Cooperative Catalytic Behavior of SnO2 and NiWO4 over BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication of Triple-Layer Photoanodes
2.2. Material Characterization of NiWO4/BiVO4/SnO2 Photoanodes
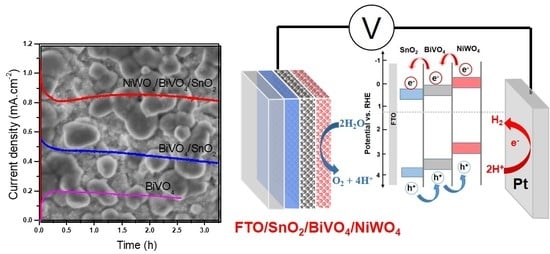
2.3. Photo-Electrochemical Behavior of Photoanodes
3. Experimental
3.1. Chemicals
3.2. Preparation of Photoanodes
3.2.1. Electrodeposition of SnO2 onto FTO Substrates
3.2.2. Electrodeposition of BiVO4 onto SnO2/FTO Substrates
3.2.3. Preparation of NiWO4 on BiVO4/SnO2 Photoanodes
3.3. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruth, M.; Joseck, F. Hydrogen Threshold Cost Calculation; United States Department of Energy: Washington, DC, USA, 2011. [Google Scholar]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for Solar Fuels and Chemicals. Nat. Mater. 2017, 16, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Jaramillo, Energy Environ. Science 2013, 6, 1983–2002. [Google Scholar]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Arunachalam, P.; Al-Mayouf, A. Photoelectrochemical Water Splitting. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Woodhead Publishing, 2019; pp. 585–606. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Arunachalam, P.; Amer, M.S.; Ghanem, M.A.; Al-Mayouf, A.M.; Zhao, D. Activation Effect of Silver Nanoparticles on the Photoelectrochemical Performance of Mesoporous TiO2 Nanospheres Photoanodes for Water Oxidation Reaction. Int. J. Hydrog. Enery 2017, 42, 11346–11355. [Google Scholar] [CrossRef]
- Du, C.; Yang, X.; Mayer, M.T.; Hoyt, H.; Xie, J.; McMahon, G.; Bischoping, G.; Wang, D. Hematite-based Water Splitting with Low Turn-On Voltages. Angew. Chem. Int. Ed. 2013, 52, 12692–12695. [Google Scholar] [CrossRef]
- Jang, J.W.; Du, C.; Ye, Y.; Lin, Y.; Yao, X.; Thorne, J.; Liu, E.; McMahon, G.; Zhu, J.; Javey, A. Enabling Unassisted Solar Water Splitting by Iron Oxide and Silicon. Nat. Commun. 2015, 6, 7447. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, J.; Park, T.J.; Lee, J.S. Mg-Doped WO3 as a Novel Photocatalyst for Visible Light-Induced Water Splitting. Catal. Lett. 2002, 80, 53–57. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Wang, Q. Nanostructure-Based WO3 Photoanodes for Photoelectrochemical Water Splitting. Phys. Chem. Chem. Phys. 2012, 14, 7894–7911. [Google Scholar] [CrossRef]
- Suarez, C.M.; Hernandez, S.; Russo, N. BiVO4 as Photocatalyst for Solar Fuels Production Through Water Splitting: A Short Review. Appl. Catal. A Gen. 2015, 504, 158–170. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Ghanem, M.A.; Al-Mayouf, A.M.; Gimenez, S.; Bisquert, J.; Herraiz-Cardona, I. Cooperative Catalytic Effect of ZrO2 and α-Fe2O3 Nanoparticles on BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting. ChemSusChem 2016, 9, 2779–2783. [Google Scholar] [CrossRef] [PubMed]
- Shaddad, M.N.; Arunachalam, P.; Labis, J.; Hezam, M.; Al-Mayouf, A.M. Fabrication of Robust Nanostructured (Zr)BiVO4/Nickel Hexacyanoferrate Core/Shell Photoanodes for Solar Water Splitting. Appl. Catal. B Env. 2019, 244, 863–870. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, K.; Shin, K.; Ma, M.; Kwon, J.; Choi, I.T.; Kim, J.K.; Kim, H.K.; Wang, D.H.; Park, J.H. Unassisted Photoelectrochemical Water Splitting Beyond 5.7% Solar-To-Hydrogen Conversion Efficiency by a Wireless Monolithic Photoanode/Dye-Sensitised Solar Cell Tandem Device. Nano Energy 2015, 13, 182–191. [Google Scholar] [CrossRef]
- Malathi, A.; Vasanthakumar, V.; Arunachalam, P.; Madhavan, J.; Ghanem, M.A. A Low Cost Additive-Free Facile Synthesis of BiFeWO6/BiVO4 Nanocomposite with Enhanced Visible-Light Induced Photocatalytic Activity. J. Colloid Interface Sci. 2017, 506, 553–563. [Google Scholar] [CrossRef]
- Peng, B.; Xia, M.; Li, C.; Yue, C.; Diao, P. Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting. Catalysts 2018, 8, 663. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A Review on BiVO4 Photocatalyst: Activity Enhancement Methods for Solar Photocatalytic Applications. Appl. Catal. A Gen 2018, 555, 47–74. [Google Scholar]
- Kudo, A.; Omori, K.; Kato, H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem Mater 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.S. Progress in Bismuth Vanadate Photoanodes for Use in Solar Water Oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic Generation of Hydrogen by Core-Shell WO3/BiVO4 Nanorods with Ultimate Water Splitting Efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-Complete Suppression of Surface Recombination in Solar Photoelectrolysis by “Co-Pi” Catalyst-Modified W: BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kafizas, A.; Pendlebury, S.R.; Le Formal, F.; Durrant, J.R. Photoinduced absorption spectroscopy of CoPi on BiVO4: the function of CoPi during water oxidation. Adv. Funct. Mater. 2016, 26, 4951–4960. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Gratzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. Chemsuschem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- Ma, Y.; Le Formal, F.; Kafizas, A.; Pendlebury, S.R.; Durrant, J.R. Efficient Suppression of Back Electron/Hole Recombination in Cobalt Phosphate Surface-Modified Undoped Bismuth Vanadate Photoanodes. J. Mater. Chem. A 2015, 3, 20649–20657. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel–Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Al-Sharif, M.S.; Arunachalam, P.; Abiti, T.; Amer, M.S.; Al-Shalwi, M.; Ghanem, M.A. Mesoporous cobalt phosphate electrocatalyst prepared using liquid crystal template for methanol oxidation reaction in alkaline solution. Arabian J. Chem. 2018. [Google Scholar] [CrossRef]
- Arunachalam, P.; Al-Mayouf, A.; Ghanem, M.A.; Shaddad, M.N.; Weller, M.T. Photoelectrochemical Oxidation of Water Using La(Ta, Nb)O2N Modified Electrodes. Int. J. Hydrog. Energy 2016, 41, 11644–11652. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Arunachalam, P.; Al-Mayouf, A.M.; Ghanem, M.A.; Alharthi, A.I. Enhanced Photoelectrochemical Oxidation of Alkali Water over Cobalt Phosphate (Co-Pi) Catalyst-Modified ZnLaTaON2 Photoanodes. Ionics 2019, 25, 737–745. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Cardenas-Morcoso, D.; Arunachalam, P.; Garcia-Tecedor, M.; Ghanem, M.A.; Bisquert, J.; Al–Mayouf, A.; Gimenez, S. Enhancing the Optical Absorption and Interfacial Properties of BiVO4 with Ag3PO4 Nanoparticles for Efficient Water Splitting. J. Phys. Chem. C 2018, 122, 11608–11615. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Arunachalam, P.; Alothman, A.A.; Beagan, A.M.; Alshalwi, M.N.; Al-Mayouf, A.M. Synergetic Catalytic Behavior of AgNi-OH-Pi Nanostructures on Zr: BiVO4 Photoanode for Improved Stability and Photoelectrochemical Water Splitting Performance. J. Catal. 2019, 371, 10–19. [Google Scholar] [CrossRef]
- Kuang, Y.; Jia, Q.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A Front-Illuminated Nanostructured Transparent BiVO4 Photoanode for> 2% Efficient Water Splitting. Adv. Energy Mater. 2016, 6, 1501645. [Google Scholar] [CrossRef]
- Milbrat, A.; Vijselaar, W.; Guo, Y.; Mei, B.; Huskens, J.; Mul, G. Integration of Molybdenum-Doped, Hydrogen-Annealed BiVO4 with Silicon Microwires for Photoelectrochemical Applications. ACS Sustain. Chem. Eng. 2019, 7, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 Electrodes for Enhanced Photoactivity of Water Oxidation. Energy Env. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Chatchai, P.; Murakami, Y.; Kishioka, S.Y.; Nosaka, A.; Nosaka, Y. FTO / SnO2 /BiVO4 Composite Photoelectrode for Water Oxidation under Visible Light Irradiation. Electrochem. Solid State Lett. 2008, 11, H160–H163. [Google Scholar] [CrossRef]
- Lee, M.G.; Kim, D.H.; Sohn, W.; Moon, C.W.; Park, H.; Lee, S.; Jang, H.W. Conformally Coated BiVO4 Nanodots on Porosity-Controlled WO3 Nanorods Asllllll Highly Efficient Type II Heterojunction Photoanodes for Water Oxidatlion. Nano Energy 2016, 28, 250–260. [Google Scholar] [CrossRef]
- Liang, Y.; Tsubota, T.; Mooij, L.P.; Van De Krol, R. Highly Improved Quantum Efficiencies for Thin Film BiVO4 Photoanodes. J. Phys. Chem. C 2011, 115, 17594–17598. [Google Scholar] [CrossRef]
- Byun, S.; Kim, B.; Jeon, S.; Shin, B. Effects of a SnO2 Hole Blocking Layer in a BiVO4-Based Photoanode on Photoelectrocatalytic Water Oxidation. J. Mater. Chem. A 2017, 5, 6905–6913. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-Doped Hematite Nanostructures for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef]
- Shinde, P.S.; Choi, S.H.; Kim, Y.; Ryu, J.; Jang, J.S. Onset Potential Behavior in α-Fe2O3 Photoanodes: The Influence of Surface and Diffusion Sn Doping on the Surface States. Phys. Chem. Chem. Phys. 2016, 18, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Bohn, C.D.; Agrawal, A.K.; Walter, E.C.; Vaudin, M.D.; Herzing, A.A.; Haney, P.M.; Talin, A.A.; Szalai, V.A. Effect of Tin Doping on α-Fe2O3 Photoanodes for Water Splitting. J. Phys. Chem. C 2012, 116, 15290–15296. [Google Scholar] [CrossRef]
- Frydrych, J.; Machala, L.; Tucek, J.; Siskova, K.; Filip, J.; Pechousek, J.; Safarova, K.; Vondracek, M.; Seo, J.H.; Schneeweiss, O.; et al. Facile Fabrication of Tin-Doped Hematite Photoelectrodes—Effect of Doping on Magnetic Properties and Performance for Light-inDuced Water Splitting. J. Mater. Chem. 2012, 22, 23232. [Google Scholar] [CrossRef]
- Dunn, H.K.; Feckl, J.M.; Muller, A.; Fattakhova-Rohlfing, D.; Morehead, S.G.; Roos, J.; Peter, L.M.; Scheu, C.; Bein, T. Tin Doping Speeds Up Hole Transfer During Light-Driven Water Oxidation at Hematite Photoanodes. Phys. Chem. Chem. Phys. 2014, 16, 24610–24620. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Farsi, H.; Moghiminia, S.; Zubkov, T.; Lightcap, I.V.; Riley, A.; Peters, D.G.; Li, Z. Nickel Tungstate (NiWO4) Nanoparticles/Graphene Composites: Preparation and Photoelectrochemical Applications. Semicond. Sci. Technol. 2018, 33, 055008. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Ahmed, J.; Ahamad, T.; Arunachalam, P.; Ahmad, T.; Khan, A. Bifunctional Electro-Catalytic Performances of CoWO4 Nanocubes for Water Redox Reactions (OER/ORR). RSC Adv. 2017, 7, 45615–45623. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Ahamad, T.; Alhokbany, N.; Arunachalam, P.; Al-Mayouf, A.M.; Ahmad, T. Synthesis, Characterization, Multifunctional Electrochemical (OGR/ORR/SCs) and Photodegradable Activities of ZnWO4 Nanobricks. J. Sol Gel Sci. Technol. 2018, 87, 137–146. [Google Scholar] [CrossRef]
- Srirapu, V.K.V.P.; Kumar, A.; Srivastava, P.; Singh, R.N.; Sinha, A.S.K. Nanosized CoWO4 and NiWO4 as Efficient Oxygen-Evolving. Electrocatal. Electrochim. Acta 2016, 209, 75–84. [Google Scholar] [CrossRef]
- Bai, S.; Cao, M.; Jin, Y.; Dai, X.; Liang, X.; Ye, Z.; Li, M.; Cheng, J.; Xiao, X.; Wu, Z. Low Temperature Combustion-Synthesized Nickel Oxide Thin Films as Hole-Transport Interlayers for Solution-Processed Optoelectronic Devices. Adv. Energy Mater. 2014, 4, 1301460. [Google Scholar] [CrossRef]
- Teng, W.; Li, X.; Zhao, Q.; Chen, G. Fabrication of Ag/Ag3PO4/TiO2 Heterostructure Photoelectrodes for Efficient Decomposition of 2-Chlorophenol under Visible Light Irradiation. J. Mater. Chem. A 2013, 1, 9060–9068. [Google Scholar] [CrossRef]
- Lei, Z.; Bai, J.; Li, Y.; Wang, Z.; Zhao, C. Fabrication of Nanoporous Nickel-Iron Hydroxylphosphate Composite as Bifunctional and Reversible Catalyst for Highly Efficient Intermittent Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 35837–35846. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Huang, Y.; Xu, X.; Din, M.A.U.; Xie, G.; Wang, X. Iron Hydroxide Modified Nickel Hydroxylphosphate Single-Wall Nanotubes as Efficient Electrocatalysts for Oxygen Evolution Reactions. ACS Appl. Mater. Interfaces 2018, 10, 9407–9414. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.J.; Kang, H.J.; Kong, K.J.; Lee, Y.S.; Park, H.; Lee, Y.; Buonassisi, T.; Gleason, K.K.; Lee, J.S. Phase Transition-Induced Band Edge Engineering of BiVO4 to Split Pure Water Under Visible Light. Proc. Natl. Acad. Sci. USA 2015, 112, 13774–13778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, Y.; Zhang, J.; Rao, P.M. Photoanode with Enhanced Performance Achieved by Coating BiVO4 onto ZnO-Templated Sb-Doped SnO2 Nanotube Scaffold. ACS Appl. Mater. Interfaces 2017, 9, 11356–11362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, C.; Giri, B.; Allen, P.; Xu, X.; Joshi, H.; Fan, Y.Y.; Titova, L.V.; Rao, P.M. High Light Absorption and Charge Separation Efficiency at Low Applied Voltage from Sb-Doped SnO2/BiVO4 Core/Shell Nanorod-Array Photoanodes. Nano Lett. 2016, 16, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Z.; Xu, Y.; Sun, J.; Hong, W.; Liu, X.; Wang, J.; Yang, S. Simple Synthesis of Amorphous NiWO4 Nanostructure and its Application as a Novel Cathode Material for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 8044–8052. [Google Scholar] [CrossRef]
- Salvati, J., Jr.; Makovsky, L.E.; Stencel, J.M.; Brown, F.R.; Hercules, D.M. Surface Spectroscopic Study of Tungsten-Alumina Catalysts Using X-ray Photoelectron, Ion Scattering, And Raman Spectroscopies. J. Phys. Chem. 1981, 85, 3700–3707. [Google Scholar] [CrossRef]
- Li, C.P.; Proctor, A.; Hercules, D.M. Curve Fitting Analysis of ESCA Ni 2p Spectra of Nickel-Oxygen Compounds and Ni/Al2O3 Catalysts. Appl. Spectrosc. 1984, 38, 880–886. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaddad, M.N.; Arunachalam, P.; Hezam, M.; Al-Mayouf, A.M. Cooperative Catalytic Behavior of SnO2 and NiWO4 over BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting Performance. Catalysts 2019, 9, 879. https://doi.org/10.3390/catal9110879
Shaddad MN, Arunachalam P, Hezam M, Al-Mayouf AM. Cooperative Catalytic Behavior of SnO2 and NiWO4 over BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting Performance. Catalysts. 2019; 9(11):879. https://doi.org/10.3390/catal9110879
Chicago/Turabian StyleShaddad, Maged N., Prabhakarn Arunachalam, Mahmoud Hezam, and Abdullah M. Al-Mayouf. 2019. "Cooperative Catalytic Behavior of SnO2 and NiWO4 over BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting Performance" Catalysts 9, no. 11: 879. https://doi.org/10.3390/catal9110879
APA StyleShaddad, M. N., Arunachalam, P., Hezam, M., & Al-Mayouf, A. M. (2019). Cooperative Catalytic Behavior of SnO2 and NiWO4 over BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting Performance. Catalysts, 9(11), 879. https://doi.org/10.3390/catal9110879