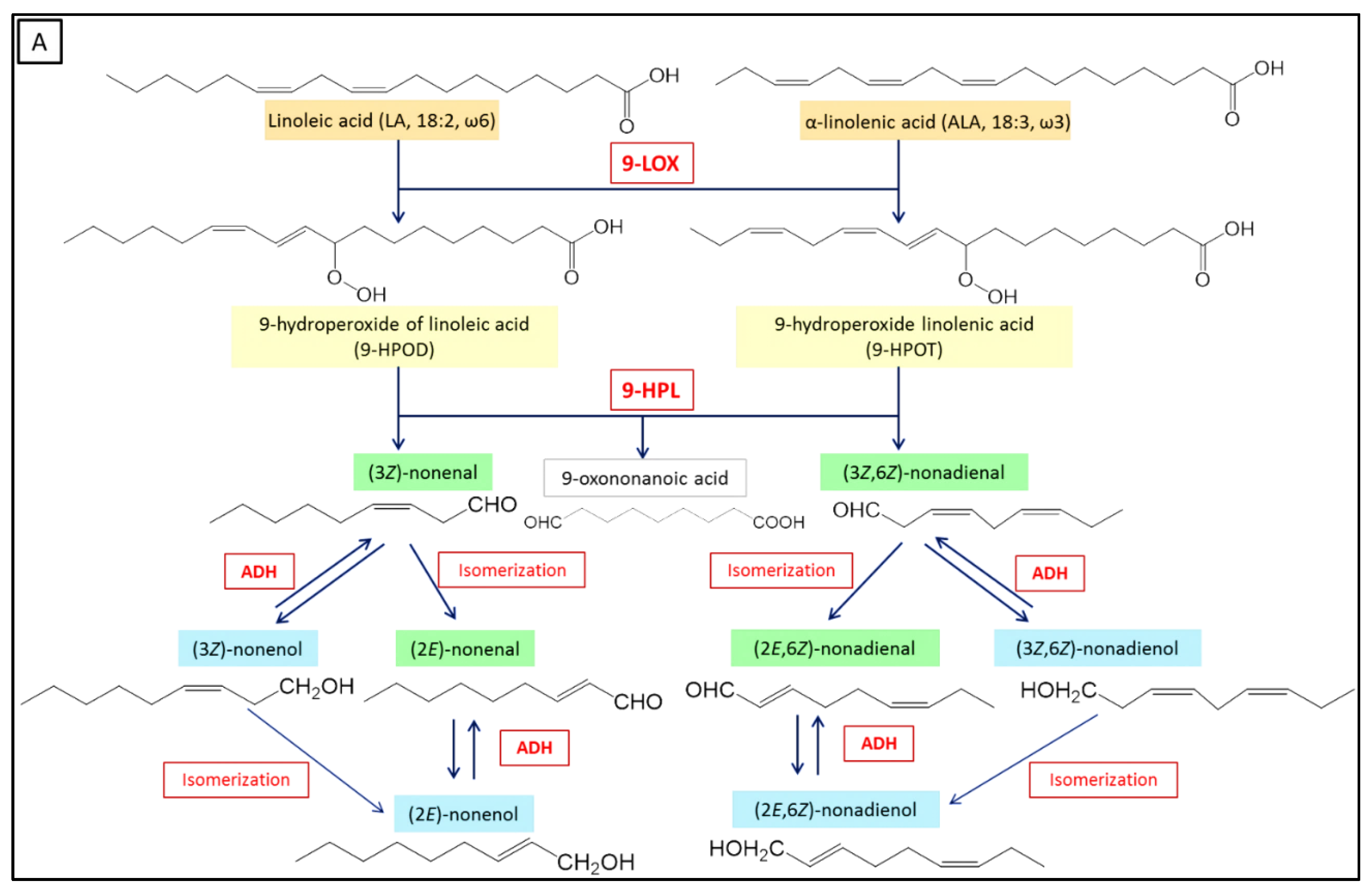
3.4. Isomerization, Dehydrogenation and Esterification Reactions of HPL Products
4. Industrial Purpose and Use of GLVs
4.1. Flavoring Interest of GLVs
4.2. Industrial Use of GLVs
4.3. Production of GLVs: Synthetic versus Natural Flavors
5. Potential of the Use of the LOX Pathway for Biotechnological Production of Natural GLVs
5.1. Release of Fatty Acids from Triacylgycerols
5.2. Transformation of Polyunsatured Fatty Acids into Hydroperoxides
5.3. GLVs Synthesis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hatanaka, A.; Kajiwara, T.; Sekiya, J. Biosynthetic pathway for C6-aldehydes formation from linolenic acid in green leaves. Chem. Phys. Lipids 1987, 44, 341–361. [Google Scholar] [CrossRef]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Blee, E.; Joyard, J. Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol. 1996, 110, 445–454. [Google Scholar] [CrossRef]
- Bate, N.J.; Rothstein, S.J. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998, 16, 561–569. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Grechkin, A.N. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog. Lipid Res. 1998, 37, 317–352. [Google Scholar] [CrossRef]
- Blee, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef]
- Blee, E. Phytooxylipins and plant defense reactions. Prog. Lipid Res. 1998, 37, 33–72. [Google Scholar] [CrossRef]
- Griffiths, G. Biosynthesis and analysis of plant oxylipins. Free Radic. Res. 2015, 49, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Drouet, P.; Thomas, D.; Legoy, M.D. Production of 13(S)-hydroperoxy-9(Z),11(E)-octadecadienoic acid using soybean lipoxygenase 1 in a biphasic octane-water system. Tetrahedron Lett. 1994, 35, 3923–3926. [Google Scholar] [CrossRef]
- Fauconnier, M.L.; Marlier, M. An efficient procedure for the production of fatty acid hydroperoxides from hydrolyzed flax seed oil and soybean lipoxygenase. Biotechnol. Tech. 1996, 10, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Andre, E.; Hou, K.-W. Présence d’une oxydase des lipides ou lipoxydase dans la graine de soja. Comptes Rendus de l’Académie des Sciences (Paris) 1932, 194, 645–647. [Google Scholar]
- Gardner, H.W. Recent investigations into the lipoxygenase pathway of plants. Biochim. Biophys. Acta 1991, 1084, 221–239. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef]
- Ishiguro, S.; Kawai-Oda, A.; Ueda, J.; Nishida, I.; Okada, K. The defective in anther dehiscence1 Gene Encodes a Novel Phospholipase A1 Catalyzing the Initial Step of Jasmonic Acid Biosynthesis, Which Synchronizes Pollen Maturation, Anther Dehiscence, and Flower Opening in Arabidopsis. Plant Cell 2001, 13, 2191–2210. [Google Scholar] [CrossRef]
- Vliegenthart, J.F.G.; Veldink, G.A. Substrates and products of lipoxygenase catalysis. Stud. Nat. Prod. Chem. 1991, 9, 559–589. [Google Scholar]
- Schwab, W. Biosynthesis of Plant Flavors: Analysis and Biotechnological Approach. In Flavor Chemistry; American Chemical Society: Washington, DC, USA, 2000; Volume 756, pp. 72–86. [Google Scholar]
- Vick, B.A.; Zimmerman, D.C. Pathways of Fatty Acid hydroperoxide metabolism in spinach leaf chloroplasts. Plant Physiol. 1987, 85, 1073–1078. [Google Scholar] [CrossRef]
- Hughes, R.K.; De Domenico, S.; Santino, A. Plant cytochrome CYP74 family: Biochemical features, endocellular localisation, activation mechanism in plant defence and improvements for industrial applications. ChemBioChem 2009, 10, 1122–1133. [Google Scholar] [CrossRef]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Schilmiller, A.L.; McCaig, B.C.; Howe, G.A. Identification of a jasmonate-regulated allene oxide synthase that metabolizes 9-hydroperoxides of linoleic and linolenic acids. J. Biol. Chem. 2002, 277, 46051–46058. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, I.; Hause, B.; Maucher, H.; Pitzschke, A.; Miersch, O.; Ziegler, J.; Ryan, C.A.; Wasternack, C. Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato - amplification in wound signalling. Plant J. 2003, 33, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Grechkin, A.N.; Hamberg, M. Divinyl ether synthase from garlic (Allium sativum L.) bulbs: Sub-cellular localization and substrate regio- and stereospecificity. FEBS Lett. 1996, 388, 112–114. [Google Scholar] [CrossRef]
- Grechkin, A.N. Hydroperoxide lyase and divinyl ether synthase. Prostaglandins Other Lipid Mediat. 2002, 68–69, 457–470. [Google Scholar] [CrossRef]
- Grechkin, A.N.; Mukhtarova, L.S.; Hamberg, M. Detection of an enol intermediate in the hydroperoxide lyase chain cleavage reaction. FEBS Lett. 2003, 549, 31–34. [Google Scholar] [CrossRef]
- Grechkin, A.N.; Hamberg, M. The “heterolytic hydroperoxide lyase” is an isomerase producing a short-lived fatty acid hemiacetal. Biochim. Biophys. Acta 2004, 1636, 47–58. [Google Scholar] [CrossRef]
- Grechkin, A.N.; Bruhlmann, F.; Mukhtarova, L.S.; Gogolev, Y.V.; Hamberg, M. Hydroperoxide lyases (CYP74C and CYP74B) catalyze the homolytic isomerization of fatty acid hydroperoxides into hemiacetals. Biochim. Biophys. Acta 2006, 1761, 1419–1428. [Google Scholar] [CrossRef]
- Gobel, C.; Feussner, I.; Schmidt, A.; Scheel, D.; Sanchez-Serrano, J.; Hamberg, M.; Rosahl, S. Oxylipin profiling reveals the preferential stimulation of the 9-lipoxygenase pathway in elicitor-treated potato cells. J. Biol. Chem. 2001, 276, 6267–6273. [Google Scholar] [CrossRef]
- Hamberg, M. An epoxy alcohol synthase pathway in higher plants: Biosynthesis of antifungal trihydroxy oxylipins in leaves of potato. Lipids 1999, 34, 1131–1142. [Google Scholar] [CrossRef]
- Kuhn, H.; Wiesner, R.; Rathmann, J.; Schewe, T. Formation of ketodienoic fatty acids by the pure pea lipoxygenase-1. Eicosanoids 1991, 4, 9–14. [Google Scholar] [PubMed]
- Conconi, A.; Miquel, M.; Browse, J.A.; Ryan, C.A. Intracellular Levels of Free Linolenic and Linoleic Acids Increase in Tomato Leaves in Response to Wounding. Plant Physiol. 1996, 111, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.C.; Schwab, W. Cloning and characterization of a 9-lipoxygenase gene induced by pathogen attack from Nicotiana benthamiana for biotechnological application. BMC Biotechnol. 2011, 11, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta 2005, 1734, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Kramell, R.; Atzorn, R.; Schneider, G.; Miersch, O.; Brückner, C.; Schmidt, J.; Sembdner, G.; Parthier, B. Occurrence and identification of jasmonic acid and its amino acid conjugates induced by osmotic stress in barley leaf tissue. J. Plant Growth Regul. 1995, 14, 29. [Google Scholar] [CrossRef]
- Conconi, A.; Smerdon, M.J.; Howe, G.A.; Ryan, C.A. The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature 1996, 383, 826–829. [Google Scholar] [CrossRef]
- Porta, H.; Rueda-Benitez, P.; Campos, F.; Colmenero-Flores, J.M.; Colorado, J.M.; Carmona, M.J.; Covarrubias, A.A.; Rocha-Sosa, M. Analysis of lipoxygenase mRNA accumulation in the common bean (Phaseolus vulgaris L.) during development and under stress conditions. Plant Cell Physiol. 1999, 40, 850–858. [Google Scholar] [CrossRef]
- Savchenko, T.; Dehesh, K. Drought stress modulates oxylipin signature by eliciting 12-OPDA as a potent regulator of stomatal aperture. Plant Signal. Behav. 2014, 9, e28304. [Google Scholar] [CrossRef] [Green Version]
- Savchenko, T.V.; Zastrijnaja, O.M.; Klimov, V.V. Oxylipins and plant abiotic stress resistance. Biochemistry (Moscow) 2014, 79, 362–375. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerre-Tugaye, M.T.; Rosahl, S.; et al. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Hatanaka, A. Green-leaf-derived C6-aroma compounds with potent antibacterial action that act on both gram-negative and gram-positive bacteria. J. Agric. Food Chem. 2002, 50, 7639–7644. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.; Juttner, F.; Slusarenko, A.J. Volatile products of the lipoxygenase pathway evolved from Phaseolus vulgaris (L.) leaves inoculated with Pseudomonas syringae pv phaseolicola. Plant Physiol. 1993, 101, 13–24. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 2008, 69, 2127–2132. [Google Scholar] [CrossRef]
- Matsui, K.; Minami, A.; Hornung, E.; Shibata, H.; Kishimoto, K.; Ahnert, V.; Kindl, H.; Kajiwara, T.; Feussner, I. Biosynthesis of fatty acid derived aldehydes is induced upon mechanical wounding and its products show fungicidal activities in cucumber. Phytochemistry 2006, 67, 649–657. [Google Scholar] [CrossRef]
- Creelman, R.A.; Tierney, M.L.; Mullet, J.E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc. Natl. Acad. Sci. USA 1992, 89, 4938–4941. [Google Scholar] [CrossRef]
- Zimmerman, D.C.; Coudron, C.A. Identification of traumatin, a wound hormone, as 12-Oxo-trans-10-dodecenoic acid. Plant Physiol. 1979, 63, 536–541. [Google Scholar] [CrossRef]
- Chehab, E.W.; Kaspi, R.; Savchenko, T.; Rowe, H.; Negre-Zakharov, F.; Kliebenstein, D.; Dehesh, K. Distinct roles of jasmonates and aldehydes in plant-defense responses. Public Libr. Sci. One 2008, 3, e1904. [Google Scholar] [CrossRef]
- Royo, J.; Leon, J.; Vancanneyt, G.; Albar, J.P.; Rosahl, S.; Ortego, F.; Castanera, P.; Sanchez-Serrano, J.J. Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests. Proc. Natl. Acad. Sci. USA 1999, 96, 1146–1151. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, R.; Arimura, G.; Takabayashi, J.; Shimoda, T.; Nishioka, T. Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 2000, 41, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.; Boland, W.; Mithofer, A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol. 2005, 167, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, M.; Bachmann, A.; Weichert, H.; Kolbe, A.; Balkenhohl, T.; Wasternack, C.; Feussner, I. Formation of lipoxygenase-pathway-derived aldehydes in barley leaves upon methyl jasmonate treatment. Eur. J. Biochem. 1999, 260, 885–895. [Google Scholar] [CrossRef]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef] [Green Version]
- Arimura, G.-I.; Ozawa, R.; Horiuchi, J.-I.; Nishioka, T.; Takabayashi, J. Plant–plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem. Syst. Ecol. 2001, 29, 1049–1061. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2007, 49, 194–207. [Google Scholar] [CrossRef]
- Allmann, S.; Baldwin, I.T. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 2010, 329, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D. Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 1998, 3, 419–426. [Google Scholar] [CrossRef]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, K.; Kurishita, S.; Hisamitsu, A.; Kajiwara, T. A lipid-hydrolysing activity involved in hexenal formation. Biochem. Soc. Trans. 2000, 28, 857–860. [Google Scholar] [CrossRef]
- Narvaez-Vasquez, J.; Florin-Christensen, J.; Ryan, C.A. Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell 1999, 11, 2249–2260. [Google Scholar] [CrossRef]
- Wang, C.; Zien, C.A.; Afitlhile, M.; Welti, R.; Hildebrand, D.F.; Wang, X. Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in arabidopsis. Plant Cell 2000, 12, 2237–2246. [Google Scholar] [CrossRef]
- Hyun, Y.; Choi, S.; Hwang, H.J.; Yu, J.; Nam, S.J.; Ko, J.; Park, J.Y.; Seo, Y.S.; Kim, E.Y.; Ryu, S.; et al. Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev. Cell 2008, 14, 183–192. [Google Scholar] [CrossRef]
- Yang, W.Y.; Zheng, Y.; Bahn, S.C.; Pan, X.Q.; Li, M.Y.; Vu, H.S.; Roth, M.R.; Scheu, B.; Welti, R.; Hong, Y.Y.; et al. The patatin-containing phospholipase A pPLAIIalpha modulates oxylipin formation and water loss in Arabidopsis thaliana. Mol. Plant 2012, 5, 452–460. [Google Scholar] [CrossRef]
- Bonaventure, G.; Schuck, S.; Baldwin, I.T. Revealing complexity and specificity in the activation of lipase-mediated oxylipin biosynthesis: A specific role of the Nicotiana attenuata GLA1 lipase in the activation of jasmonic acid biosynthesis in leaves and roots. Plant Cell Environ. 2011, 34, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, B.A.; Müller, A.; Hennig, P.; Gebhardt, S.; Schubert-Zsilavecz, M.; Weiler, E.W. A novel class of oxylipins, sn1-O-(12-oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl Diglyceride, from Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 12832–12838. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; von Reuss, S.H.; Tasaka, H.; Nomura, M.; Mochizuki, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Boland, W.; Takabayashi, J.; et al. Traumatin- and dinortraumatin-containing galactolipids in Arabidopsis: Their formation in tissue-disrupted leaves as counterparts of green leaf volatiles. J. Biol. Chem. 2013, 288, 26078–26088. [Google Scholar] [CrossRef] [PubMed]
- Mwenda, C.M.; Matsui, K. The importance of lipoxygenase control in the production of green leaf volatiles by lipase-dependent and independent pathways. Plant Biotechnol. 2014, 31, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, A.; Iijima, Y.; Aoki, K.; Shibata, D.; Sugimoto, K.; Takabayashi, J.; Matsui, K. Monogalactosyl diacylglycerol is a substrate for lipoxygenase: Its implications for oxylipin formation directly from lipids. J. Plant Interact. 2011, 6, 93–97. [Google Scholar] [CrossRef]
- Siedow, J.N. Plant lipoxygenase: Structure and function. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Perez, A.G.; Sanz, C.; Olias, R.; Olias, J.M. Lipoxygenase and hydroperoxide lyase activities in ripening strawberry fruits. J. Agric. Food Chem. 1999, 47, 249–253. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef]
- Feussner, I.; Hause, B.; Vörös, K.; Parthier, B.; Wasternack, C. Jasmonate-induced lipoxygenase forms are localized in chloroplasts of barley leaves (Hordeum vulgare cv. Salome). Plant J. 1995, 7, 949–957. [Google Scholar] [CrossRef]
- Fauconnier, M.-L.; Marlier, M. Les lipoxygénases du soja. Biotechnol. Agron. Société Environ. 1997, 1, 125–141. [Google Scholar]
- Ivanov, I.; Heydeck, D.; Hofheinz, K.; Roffeis, J.; O’Donnell, V.; Kuhn, H.; Walther, M. Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 2010, 503, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C.; Kindl, H.; Kühn, H. Lipoxygenase-catalyzed oxygenation of storage lipids is implicated in lipid mobilization during germination. Proc. Natl. Acad. Sci. USA 1995, 92, 11849–11853. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.A.; Weichert, H.; Fischer, A.M.; Feussner, I.; Grimes, H.D. Activity of soybean lipoxygenase isoforms against esterified fatty acids indicates functional specificity. Arch. Biochem. Biophys. 2001, 388, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Belkner, J.; Wiesner, R.; Kuhn, H.; Lankin, V.Z. The oxygenation of cholesterol esters by the reticulocyte lipoxygenase. FEBS Lett. 1991, 279, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Liavonchanka, A.; Feussner, I. Lipoxygenases: Occurrence, functions and catalysis. J. Plant Physiol. 2006, 163, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Palmieri-Thiers, C.; Canaan, S.P.; Brunini, V.; Lorenzi, V.; Tomi, F.L.; Desseyn, J.-L.; Garscha, U.; Oliw, E.H.; Berti, L.; Maury, J. A lipoxygenase with dual positional specificity is expressed in olives (Olea europaea L.) during ripening. Biochim. Biophys. Acta 2009, 1791, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Palmieri-Thiers, C.; Alberti, J.-C.; Canaan, S.P.; Brunini, V.; Gambotti, C.; Tomi, F.L.; Oliw, E.H.; Berti, L.; Maury, J. Identification of putative residues involved in the accessibility of the substrate-binding site of lipoxygenase by site-directed mutagenesis studies. Arch. Biochem. Biophys. 2011, 509, 82–89. [Google Scholar] [CrossRef]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 15003. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, S.; Sugimoto, K.; Koeduka, T.; Matsui, K. Arabidopsis lipoxygenase 2 is essential for formation of green leaf volatiles and five-carbon volatiles. FEBS Lett. 2016, 590, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. [53] Lipoxygenase from soybeans: EC 1.13.11.12 Linoleate:oxygen oxidoreductase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 71, pp. 441–451. [Google Scholar]
- Minor, W.; Steczko, J.; Bolin, J.T.; Otwinowski, Z.; Axelrod, B. Crystallographic determination of the active site iron and its ligands in soybean lipoxygenase L-1. Biochemistry 1993, 32, 6320–6323. [Google Scholar] [CrossRef]
- Minor, W.; Steczko, J.; Stec, B.; Otwinowski, Z.; Bolin, J.T.; Walter, R.; Axelrod, B. Crystal structure of soybean lipoxygenase L-1 at 1.4 A resolution. Biochemistry 1996, 35, 10687–10701. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, N.; Solomon, E.I. Density-functional investigation on the mechanism of H-atom abstraction by lipoxygenase. J. Biol. Inorg. Chem. 2003, 8, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Gillmor, S.A.; Villasenor, A.; Fletterick, R.; Sigal, E.; Browner, M.F. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat. Struct. Biol. 1997, 4, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Borngräber, S.; Browner, M.; Gillmor, S.; Gerth, C.; Anton, M.; Fletterick, R.; Kühn, H. Shape and Specificity in Mammalian 15-Lipoxygenase Active Site The Functional Interplay Of Sequence Determinants For The Reaction Specificity. J. Biol. Chem. 1999, 274, 37345–37350. [Google Scholar] [CrossRef]
- Hughes, R.K.; Lawson, D.M.; Hornostaj, A.R.; Fairhurst, S.A.; Casey, R. Mutagenesis and modelling of linoleate-binding to pea seed lipoxygenase. Eur. J. Biochem. 2001, 268, 1030–1040. [Google Scholar] [CrossRef]
- Gardner, H.W. Soybean lipoxygenase-1 enzymically forms both (9S)- and (13S)-hydroperoxides from linoleic acid by a pH-dependent mechanism. Biochim. Biophys. Acta 1989, 1001, 274–281. [Google Scholar] [CrossRef]
- Hornung, E.; Walther, M.; Kühn, H.; Feussner, I. Conversion of cucumber linoleate 13-lipoxygenase to a 9-lipoxygenating species by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 4192–4197. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Yadav, J.S. Lipoxygenase biocatalysis: A survey of asymmetric oxygenation. J. Mol. Catal. B Enzym. 2003, 26, 3–28. [Google Scholar] [CrossRef]
- Coffa, G.; Imber, A.N.; Maguire, B.C.; Laxmikanthan, G.; Schneider, C.; Gaffney, B.J.; Brash, A.R. On the relationships of substrate orientation, hydrogen abstraction, and product stereochemistry in single and double dioxygenations by soybean lipoxygenase-1 and its Ala542Gly mutant. J. Biol. Chem. 2005, 280, 38756–38766. [Google Scholar] [CrossRef]
- Coffa, G.; Schneider, C.; Brash, A.R. A comprehensive model of positional and stereo control in lipoxygenases. Biochem. Biophys. Res. Commun. 2005, 338, 87–92. [Google Scholar] [CrossRef]
- Fauconnier, M.L.; Perez, A.G.; Sanz, C.; Marlier, M. Purification and characterization of tomato leaf (Lycopersicon esculentum mill.) hydroperoxide lyase. J. Agric. Food Chem. 1997, 45, 4232–4236. [Google Scholar] [CrossRef]
- Gargouri, M.; Drouet, P.; Legoy, M.D. Hydroperoxide-lyase activity in mint leaves. Volatile C6-aldehyde production from hydroperoxy-fatty acids. J. Biotechnol. 2004, 111, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Vick, B.A. The purification and characterization of fatty acid hydroperoxide lyase in sunflower. Biochim. Biophys. Acta 1999, 1436, 531–540. [Google Scholar] [CrossRef]
- Salas, J.Ì.N.J.; Sanchez, J. Hydroperoxide lyase from olive (Olea europaea) fruits. Plant Sci. 1999, 143, 19–26. [Google Scholar] [CrossRef]
- Padilla, M.N.; Hernandez, M.L.; Perez, A.G.; Sanz, C.; Martinez-Rivas, J.M. Isolation, expression, and characterization of a 13-hydroperoxide lyase gene from olive fruit related to the biosynthesis of the main virgin olive oil aroma compounds. J. Agric. Food Chem. 2010, 58, 5649–5657. [Google Scholar] [CrossRef]
- Jacopini, S.; Mariani, M.; Brunini-Bronzini de Caraffa, V.; Gambotti, C.; Vincenti, S.; Desjobert, J.-M.; Muselli, A.; Costa, J.; Berti, L.; Maury, J. Olive recombinant hydroperoxide lyase, an efficient biocatalyst for synthesis of green leaf volatiles. Appl. Biochem. Biotechnol. 2016, 179, 671–683. [Google Scholar] [CrossRef]
- Shibata, Y.; Matsui, K.; Kajiwara, T.; Hatanaka, A. Purification and Properties of Fatty Acid Hydroperoxide Lyase from Green Bell Pepper Fruits. Plant Cell Physiol. 1995, 36, 147–156. [Google Scholar]
- Husson, F.; Belin, J.M. Purification of hydroperoxide lyase from green bell pepper (Capsicum annuum L.) fruits for the generation of C6-aldehydes in vitro. J. Agric. Food Chem. 2002, 50, 1991–1995. [Google Scholar] [CrossRef]
- Matsui, K.; Ujita, C.; Fujimoto, S.; Wilkinson, J.; Hiatt, B.; Knauf, V.; Kajiwara, T.; Feussner, I. Fatty acid 9- and 13-hydroperoxide lyases from cucumber. FEBS Lett. 2000, 481, 183–188. [Google Scholar] [CrossRef]
- Matoba, T.; Hidaka, H.; Kitamura, K.; Kaizuma, N.; Kito, M. Contribution of hydroperoxide lyase activity to n-hexanal formation in soybean. J. Agric. Food Chem. 1985, 33, 856–858. [Google Scholar] [CrossRef]
- Kuroda, H.; Oshima, T.; Kaneda, H.; Takashio, M. Identification and functional analyses of two cDNAs that encode fatty acid 9-/13-hydroperoxide lyase (CYP74C) in rice. Biosci. Biotechnol. Biochem. 2005, 69, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A.; Kajiwara, T.; Matsui, K.; Toyota, H. Substrate specificity of tea leaf hydroperoxide lyase. Zeitschrift für Naturforschung 1992, 47c, 379–677. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Veldink, G.A.; Vliegenthart, J.F. Fatty acid hydroperoxide lyase: A plant cytochrome p450 enzyme involved in wound healing and pest resistance. Chembiochem 2001, 2, 494–504. [Google Scholar] [CrossRef]
- Kajiwara, T.; Sekiya, J.; Asano, M.; Hatanaka, A. Enantioselectivity of enzymatic cleavage reaction of 13-hydroperoxylinolenic acid to C6-aldehyde and C12-oxo acid in tea chloroplasts. Agric. Biol. Chem. 1982, 46, 3087–3088. [Google Scholar]
- Hatanaka, A.; Kajiwara, T.; Sekiya, J.; Imoto, M.; Inouye, S. Participation and properties of lipoxygenase and hydroperoxide lyase in volatile C6-aldehyde formation from C18-Unsaturated. Fatty acids in isolated tea chloroplasts. Plant Cell Physiol. 1982, 23, 91–99. [Google Scholar] [CrossRef]
- Kim, I.S.; Grosch, W. Partial purification and properties of a hydroperoxide lyase from fruits of pear. J. Agric. Food Chem. 1981, 29, 1220–1225. [Google Scholar] [CrossRef]
- Gardner, H.W.; Weisleder, D.; Plattner, R.D. Hydroperoxide lyase and other hydroperoxide-metabolizing activity in tissues of soybean, Glycine max. Plant Physiol. 1991, 97, 1059–1072. [Google Scholar] [CrossRef]
- Tijet, N.; Schneider, C.; Muller, B.L.; Brash, A.R. Biogenesis of volatile aldehydes from fatty acid hydroperoxides: Molecular cloning of a hydroperoxide lyase (CYP74C) with specificity for both the 9- and 13-hydroperoxides of linoleic and linolenic acids. Arch. Biochem. Biophys. 2001, 386, 281–289. [Google Scholar] [CrossRef]
- Mita, G.; Quarta, A.; Fasano, P.; De Paolis, A.; Di Sansebastiano, G.P.; Perrotta, C.; Iannacone, R.; Belfield, E.; Hughes, R.; Tsesmetzis, N.; et al. Molecular cloning and characterization of an almond 9-hydroperoxide lyase, a new CYP74 targeted to lipid bodies. J. Exp. Bot. 2005, 56, 2321–2333. [Google Scholar] [CrossRef]
- Vick, B.A.; Zimmerman, D.C. Lipoxygenase and hydroperoxide lyase in germinating watermelon seedlings. Plant Physiol. 1976, 57, 780–788. [Google Scholar] [CrossRef]
- Sekiya, J.; Kajiwara, T.; Hatanaka, A. Volatile C6-Aldehyde Formation via Hydroperoxides from C18-Unsaturated Fatty Acids in Etiolated Alfalfa and Cucumber Seedlings. Agric. Biol. Chem. 1979, 43, 969–980. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Van Dijken, A.J.; Smeekens, S.C.; Veldink, G.A.; Vliegenthart, J.F. Characterization of three cloned and expressed 13-hydroperoxide lyase isoenzymes from alfalfa with unusual N-terminal sequences and different enzyme kinetics. Eur. J. Biochem. 2000, 267, 2473–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, K.; Miyahara, C.; Wilkinson, J.; Hiatt, B.; Knauf, V.; Kajiwara, T. Fatty acid hydroperoxide lyase in tomato fruits: Cloning and properties of a recombinant enzyme expressed in Escherichia coli. Biosci. Biotechnol. Biochem. 2000, 64, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Suurmeijer, C.N.; Perez-Gilabert, M.; van Unen, D.J.; van der Hijden, H.T.; Veldink, G.A.; Vliegenthart, J.F. Purification, stabilization and characterization of tomato fatty acid hydroperoxide lyase. Phytochemistry 2000, 53, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Mu, W.; Xue, Q.; Jiang, B.; Hua, Y. Molecular cloning, expression, and enzymatic characterization of Solanum tuberosum hydroperoxide lyase. Eur. Food Res. Technol. 2012, 234, 723–731. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Gigot, C.; Fauconnier, M.-L.; Ongena, M.; Destain, J.; Du Jardin, P.; Wathelet, J.-P.; Thonart, P. Sugar beet leaves as new source of hydroperoxide lyase in a bioprocess producing green-note aldehydes. Biotechnol. Lett. 2008, 30, 1115–1119. [Google Scholar] [CrossRef]
- Fukushige, H.; Hildebrand, D.F. Watermelon (Citrullus lanatus) hydroperoxide lyase greatly increases C6 aldehyde formation in transgenic leaves. J. Agric. Food Chem. 2005, 53, 2046–2051. [Google Scholar] [CrossRef]
- De Domenico, S.; Tsesmetzis, N.; Di Sansebastiano, G.P.; Hughes, R.K.; Casey, R.; Santino, A. Subcellular localisation of Medicago truncatula 9/13-hydroperoxide lyase reveals a new localisation pattern and activation mechanism for CYP74C enzymes. BMC Plant Biol. 2007, 7, 58–70. [Google Scholar] [CrossRef]
- Zhu, B.-Q.; Xu, X.-Q.; Wu, Y.-W.; Duan, C.-Q.; Pan, Q.-H. Isolation and characterization of two hydroperoxide lyase genes from grape berries: HPL isogenes in Vitis vinifera grapes. Mol. Biol. Rep. 2012, 39, 7443–7455. [Google Scholar] [CrossRef]
- Hatanaka, A.; Kajiwara, T.; Matsui, K. Concentration of hydroperoxide lyase activities in root of cucumber seedling. Zeitschrift Naturforschung 1988, 43c, 308–310. [Google Scholar] [CrossRef]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Wathelet, J.-P.; Du Jardin, P.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. [Google Scholar]
- Howe, G.A.; Lee, G.I.; Itoh, A.; Li, L.; DeRocher, A.E. Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiol. 2000, 123, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Bate, N.J.; Sivasankar, S.; Moxon, C.; Riley, J.M.; Thompson, J.E.; Rothstein, S.J. Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiol. 1998, 117, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, J.E.; Itoh, A.; Howe, G.A. Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol. 2001, 125, 306–317. [Google Scholar] [CrossRef]
- Maucher, H.; Hause, B.; Feussner, I.; Ziegler, J.R.; Wasternack, C. Allene oxide synthases of barley (Hordeum vulgare cv. Salome): Tissue specific regulation in seedling development. Plant J. 2000, 21, 199–213. [Google Scholar] [CrossRef]
- Shibata, Y.; Matsui, K.; Kajiwara, T.; Hatanaka, A. Fatty acid hydroperoxide lyase is a heme protein. Biochem. Biophys. Res. Commun. 1995, 207, 438–443. [Google Scholar] [CrossRef]
- Tijet, N.; Wäspi, U.; Gaskin, D.J.; Hunziker, P.; Muller, B.L.; Vulfson, E.N.; Slusarenko, A.; Brash, A.R.; Whitehead, I.M. Purification, molecular cloning, and expression of the gene encoding fatty acid 13-hydroperoxide lyase from guava fruit (Psidium guajava). Lipids 2000, 35, 709–720. [Google Scholar] [CrossRef]
- Schreier, P.; Lorenz, G. Separation, partial purification and characterization of a fatty acid hydroperoxide cleaving enzyme from apple and tomato Fruits. Zeitschrift Naturforschung C 1982, 37, 165–173. [Google Scholar] [CrossRef]
- Olias, J.M.; Rios, J.J.; Valle, M.; Zamora, R.; Sanz, L.C.; Axelrod, B. Fatty acid hydroperoxide lyase in germinating soybean seedlings. J. Agric. Food Chem. 1990, 38, 624–630. [Google Scholar] [CrossRef]
- Matsui, K.; Toyota, H.; Kajiwara, T.; Kakuno, T.; Hatanaka, A. Fatty acid hydroperoxide cleaving enzyme, hydroperoxide lyase, from tea leaves. Phytochemistry 1991, 30, 2109–2113. [Google Scholar] [CrossRef]
- Gomi, K.; Yamasaki, Y.; Yamamoto, H.; Akimitsu, K. Characterization of a hydroperoxide lyase gene and effect of C6-volatiles on expression of genes of the oxylipin metabolism in Citrus. J. Plant Physiol. 2003, 160, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Kong, X.; Zhang, C.; Jiang, B.; Hua, Y. Purification and characterization of hydroperoxide lyase from amaranth tricolor (Amaranthus mangostanus L.) leaves. Eur. Food Res. Technol. 2010, 231, 865–871. [Google Scholar] [CrossRef]
- Matsui, K.; Shibutani, M.; Hase, T.; Kajiwara, T. Bell pepper fruit fatty acid hydroperoxide lyase is a cytochrome P450 (CYP74B). FEBS Lett. 1996, 394, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Gomez, M.P.; Kermasha, S.; Nicaud, J.-M.; Belin, J.-M.; Husson, F. Predicted secondary structure of hydroperoxide lyase from green bell pepper cloned in the yeast Yarrowia lipolytica. J. Mol. Catal. B Enzym. 2010, 65, 63–67. [Google Scholar] [CrossRef]
- Panagakou, I.; Touloupakis, E.; Ghanotakis, D.F. Structural characterization of hydroperoxide lyase in dodecyl maltoside by using circular dichroism. Protein J. 2013, 32, 1–6. [Google Scholar] [CrossRef]
- Lee, D.S.; Nioche, P.; Hamberg, M.; Raman, C.S. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 2008, 455, 363–368. [Google Scholar] [CrossRef]
- Li, L.; Chang, Z.; Pan, Z.; Fu, Z.Q.; Wang, X. Modes of heme binding and substrate access for cytochrome P450 CYP74A revealed by crystal structures of allene oxide synthase. Proc. Natl. Acad. Sci. USA 2008, 105, 13883–13888. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.-H.; Chen, S.-X.; Wang, C.-Y.; Zhang, R.-R.; Cheng, S.-Q.; Meng, H.-W.; Shen, X.-Q. Isolation, expression, and characterization of a hydroperoxide lyase gene from cucumber. Int. J. Mol. Sci. 2013, 14, 22082–22101. [Google Scholar] [CrossRef]
- Toporkova, Y.Y.; Gogolev, Y.V.; Mukhtarova, L.S.; Grechkin, A.N. Determinants governing the CYP74 catalysis: Conversion of allene oxide synthase into hydroperoxide lyase by site-directed mutagenesis. FEBS Lett. 2008, 582, 3423–3428. [Google Scholar] [CrossRef] [Green Version]
- Hughes, R.K.; Yousafzai, F.K.; Ashton, R.; Chechetkin, I.R.; Fairhurst, S.A.; Hamberg, M.; Casey, R. Evidence for communality in the primary determinants of CYP74 catalysis and of structural similarities between CYP74 and classical mammalian P450 enzymes. Proteins 2008, 72, 1199–1211. [Google Scholar] [CrossRef]
- Hughes, R.K.; Belfield, E.J.; Muthusamay, M.; Khan, A.; Rowe, A.; Harding, S.E.; Fairhurst, S.A.; Bornemann, S.; Ashton, R.; Thorneley, R.N.; et al. Characterization of Medicago truncatula (barrel medic) hydroperoxide lyase (CYP74C3), a water-soluble detergent-free cytochrome P450 monomer whose biological activity is defined by monomer-micelle association. Biochem. J. 2006, 395, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Brash, A.R. Mechanistic aspects of CYP74 allene oxide synthases and related cytochrome P450 enzymes. Phytochemistry 2009, 70, 1522–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoeun, S.; Sukhanov, A.; Han, O. Separation of enzymatic functions and variation of spin state of rice allene oxide synthase-1 by mutation of Phe-92 and Pro-430. Bioorg. Chem. 2016, 68, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Shibata, Y.; Kajiwara, T.; Hatanaka, A. Separation of 13- and 9-hydroperoxide lyase activities in cotyledons of cucumber seedling. Zeitschrift Naturforschung 1989, 44, 883–885. [Google Scholar] [CrossRef]
- Matsui, K.; Kajiwara, T.; Hatanaka, A. Inactivation of tea leaf hydroperoxide lyase by fatty acid hydroperoxide. J. Agric. Food Chem. 1992, 40, 175–178. [Google Scholar] [CrossRef]
- Kemal, C.; Louis-Flamberg, P.; Krupinski-Olsen, R.; Shorter, A.L. Reductive inactivation of soybean lipoxygenase 1 by catechols: A possible mechanism for regulation of lipoxygenase activity. Biochemistry 1987, 26, 7064–7072. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Veldink, G.A.; Vliegenthart, J.F. Alfalfa contains substantial 9-hydroperoxide lyase activity and a 3Z:2E-enal isomerase. FEBS Lett. 1999, 443, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Salas, J.J.; Sanchez, J.; Ramli, U.S.; Manaf, A.M.; Williams, M.; Harwood, J.L. Biochemistry of lipid metabolism in olive and other oil fruits. Prog. Lipid Res. 2000, 39, 151–180. [Google Scholar] [CrossRef]
- Matsui, K.; Sugimoto, K.; Mano, J.I.; Ozawa, R.; Takabayashi, J. Differential metabolisms of Green Leaf Volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. Public Libr. Sci. ONE 2012, 7, e36433. [Google Scholar] [CrossRef]
- Hatanaka, A.; Kajiwara, T.; Sekiya, J. Fatty Acid Hydroperoxide Lyase in Plant Tissues. In Biogeneration of Aromas; American Chemical Society: Cambridge, MA, USA, 1986; Volume 317, pp. 167–175. [Google Scholar]
- Weichert, H.; Kolbe, A.; Kraus, A.; Wasternack, C.; Feussner, I. Metabolic profiling of oxylipins in germinating cucumber seedlings--lipoxygenase-dependent degradation of triacylglycerols and biosynthesis of volatile aldehydes. Planta 2002, 215, 612–619. [Google Scholar] [CrossRef]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. J. Agric. Food Chem. 2009, 57, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.; Schieberle, P. Characterization of the Key Aroma Compounds in the Beverage Prepared from Darjeeling Black Tea: Quantitative Differences between Tea Leaves and Infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef]
- Alasalvar, C.; Topal, B.; Serpen, A.; Bahar, B.; Pelvan, E.; Gökmen, V. Flavor Characteristics of Seven Grades of Black Tea Produced in Turkey. J. Agric. Food Chem. 2012, 60, 6323–6332. [Google Scholar] [CrossRef] [PubMed]
- Kotseridis, Y.; Baumes, R. Identification of Impact Odorants in Bordeaux Red Grape Juice, in the Commercial Yeast Used for Its Fermentation, and in the Produced Wine. J. Agric. Food Chem. 2000, 48, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.T.; Alonso, M.V.; Rios, J.J.; Aparicio, R. Virgin Olive Oil Aroma: Relationship between Volatile Compounds and Sensory Attributes by Chemometrics. J. Agric. Food Chem. 1995, 43, 2925–2931. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef]
- Olias, J.M.; Perez, A.G.; Rios, J.J.; Sanz, L.C. Aroma of virgin olive oil: Biogenesis of the “green” odor notes. J. Agric. Food Chem. 1993, 41, 2368–2373. [Google Scholar] [CrossRef]
- Padilla, M.N.; Hernández, M.L.; Sanz, C.; Martínez-Rivas, J.M. Functional characterization of two 13-lipoxygenase genes from olive fruit in relation to the biosynthesis of volatile compounds of virgin olive oil. J. Agric. Food Chem. 2009, 57, 9097–9107. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.E.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Kubo, A.; Lunde, C.S.; Kubo, I. Antimicrobial activity of the olive oil flavor compounds. J. Agric. Food Chem. 1995, 43, 1629–1633. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.; Kubo, A.; Nihei, K.; Lunde, C.S. Modes of antifungal action of (2E)-alkenals against Saccharomyces cerevisiae. J. Agric. Food Chem. 2003, 51, 3951–3957. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.A. Flavours: The Legal Framework. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 15–24. [Google Scholar]
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.M.; Rabenhorst, J. Applied biocatalysis for the synthesis of natural flavour compounds—Current industrial processes and future prospects. Biotechnol. Lett. 2004, 26, 463–472. [Google Scholar] [CrossRef]
- Kanisawa, T.; Itoh, H. Method for Preparing Green Aroma Compounds. U.S. Patent 4769243A, 6 September 1988. [Google Scholar]
- Goers, S.K.; Ghossi, P.; Patterson, J.T.; Young, C.L. Process for Producing a Green Leaf Essence. Patent CA 1309615C, 3 November 1992. [Google Scholar]
- Sekiya, J.; Monma, T.; Kajiwara, T.; Hatanaka, A. Changes in Activities of Lipoxygenase and Hydroperoxide Lyase during Seed Development of Soybean. Agric. Biol. Chem. 1986, 50, 521–522. [Google Scholar]
- Trawatha, S.E.; TeKrony, D.M.; Hildebrand, D.F. Relationship of Soybean Seed Quality to Fatty Acid and C6-Aldehyde Levels during Storage. Crop Sci. 1995, 35, 1415–1422. [Google Scholar] [CrossRef]
- Whitehead, I.M.; Slusarenko, A.J.; Waspi, U.; Gaskin, D.J.H.; Brash, A.R.; Tijet, N. Guava (Psidium Guajava) 13-Hydroperoxide Lyase and Uses Thereof. U.S. Patent 6,780,621 B2, 24 August 2004. [Google Scholar]
- Fu, X.; Zhu, X.; Gao, K.; Duan, J. Oil and fat hydrolysis with lipase from Aspergillus sp. J. Am. Oil Chem. Soc. 1995, 72, 527–531. [Google Scholar] [CrossRef]
- Gargouri, M.; Akacha, N.B.; Kotti, F.; Ben Rejeb, I. Lipoxygenase pathway: Valorization of plant oils and aroma biosynthesis. Biotechnol. Agron. Société Environ. 2008, 12, 185–202. [Google Scholar]
- Kosugi, Y.; Tanaka, H.; Tomizuka, N. Continuous hydrolysis of oil by immobilized lipase in a countercurrent reactor. Biotechnol. Bioeng. 1990, 36, 617–622. [Google Scholar] [CrossRef]
- Xi, W.-W.; Xu, J.-H. Preparation of enantiopure (S)-ketoprofen by immobilized Candida rugosa lipase in packed bed reactor. Process Biochem. 2005, 40, 2161–2166. [Google Scholar] [CrossRef]
- Gargouri, M.; Legoy, M.D. Bienzymatic reaction for hydroperoxide production in a multiphasic system. Enzym. Microb. Technol. 1997, 21, 79–84. [Google Scholar] [CrossRef]
- Kim, K.H.; Kwon, D.Y.; Rhee, J.S. Effects of organic solvents on lipase for fat splitting. Lipids 1984, 19, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kawasaki, M.; Shiomori, K.; Baba, Y.; Hano, T. Hydrolysis kinetics of olive oil with lipase in a transfer cell. J. Ferment. Bioeng. 1994, 77, 283–287. [Google Scholar] [CrossRef]
- Yang, F.; Russell, A.J. A comparison of lipase-catalyzed ester hydrolysis in reverse micelles, organic solvents, and biphasic systems. Biotechnol. Bioeng. 1995, 47, 60–70. [Google Scholar] [CrossRef]
- H-Kittikun, A.; Prasertsan, P.; Sungpud, C. Continuous production of fatty acids from palm olein by immobilized lipase in a two-phase system. J. Am. Oil Chem. Soc. 2000, 77, 599–603. [Google Scholar] [CrossRef]
- Tiss, A.; Carrière, F.; Verger, R. Effects of Gum Arabic on Lipase Interfacial Binding and Activity. Anal. Biochem. 2001, 294, 36–43. [Google Scholar] [CrossRef]
- Mtibaa, H.; Fendri, A.; Sayari, A.; Ben Salah, A.; Mejdoub, H.; Gargouri, Y. La lipase de Candida rugosa: Caractérisation biochimique. Oléagineux Corps Gras Lipides 2002, 9, 43–47. [Google Scholar] [CrossRef]
- Piazza, G.J. Lipoxygenase catalyzed hydroperoxide formation in microemulsions containing nonionic surfactant. Biotechnol. Lett. 1992, 14, 1153–1158. [Google Scholar] [CrossRef]
- Rodakiewicz-Nowak, J.; Maslakiewicz, P.; Haber, J. The effect of linoleic acid on pH inside sodium bis(2-ethylhexyl)sulfosuccinate reverse micelles in isooctane and on the enzymic activity of soybean lipoxygenase. Eur. J. Biochem. 1996, 238, 549–553. [Google Scholar] [CrossRef]
- Gargouri, M.; Drouet, P.; Hervagault, J.F.; Legoy, M.D. Investigation of behavior of an enzyme in a biphasic system: Soybean lipoxygenase-1. Biotechnol. Bioeng. 1996, 51, 573–580. [Google Scholar] [CrossRef]
- Kaewthong, W.; Sirisansaneeyakul, S.; Prasertsan, P.; H-Kittikun, A. Continuous production of monoacylglycerols by glycerolysis of palm olein with immobilized lipase. Process Biochem. 2005, 40, 1525–1530. [Google Scholar] [CrossRef]
- Gargouri, M.; Legoy, M.D. The kinetic behaviour of a two-enzyme system in biphasic media: Coupling hydrolysis and lipoxygenation. Biochim. Biophys. Acta 1997, 1337, 227–232. [Google Scholar] [CrossRef]
- Kermasha, S.; Dioum, N.; Bisakowski, B.; Vega, M. Biocatalysis by immobilized lipoxygenase in a ternary micellar system. J. Mol. Catal. B Enzym. 2002, 19–20, 305–317. [Google Scholar] [CrossRef]
- Karadag, H.; Bilgin, R.; Tukel, S. Immobilization of Soybean Lipoxygenase Onto Polyacrylamide Gel. Biotechnol. Biotechnol. Equip. 2006, 20, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, M.C.; Amrutha, N.; Tavanandi, H.A.; Raghavarao, K.S.M.S. Stabilization of Lipoxygenase-1 from Glycine max by Microencapsulation. Dry. Technol. 2015, 33, 493–501. [Google Scholar] [CrossRef]
- Christopher, J.; Axelrod, B. On the different positional specificities of peroxidation of linoleate shown by two isozymes of soybean lipoxygenase. Biochem. Biophys. Res. Commun. 1971, 44, 731–736. [Google Scholar] [CrossRef]
- Muller, B.; Gautier, A.; Dean, C.; Kuhn, J.C. Process for the Enzymatic Preparation of Aliphatic Alcohols and Aldehydes from Linoleic Acid, Linoleic Acid, or a Natural Precursor. U.S. Patent No. 5,464,761, 1995. [Google Scholar]
- Noordermeer, M.A.; Van Der Goot, W.; Van Kooij, A.J.; Veldsink, J.W.; Veldink, G.A.; Vliegenthart, J.F. Development of a biocatalytic process for the production of c6-aldehydes from vegetable oils by soybean lipoxygenase and recombinant hydroperoxide lyase. J. Agric. Food Chem. 2002, 50, 4270–4274. [Google Scholar] [CrossRef]
- Márczy, J.S.; Németh, Á.S.; Samu, Z.; Háger-Veress, Á.; Szajáni, B. Production of hexanal from hydrolyzed sunflower oil by lipoxygenase and hydroperoxide lyase enzymes. Biotechnol. Lett. 2002, 24, 1673–1675. [Google Scholar] [CrossRef]
- Nemeth, A.S.; Marczy, J.S.; Samu, Z.; Hager-Veress, A.; Szajani, B. Biocatalytic production of 2(E)-hexenal from hydrolysed linseed oil. Enzyme Microb. Technol. 2004, 34, 667–672. [Google Scholar] [CrossRef]
- Galliard, T.; Phillips, D.R. Lipoxygenase from potato tubers. Partial purification and properties of an enzyme that specifically oxygenates the 9-position of linoleic acid. Biochem. J. 1971, 124, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Sekiya, J.; Aoshima, H.; Kajiwara, T.; Togo, T.; Hatanaka, A. Purification and Some Properties of Potato Tuber Lipoxygenase and Detection of Linoleic Acid Radical in the Enzyme Reaction. Agric. Biol. Chem. 1977, 41, 827–832. [Google Scholar]
- Gargouri, M.; Legoy, M. A two-enzyme system for the transformation of unsaturated oils to 9(S)-hydroperoxy fatty acids. Biotechnol. Lett. 2002, 24, 915–918. [Google Scholar] [CrossRef]
- Kerler, J.; Kohlen, E.; Fitz, W.; Vliet, A.V.D.; Winkel, C. Method for the Enzymatic Preparation of Aldehydes Rich Aromas c6-c10. U.S. Patent No. 6,864,072, 8 March 2005. [Google Scholar]
- Wang, Z. Hock Rearrangement. In Comprehensive Organic Name Reactions and Reagents; American Cancer Society: Atlanta, GA, USA, 2010; pp. 1438–1442. [Google Scholar]
- Brunerie, P. Procédé de synthèse du cis-3-hexene-1-ol à partir d’acide gras insaturé. Patent EP 0481147B1, 10 June 1998. [Google Scholar]
- Schade, F.; Thompson, J.; Legge, R. Use of a plant-derived enzyme template for the production of the green-note volatile hexanal. Biotechnol. Bioeng. 2003, 84, 265–273. [Google Scholar] [CrossRef]
- Akacha, N.B.; Boubaker, O.; Gargouri, M. Production of hexenol in a two-enzyme system: Kinetic study and modelling. Biotechnol. Lett. 2005, 27, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, M.; Akacha, N.B.; Legoy, M.D. Coupled hydroperoxide lyase and alcohol dehydrogenase for selective synthesis of aldehyde or alcohol. Appl. Biochem. Biotechnol. 2004, 119, 171–180. [Google Scholar] [CrossRef]
- Akacha, N.B.; Gargouri, M. Enzymatic synthesis of green notes with hydroperoxide-lyase from olive leaves and alcohol-dehydrogenase from yeast in liquid/gas reactor. Process Biochem. 2009, 44, 1122–1127. [Google Scholar] [CrossRef]
- Hausler, A.; Ehret, C.; Binggeli, E. Process for the Production of Degradation Products of Fatty Acids. U.S. Patent No. 6150145, 21 November 2000. [Google Scholar]
- Tan, Y.; Siebert, K.J. Quantitative Structure−Activity Relationship Modeling of Alcohol, Ester, Aldehyde, and Ketone Flavor Thresholds in Beer from Molecular Features. J. Agric. Food Chem. 2004, 52, 3057–3064. [Google Scholar] [CrossRef]
- Brunerie, P.; Koziet, Y. Process for Producing Natural cis-3-Hexenol from Unsaturated Fatty Acids. U.S. Patent 5620879A, 15 April 1997. [Google Scholar]
- Hall, C.E.; Karboune, S.; Florence, H.; Kermasha, S. Stabilization of an enzymatic extract from Penicillium camemberti containing lipoxygenase and hydroperoxide lyase activities. Process Biochem. 2008, 43, 258–264. [Google Scholar] [CrossRef]
- Akacha, N.B.; Karboune, S.; Gargouri, M.; Kermasha, S. Activation and stabilization of the hydroperoxide lyase enzymatic extract from mint leaves (Mentha spicata) using selected chemical additives. Appl. Biochem. Biotechnol. 2010, 160, 901–911. [Google Scholar] [CrossRef]
- Long, Z.; Kong, X.; Zhang, C.; Hua, Y. Stability of hydroperoxide lyase activity from Amaranthus tricolor (Amaranthus mangostanus L.) leaves: Influence of selected additives. J. Sci. Food Agric. 2010, 90, 729–734. [Google Scholar]
- Hornostaj, A.R.; Robinson, D.S. Purification of hydroperoxide lyase from pea seeds. Food Chem. 2000, 71, 241–247. [Google Scholar] [CrossRef]
- Gianfreda, L.; Scarfi, M.R. Enzyme stabilization: State of the art. Mol. Cell. Biochem. 1991, 100, 97–128. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Stumpe, M.; Matsui, K.; Kajiwara, T.; Feussner, I. Kinetics of barley FA hydroperoxide lyase are modulated by salts and detergents. Lipids 2003, 38, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hua, Y.; Kong, X.; Zhang, C.; Chen, Y. Covalent immobilization of hydroperoxide lyase on chitosan hybrid hydrogels and production of C6 aldehydes by immobilized enzyme. J. Mol. Catal. B Enzym. 2013, 95, 89–98. [Google Scholar] [CrossRef]
- Liu, Q.; Hua, Y. Continuous synthesis of hexanal by immobilized hydroperoxide lyase in packed-bed reactor. Bioprocess Biosyst. Eng. 2015, 38, 2439–2449. [Google Scholar] [CrossRef]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Muhovski, Y.; Wathelet, J.-P.; du Jardin, P.; Thonart, P. Optimization and scaling up of a biotechnological synthesis of natural green leaf volatiles using Beta vulgaris hydroperoxide lyase. Process Biochem. 2012, 47, 2547–2551. [Google Scholar] [CrossRef]
- Jacopini, S.; Vincenti, S.; Mariani, M.; Brunini-Bronzini de Caraffa, V.; Gambotti, C.; Desjobert, J.M.; Muselli, A.; Costa, J.; Tomi, F.; Berti, L.; et al. Activation and Stabilization of Olive Recombinant 13-Hydroperoxide Lyase Using Selected Additives. Appl. Biochem. Biotechnol. 2017, 182, 1000–1013. [Google Scholar] [CrossRef]
- Brühlmann, F.; Bosijokovic, B.; Ullmann, C.; Auffray, P.; Fourage, L.; Wahler, D. Directed evolution of a 13-hydroperoxide lyase (CYP74B) for improved process performance. J. Biotechnol. 2013, 163, 339–345. [Google Scholar] [CrossRef]
- Brühlmann, F.; Bosijokovic, B. Efficient Biochemical Cascade for Accessing Green Leaf Alcohols. Org. Process Res. Dev. 2016, 20, 1974–1978. [Google Scholar] [CrossRef]
- Bourel, G.; Nicaud, J.-M.; Nthangeni, B.; Santiago-Gomez, P.; Belin, J.-M.; Husson, F. Fatty acid hydroperoxide lyase of green bell pepper: Cloning in Yarrowia lipolytica and biogenesis of volatile aldehydes. Enzym. Microb. Technol. 2004, 35, 293–299. [Google Scholar] [CrossRef]
- Santiago-Gomez, M.P.; Thanh, H.T.; De Coninck, J.L.; Cachon, R.M.; Kermasha, S.L.; Belin, J.-M.; Gervais, P.; Husson, F. Modeling hexanal production in oxido-reducing conditions by the yeast Yarrowia lipolytica. Process Biochem. 2009, 44, 1013–1018. [Google Scholar] [CrossRef]
- Atwal, A.S.; Bisakowski, B.; Richard, S.; Robert, N.; Lee, B. Cloning and secretion of tomato hydroperoxide lyase in Pichia pastoris. Process Biochem. 2005, 40, 95–102. [Google Scholar] [CrossRef]
- Buchhaupt, M.; Guder, J.C.; Etschmann, M.M.W.; Schrader, J. Synthesis of green note aroma compounds by biotransformation of fatty acids using yeast cells coexpressing lipoxygenase and hydroperoxide lyase. Appl. Microbiol. Biotechnol. 2012, 93, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-C.; Studart-Witkowski, C.; Schwab, W. Overexpression of hydroperoxide lyase gene in Nicotiana benthamiana using a viral vector system. Plant Biotechnol. J. 2010, 8, 783–795. [Google Scholar] [CrossRef] [PubMed]


| FONCTION | COMPOUND | ODOR |
|---|---|---|
| ALDEHYDES | hexanal | Green, apple, cut grass |
| (3Z)-hexenal | Green leaves, grassy, green, apple-like, leaf-like, cut grass | |
| (2E)-hexenal | Green, fruity, sweet | |
| (3Z)-nonenal | Cucumber-like, green | |
| (2E)-nonenal | Fatty, cut grass | |
| (3Z,6Z)-nonadienal | Cucumber-like, melon odor | |
| (2E,6Z)-nonadienal | Cucumber-like | |
| ALCOHOLS | hexanol | Fruity, aromatic, soft, cut grass |
| (3Z)-hexenol | Banana, leaf-like, green-fruity, pungent | |
| (2E)-hexenol | Green, grassy, fruity, fatty, pungent | |
| (3Z)-nonenol | Fresh, waxy, green melon odor | |
| (2E)-nonenol | Melon odor, waxy, green odor | |
| (3Z,6Z)-nonadienol | Watermelon odor | |
| (2E),(6Z)-nonadienol | Cut grass, cucumber-like | |
| ESTERS | Hexyl acetate | Sweet, fruity, floral |
| (3Z)-hexenyl acetate | Green-banana, fruity, Green, green leaves, floral, ester |
 GLVs produced from linoleic acid.
GLVs produced from linoleic acid.  GLVs produced from linolenic acid.
GLVs produced from linolenic acid.| Biocatalytic Step | Initial Substrate | Biocatalyst | Method Peculiarity | Product (Concentration and/or Yield in %) | Reference |
|---|---|---|---|---|---|
| Hydroperoxides synthesis | LA (20 g·L−1) | Soybean LOX1 | Biphasic medium (octane:borate buffer pH 9.6, 1:8) | 13-HPOD (60.2% yield) | Drouet [12] |
| Hydrolyzed flax seed oil (54 g·L−1) | LOX extracted from soybean seed | Bioreactor without addition of any solvent or surfactant | 13-HPOT (71.5% yield) | Fauconnier and Marlier [13] | |
| LA (100 mM) contained in hydrolyzed sunflower oil | LOX1 isolated from defatted soybean flour | - | 13-HPOD (68.7 mM, 72% yield) | Márczy, et al. [206] | |
| ALA (100 mM) contained in hydrolyzed linseed oil | LOX1 isolated from soybean flour | - | 13-HPOT (57 mM, 62% yield) | Nemeth, et al. [207] | |
| GLVs synthesis | Linseed oil (250 g) | LOX2 isoform of soybean flour | Heat treatment (90 to 180 °C) under acidic conditions to promote cleavage of HPOs | (2E)-hexenal (20,150 ppm) (3Z)-hexenal (10,380 ppm) (2E,6Z)-nonadienal (8900 ppm) | Kerler, et al. [211] |
| Sunflower oil (250 g) | (3Z)-hexenal (125 ppm) hexanal (5250 ppm) | ||||
| 13-HPOD (15 mM) | HPL isolated from spinach leaf | Hexanal isolation by repeated steam distillation | Hexanal (8.2 mM, 54% yield) | Márczy, et al. [206] | |
| 13-HPOT (20 mM) | HPL of a homogenate from green bell pepper fruits | Hexanal isolation by repeated steam distillation | (3Z)-hexenal (5.9 mM) (2E)-hexenal (1.6 mM ) (37% yield for the hexenal isomers together) | Nemeth, et al. [207] | |
| Linseed oil (3 g·L−1) hydrolyzed by immobilized Thermomyces lanuginosa lipase | Soybean flour and HPL of a homogenate from crushed sugar beet leaves | All reactions in the same bioreactor | (3Z)-hexenal (80% yield) and Hexanal (70% yield) | Rabetafika, et al. [128] | |
| - | - | - | - | - | |
| LA (10.7 mM) | Immobilized enzymes extracted from one gram of tomato leaves | Immobilization in an alginate and use of a packed-bed bioreactor | Hexanal (80.2 μg·g−1 of fresh weight, 0.1% yield *) | Schade, et al. [214] | |
| Chemically hydrolyzed linseed oil | - LOX of a homogenate from soybean seeds - HPL of homogenate from olive leaves - Saccharomyces cerevisiae yeast containing ADH activity | Enzymatic liquid/gas reactor for coupling GLVs synthesis and extraction | (3Z)- and (2E)-hexenals (0.36 g·kg−1 of reaction medium, 50% yield) (3Z)-hexenol (3.54 g·kg−1 of olive leaves, 47.7% yield) | Akacha and Gargouri [217] | |
| Sunflower oil or linseed oil or commercial mixture of FAs | - Soybean flour as LOX source - Guava homogenate containing HPL - Saccharomyces cerevisiae yeast containing ADH activity | Steam distillation and/or extraction of GLVvs with an inert organic solvent | Hexanal (5 g·kg−1 of reaction medium, 35.8% yield), (3Z)-hexenol (4.2 g·kg−1 of reaction medium, 41.9% yield) and (2E)-hexenal (1.5 g·kg−1 of reaction medium, 20% yield) | Muller, et al. [204] | |
| Flaxseed oil | - Candida cylindracea lipase - Shreds of violet leaves - Baker’s yeast cells | - | (2E,6Z)-nonadienal (661 mg·kg−1 of plant material) and (2E,6Z)-nonadienol (44 mg·kg−1 of plant material) | Hausler, et al. [218] | |
| Hydrolyzed safflower and linseed oils | Soybean flour containing 13-LOX and Alfalfa recombinant 13-HPL expressed in E. coli | - | Hexanal (50% yield) and (3Z)- and (2E)-hexenal (26% yield) | Noordermeer, et al. [205] | |
| 13-HPOT (10 mM) | Sugar beet HPL extracted from leaves or expressed by recombinant E. coli strains | Fed-batch substrate addition and a continuous extraction of volatiles | 3.46 mM of C6 aldehydes with the HPL extracted from leaves or 5.5 mM of C6 aldehydes with recombinant HPL | Gigot, et al. [229] | |
| 13-HPOD (17.6 mM) | Recombinant guava HPL expressed in E. coli | - | Hexanal (14g·L−1 of bacterial lysate, 3.95% yield *) | Whitehead, et al. [183] | |
| 13-HPOD and 13-HPOT (119 mM) | Green pepper recombinant HPL expressed in growing Yarrowia lipolytica | - | Hexanal (6mM) | Santiago-Gomez, et al. [234] | |
| 13-HPOT (257 mM) | Engineered recombinant guava HPL (improved by directed evolution) and recombinant ketoreductase | - | (3Z)-hexenol (8 g·L−1, 41% yield) at high isomeric purity (>99%) | Brühlmann and Bosijokovic [232] | |
| 13-HPOD (6 mM) and 13-HPOT (6 mM) | Recombinant olive HPL expressed in E. coli | - | Hexanal (5.61 mM, 93.5% yield) and (3Z)-hexenal (4.39 mM, 73% yield) | Jacopini, et al. [108] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts 2019, 9, 873. https://doi.org/10.3390/catal9100873
Vincenti S, Mariani M, Alberti J-C, Jacopini S, Brunini-Bronzini de Caraffa V, Berti L, Maury J. Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts. 2019; 9(10):873. https://doi.org/10.3390/catal9100873
Chicago/Turabian StyleVincenti, Sophie, Magali Mariani, Jean-Christophe Alberti, Sabrina Jacopini, Virginie Brunini-Bronzini de Caraffa, Liliane Berti, and Jacques Maury. 2019. "Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway" Catalysts 9, no. 10: 873. https://doi.org/10.3390/catal9100873
APA StyleVincenti, S., Mariani, M., Alberti, J. -C., Jacopini, S., Brunini-Bronzini de Caraffa, V., Berti, L., & Maury, J. (2019). Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts, 9(10), 873. https://doi.org/10.3390/catal9100873





