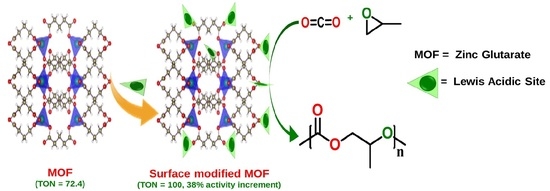
Surface Modification of a MOF-based Catalyst with Lewis Metal Salts for Improved Catalytic Activity in the Fixation of CO2 into Polymers
Abstract
1. Introduction
2. Results and Discussion
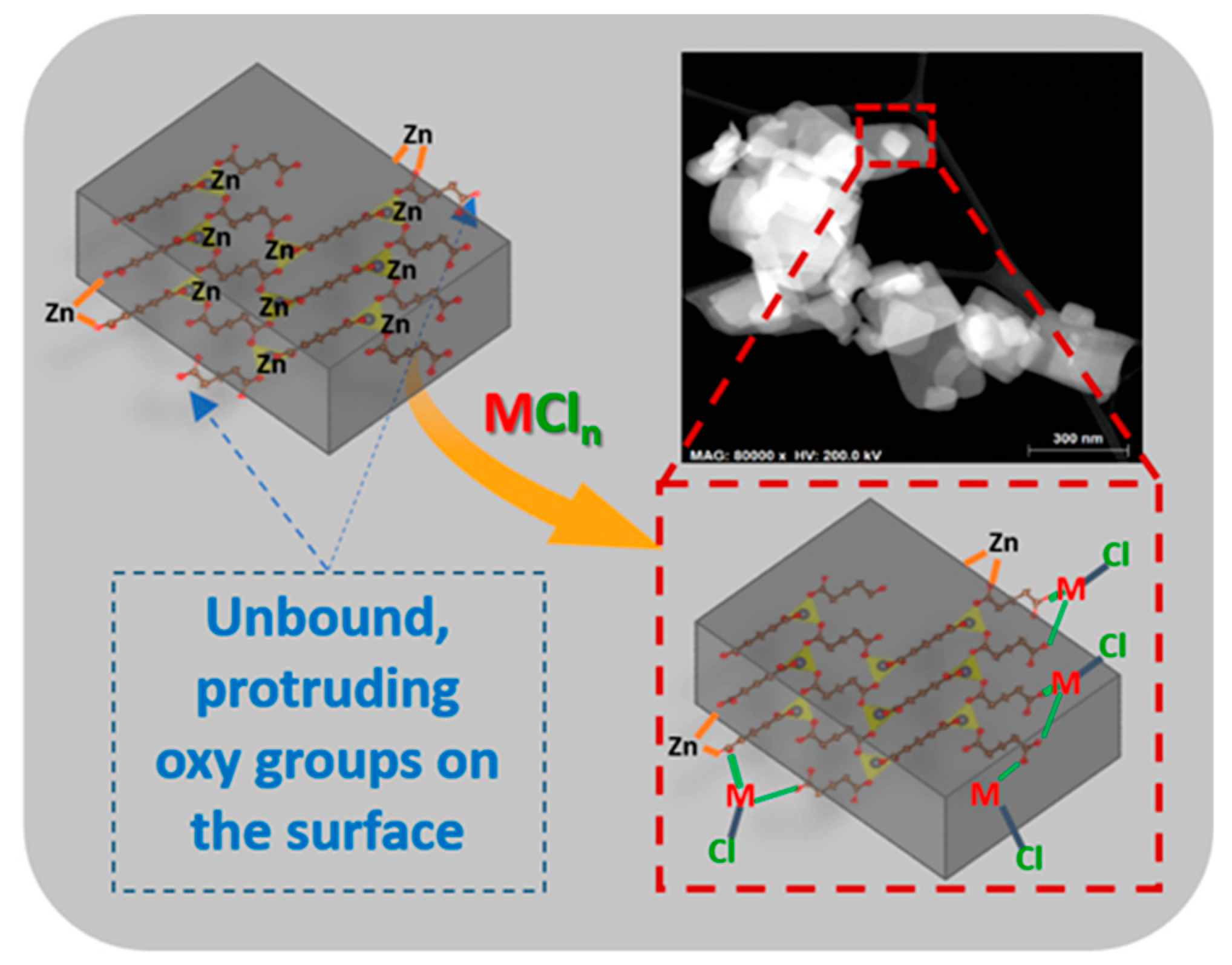
2.1. Synthesis and Characterization of Catalysts
2.2. Catalytic Activity Studies
2.3. Properties of Polymers
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of std-ZnGA
3.3. General Procedure for Preparing Metal Treated Catalysts
3.3.1. Preparation of Metal Chloride Stock Solutions
3.3.2. Metal Treatment of std-ZnGA
3.4. General Procedure for the Copolymerization of CO2 and PO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Keeling Curve. Scripps Institution of Oceanography at University of California at San Diego. Available online: https://scripps.ucsd.edu/programs/keelingcurve/ (accessed on 13 September 2017).
- Scott, D.A.; Christopher, M.B.; Geoffrey, W.C. Carbon Dioxide as a Renewable C1 Feedstock: Synthesis and Characterization of Polycarbonates from the Alternating Copolymerization of Epoxides and CO2. In Feedstocks for the Future; American Chemical Society: Washington, DC, USA, 2006; Volume 921, pp. 116–129. [Google Scholar]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Spinner, N.S.; Vega, J.A.; Mustain, W.E. Recent progress in the electrochemical conversion and utilization of CO2. Catal. Sci. Technol. 2012, 2, 19–28. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, S.; Shawat, E.; Nessim, G.D.; Jain, S.L. Amino-functionalized carbon nanofibres as an efficient metal free catalyst for the synthesis of quinazoline-2,4(1H,3H)-diones from CO2 and 2-aminobenzonitriles. RSC Adv. 2015, 5, 24670–24674. [Google Scholar] [CrossRef]
- Park, K.; Gunasekar, G.H.; Prakash, N.; Jung, K.-D.; Yoon, S. A Highly Efficient Heterogenized Iridium Complex for the Catalytic Hydrogenation of Carbon Dioxide to Formate. ChemSusChem 2015, 8, 3410–3413. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Park, K.; Jung, K.-D.; Yoon, S. Recent developments in the catalytic hydrogenation of CO2 to formic acid/formate using heterogeneous catalysts. Inorg. Chem. Front. 2016, 3, 882–895. [Google Scholar] [CrossRef]
- Kim, S.-H.; Chung, G.-Y.; Kim, S.-H.; Vinothkumar, G.; Yoon, S.-H.; Jung, K.-D. Electrochemical NADH regeneration and electroenzymatic CO2 reduction on Cu nanorods/glassy carbon electrode prepared by cyclic deposition. Electrochim. Acta 2016, 210, 837–845. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Park, K.; Ganesan, V.; Lee, K.; Kim, N.-K.; Jung, K.-D.; Yoon, S. A Covalent Triazine Framework, Functionalized with Ir/N-Heterocyclic Carbene Sites, for the Efficient Hydrogenation of CO2 to Formate. Chem. Mater. 2017, 29, 6740–6748. [Google Scholar] [CrossRef]
- Park, K.; Lee, K.; Kim, H.; Ganesan, V.; Cho, K.; Jeong, S.K.; Yoon, S. Preparation of covalent triazine frameworks with imidazolium cations embedded in basic sites and their application for CO2 capture. J. Mater. Chem. A 2017, 5, 8576–8582. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Park, K.; Jeong, H.; Jung, K.D.; Park, K.; Yoon, S. Molecular Rh(III) and Ir(III) Catalysts Immobilized on Bipyridine-Based Covalent Triazine Frameworks for the Hydrogenation of CO2 to Formate. Catalysts 2018, 8, 295. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Shin, J.; Jung, K.-D.; Park, K.; Yoon, S. Design Strategy toward Recyclable and Highly Efficient Heterogeneous Catalysts for the Hydrogenation of CO2 to Formate. ACS Catal. 2018, 8, 4346–4353. [Google Scholar] [CrossRef]
- Abbas, I.; Kim, H.; Shin, C.-H.; Yoon, S.; Jung, K.-D. Differences in bifunctionality of ZnO and ZrO2 in Cu/ZnO/ZrO2/Al2O3 catalysts in hydrogenation of carbon oxides for methanol synthesis. Appl. Catal. B Environ. 2019, 258, 117971. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Jung, K.-D.; Yoon, S. Hydrogenation of CO2 to Formate using a Simple, Recyclable, and Efficient Heterogeneous Catalyst. Inorg. Chem. 2019, 58, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, G.H.; Yoon, S. A phenanthroline-based porous organic polymer for the iridium-catalyzed hydrogenation of carbon dioxide to formate. J. Mater. Chem. A 2019, 7, 14019–14026. [Google Scholar] [CrossRef]
- Kim, C.; Choe, Y.-K.; Won, D.H.; Lee, U.; Oh, H.-S.; Lee, D.K.; Choi, C.H.; Yoon, S.; Kim, W.; Hwang, Y.J.; et al. Turning Harmful Deposition of Metal Impurities into Activation of Nitrogen-Doped Carbon Catalyst toward Durable Electrochemical CO2 Reduction. ACS Energy Lett. 2019, 4, 2343–2350. [Google Scholar] [CrossRef]
- Luinstra, G.A. Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: Catalysis and material properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Scharfenberg, M.; Hilf, J.; Frey, H. Functional Polycarbonates from Carbon Dioxide and Tailored Epoxide Monomers: Degradable Materials and Their Application Potential. Adv. Funct. Mater. 2018, 28, 1704302. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Borchardt, E. Material Properties of Poly(Propylene Carbonates). Adv. Polym. Sci. 2012, 245, 29–48. [Google Scholar] [CrossRef]
- Alessandra, Q.E.; Gabriele, C.; Jean-Luc, D.; Siglinda, P. Carbon Dioxide Recycling: Emerging Large-Scale Technologies with Industrial Potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar]
- Peters, M.; Koehler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T.E. Chemical Technologies for Exploiting and Recycling Carbon Dioxide into the Value Chain. ChemSusChem 2011, 4, 1216–1240. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of Carbon Dioxide and Epoxide. J. Polym. Sci. Pol. Lett. 1969, 7, 287–292. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of Carbon Dioxide and Epoxide with Organometallic Compounds. Makromol. Chem. 1969, 130, 210–220. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making plastics from carbon dioxide: Salen metal complexes as catalysts for the production of polycarbonates from epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Ang, R.R.; Sin, L.T.; Bee, S.T.; Tee, T.T.; Kadhum, A.A.H.; Rahmat, A.R.; Wasmi, B.A. A review of copolymerization of green house gas carbon dioxide and oxiranes to produce polycarbonate. J. Clean. Prod. 2015, 102, 1–17. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Philos. Trans. R. Soc. A 2016, 374, 20150085. [Google Scholar] [CrossRef]
- Sudakar, P.; Gunasekar, G.H.; Baek, I.H.; Yoon, S. Recyclable and efficient heterogenized Rh and Ir catalysts for the transfer hydrogenation of carbonyl compounds in aqueous medium. Green Chem. 2016, 18, 6456–6461. [Google Scholar] [CrossRef]
- Ganesan, V.; Yoon, S. Hyper-Cross-Linked Porous Porphyrin Aluminum(III) Tetracarbonylcobaltate as a Highly Active Heterogeneous Bimetallic Catalyst for the Ring-Expansion Carbonylation of Epoxides. ACS Appl. Mater. Interfaces 2019, 11, 18609–18616. [Google Scholar] [CrossRef]
- Padmanaban, S.; Gunasekar, G.H.; Lee, M.; Yoon, S. Recyclable Covalent Triazine Framework-based Ru Catalyst for Transfer Hydrogenation of Carbonyl Compounds in Water. ACS Sustain. Chem. Eng. 2019, 7, 8893–8899. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous Catalysis: A Central Science for a Sustainable Future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef]
- Padmanaban, S.; Kim, M.; Yoon, S. Acid-mediated surface etching of a nano-sized metal-organic framework for improved reactivity in the fixation of CO2 into polymers. J. Ind. Eng. Chem. 2019, 71, 336–344. [Google Scholar] [CrossRef]
- von der Assen, N.; Bardow, A. Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: Insights from an industrial case study. Green Chem. 2014, 16, 3272–3280. [Google Scholar] [CrossRef]
- Eberhardt, R.; Allmendinger, M.; Zintl, M.; Troll, C.; Luinstra, G.A.; Rieger, B. New zinc dicarboxylate catalysts for the CO2/propylene oxide copolymerization reaction: Activity enhancement through Zn(II)-ethylsulfinate initiating groups. Macromol. Chem. Phys. 2004, 205, 42–47. [Google Scholar] [CrossRef]
- Ree, M.; Hwang, Y.; Kim, J.S.; Kim, H.; Kim, G.; Kim, H. New findings in the catalytic activity of zinc glutarate and its application in the chemical fixation of CO2 into polycarbonates and their derivatives. Catal. Today 2006, 115, 134–145. [Google Scholar] [CrossRef]
- Wang, S.J.; Du, L.C.; Zhao, X.S.; Meng, Y.Z.; Tjong, S.C. Synthesis and characterization of alternating copolymer from carbon dioxide and propylene oxide. J. Appl. Polym. Sci. 2002, 85, 2327–2334. [Google Scholar] [CrossRef]
- Zhong, X.; Dehghani, F. Solvent free synthesis of organometallic catalysts for the copolymerisation of carbon dioxide and propylene oxide. Appl. Catal. B Environ. 2010, 98, 101–111. [Google Scholar] [CrossRef]
- Ang, R.R.; Sin, L.T.; Bee, S.T.; Tee, T.T.; Kadhum, A.A.H.; Rahmat, A.R.; Wasmi, B.A. Determination of zinc glutarate complexes synthesis factors affecting production of propylene carbonate from carbon dioxide and propylene oxide. Chem. Eng. J. 2017, 327, 120–127. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, H.; Ree, M. Hydrothermal synthesis of single-crystalline zinc glutarate and its structural determination. Chem. Mater. 2004, 16, 2981–2983. [Google Scholar] [CrossRef]
- Seth, S.; Matzger, A.J. Metal–Organic Frameworks: Examples, Counterexamples, and an Actionable Definition. Cryst. Growth Des. 2017, 17, 4043–4048. [Google Scholar] [CrossRef]
- Miralda, C.M.; Macias, E.E.; Zhu, M.; Ratnasamy, P.; Carreon, M.A. Zeolitic Imidazole Framework-8 Catalysts in the Conversion of CO2 to Chloropropene Carbonate. ACS Catal. 2012, 2, 180–183. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.-N.; Jang, H.-G.; Seo, G.; Ahn, W.-S. CO2 cycloaddition of styrene oxide over MOF catalysts. Appl. Catal. A Gen. 2013, 453, 175–180. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, G.; Gao, W.-Y.; Niu, Z.; Wojtas, L.; Ma, S. Anionic Metal–Organic Framework for Selective Dye Removal and CO2 Fixation. Eur. J. Inorg. Chem. 2016, 2016, 4373–4377. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Babu, R.; Jeong, H.M.; Hwang, G.-Y.; Jeong, G.S.; Kim, M.-I.; Kim, D.-W.; Park, D.-W. A solid solution zeolitic imidazolate framework as a room temperature efficient catalyst for the chemical fixation of CO2. Green Chem. 2016, 18, 6349–6356. [Google Scholar] [CrossRef]
- Babu, R.; Kim, S.-H.; Kathalikkattil, A.C.; Kuruppathparambil, R.R.; Kim, D.W.; Cho, S.J.; Park, D.-W. Aqueous microwave-assisted synthesis of non-interpenetrated metal-organic framework for room temperature cycloaddition of CO2 and epoxides. Appl. Catal. A Gen. 2017, 544, 126–136. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Huang, K.-W.; Ko, B.-T.; Lin, K.-Y.A. Bifunctional ZIF-78 heterogeneous catalyst with dual Lewis acidic and basic sites for carbon dioxide fixation via cyclic carbonate synthesis. J. CO2 Util. 2017, 22, 178–183. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.-B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Zhu, Q.; Meng, Y.Z.; Tjong, S.C.; Zhao, X.S.; Chen, Y.L. Thermally stable and high molecular weight poly(propylene carbonate)s from carbon dioxide and propylene oxide. Polym. Int. 2002, 51, 1079–1085. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, H.; Yoon, J.; Heo, K.; Ree, M. Synthesis of zinc glutarates with various morphologies using an amphiphilic template and their catalytic activities in the copolymerization of carbon dioxide and propylene oxide. J. Polym. Sci. Pol. Chem. 2005, 43, 4079–4088. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhu, Q.; Lu, X.L.; Meng, Y.Z. ZnGA-MMT catalyzed the copolymerization of carbon dioxide with propylene oxide. Eur. Polym. J. 2005, 41, 1108–1114. [Google Scholar] [CrossRef]
- Gao, L.J.; Luo, Y.C.; Lin, Y.J.; Su, T.; Su, R.P.; Feng, J.Y. Silica-supported zinc glutarate catalyst synthesized by rheological phase reaction used in the copolymerization of carbon dioxide and propylene oxide. J. Polym. Res. 2015, 22, 220. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Herdtweck, E.; Deglmann, P.; Ott, A.K.; Rieger, B. Mechanistic Insights into Heterogeneous Zinc Dicarboxylates and Theoretical Considerations for CO2-Epoxide Copolymerization. J. Am. Chem. Soc. 2011, 133, 13151–13161. [Google Scholar] [CrossRef]
- Sudakar, P.; Sivanesan, D.; Yoon, S. Copolymerization of Epichlorohydrin and CO2 Using Zinc Glutarate: An Additional Application of ZnGA in Polycarbonate Synthesis. Macromol. Rapid Commun. 2016, 37, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, S.; Dharmalingam, S.; Yoon, S. A Zn-MOF-Catalyzed Terpolymerization of Propylene Oxide, CO2, and β-butyrolactone. Catalysts 2018, 8, 393. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Navarro-Llobet, D.; Zhou, Z.P. Poly(propylene carbonate). 1. More about poly(propylene carbonate) formed from the copolymerization of propylene oxide and carbon dioxide employing a zinc glutarate catalyst. Macromolecules 2002, 35, 6494–6504. [Google Scholar] [CrossRef]
- Kim, J.S.; Ree, M.; Shin, T.J.; Han, O.H.; Cho, S.J.; Hwang, Y.T.; Bae, J.Y.; Lee, J.M.; Ryoo, R.; Kim, H. X-ray absorption and NMR spectroscopic investigations of zinc glutarates prepared from various zinc sources and their catalytic activities in the copolymerization of carbon dioxide and propylene oxide. J. Catal. 2003, 218, 209–219. [Google Scholar] [CrossRef]
- Rieter, W.J.; Taylor, K.M.L.; Lin, W. Surface Modification and Functionalization of Nanoscale Metal-Organic Frameworks for Controlled Release and Luminescence Sensing. J. Am. Chem. Soc. 2007, 129, 9852–9853. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Haldar, R.; Dey, R.; Maji, T.K.; Ghoshal, D. Porous coordination polymers based on functionalized Schiff base linkers: Enhanced CO2 uptake by pore surface modification. Dalton Trans. 2014, 43, 2272–2282. [Google Scholar] [CrossRef]
- McGuire, C.V.; Forgan, R.S. The surface chemistry of metal–organic frameworks. Chem. Commun. 2015, 51, 5199–5217. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Ree, M.; Bae, J.Y.; Jung, J.H.; Shin, T.J. A new copolymerization process leading to poly(propylene carbonate) with a highly enhanced yield from carbon dioxide and propylene oxide. J. Polym. Sci. Pol. Chem. 1999, 37, 1863–1876. [Google Scholar] [CrossRef]
- Ren, W.-M.; Liu, Z.-W.; Wen, Y.-Q.; Zhang, R.; Lu, X.-B. Mechanistic Aspects of the Copolymerization of CO2 with Epoxides Using a Thermally Stable Single-Site Cobalt(III) Catalyst. J. Am. Chem. Soc. 2009, 131, 11509–11518. [Google Scholar] [CrossRef] [PubMed]



| Entry | Catalyst | Zn:MCln b | TON c | Productivity Increment d (%) | Fco2 e | Selectivity (%) f | Mn g (kg/mol) | PDI g | Tgh (°C) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PPC | PC | |||||||||
| 1 | Std-ZnGA | 1:0 | 72.4 | - | 94.9 | 96.0 | 4.0 | 156.4 | 3.5 | 42 |
| 2 | ZnGA-Fe-10−3 | 1:10−3 | 82.5 | 13.9 | 94.8 | 98.0 | 2.0 | 137.6 | 2.8 | 38 |
| 3 | ZnGA-Fe-10−4 | 1:10−4 | 90.9 | 25.6 | 94.1 | 94.0 | 6.0 | 262.4 | 2.0 | 40 |
| 4 | ZnGA-Al-10−3 | 1:10−3 | 88.7 | 22.5 | 93.5 | 96.0 | 4.0 | 196.3 | 1.8 | 36 |
| 5 | ZnGA-Al-10−4 | 1:10−4 | 77.5 | 1.5 | 95.1 | 98.0 | 2.0 | 231.8 | 2.2 | 35 |
| 6 | ZnGA-Zn-10−2 | 1:10−2 | 72.0 | −0.5 | 93.7 | 98.0 | 2.0 | 99.8 | 3.9 | 42 |
| 7 | ZnGA-Zn-10−3 | 1:10−3 | 100.1 | 38.3 | 94.9 | 97.0 | 3.0 | 208.0 | 1.8 | 38 |
| 8 | ZnGA-Zn-10−4 | 1:10−4 | 80.7 | 11.5 | 91.7 | 96.0 | 4.0 | 147.8 | 2.2 | 37 |
| 9 | ZnGA-Co-10−3 | 1:10−3 | 72.0 | −0.5 | 92.2 | 95.0 | 5.0 | 144.4 | 1.9 | 34 |
| 10 | ZnGA-Co-10−4 | 1:10−4 | 73.6 | 1.7 | 93.5 | 95.0 | 5.0 | 70.7 | 2.4 | 35 |
| 11 | ZnGA-Al-1 | 1:1 | 8.1 | - | 36.2 | 91.0 | 9.0 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padmanaban, S.; Yoon, S. Surface Modification of a MOF-based Catalyst with Lewis Metal Salts for Improved Catalytic Activity in the Fixation of CO2 into Polymers. Catalysts 2019, 9, 892. https://doi.org/10.3390/catal9110892
Padmanaban S, Yoon S. Surface Modification of a MOF-based Catalyst with Lewis Metal Salts for Improved Catalytic Activity in the Fixation of CO2 into Polymers. Catalysts. 2019; 9(11):892. https://doi.org/10.3390/catal9110892
Chicago/Turabian StylePadmanaban, Sudakar, and Sungho Yoon. 2019. "Surface Modification of a MOF-based Catalyst with Lewis Metal Salts for Improved Catalytic Activity in the Fixation of CO2 into Polymers" Catalysts 9, no. 11: 892. https://doi.org/10.3390/catal9110892
APA StylePadmanaban, S., & Yoon, S. (2019). Surface Modification of a MOF-based Catalyst with Lewis Metal Salts for Improved Catalytic Activity in the Fixation of CO2 into Polymers. Catalysts, 9(11), 892. https://doi.org/10.3390/catal9110892