A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols
Abstract
:1. Introduction
- (i)
- pyrolysis under inert atmosphere, working in a temperature range between 400 and 800 °C and the consequent valorization of the bio-oil fraction produced by reforming processes [14];
- (ii)
- biomass gasification in order to produce syngas, working at temperature higher than 800 °C, in the presence of a suitable catalyst and gasification agents [15]
- (iii)
- catalytic partial oxidation [16];
- (iv)
- photocatalytic reforming [17];
- (v)
- aqueous phase reforming (APR) [16];
- (vi)
2. From Polyols to Cellulose: Unravelling all Sustainable Catalytic Routes in the H2 Production Via Aqueous Phase Reforming (APR) Reactions
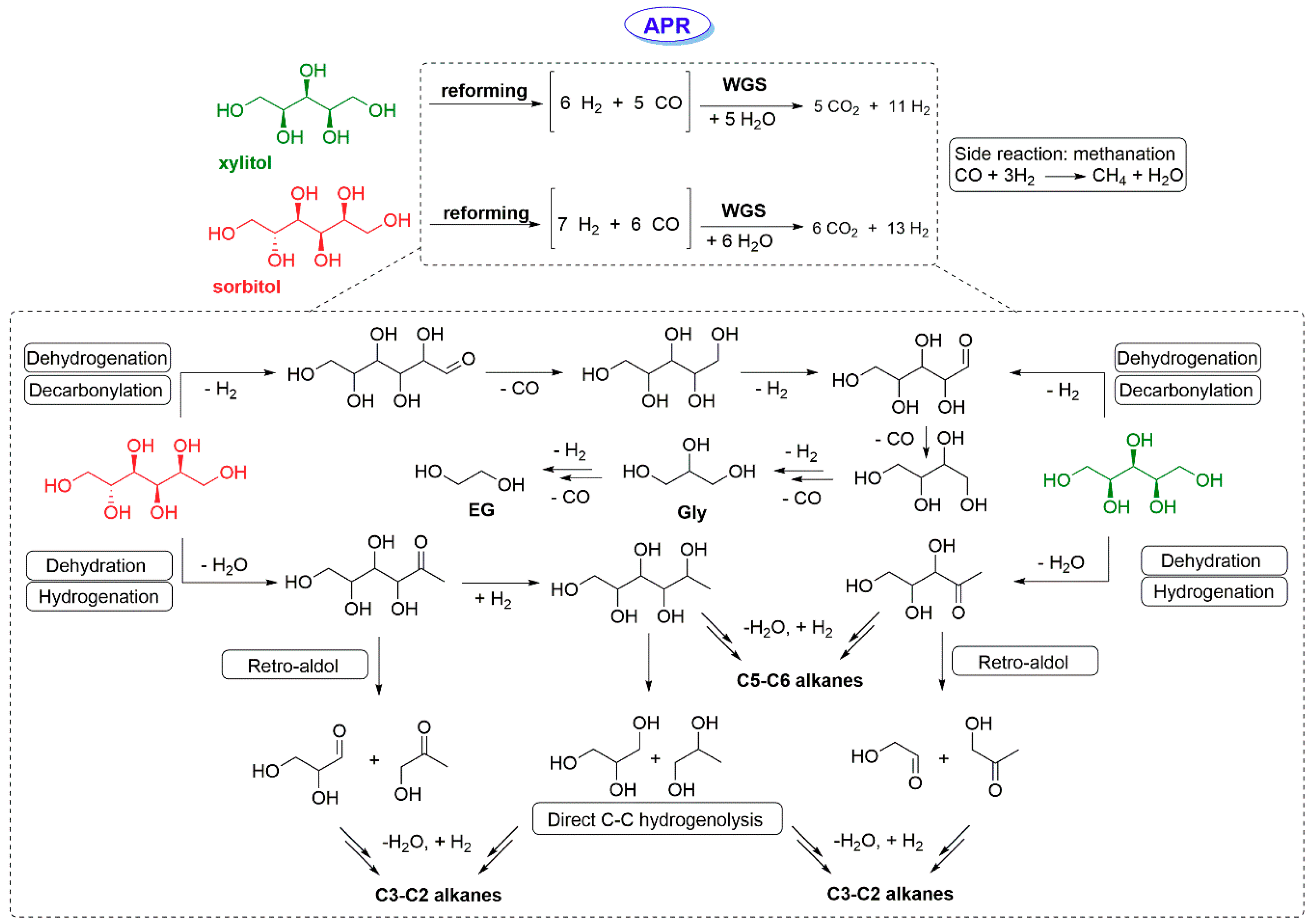
2.1. Aqueous Phase Reforming of C5 and C6 Polyols
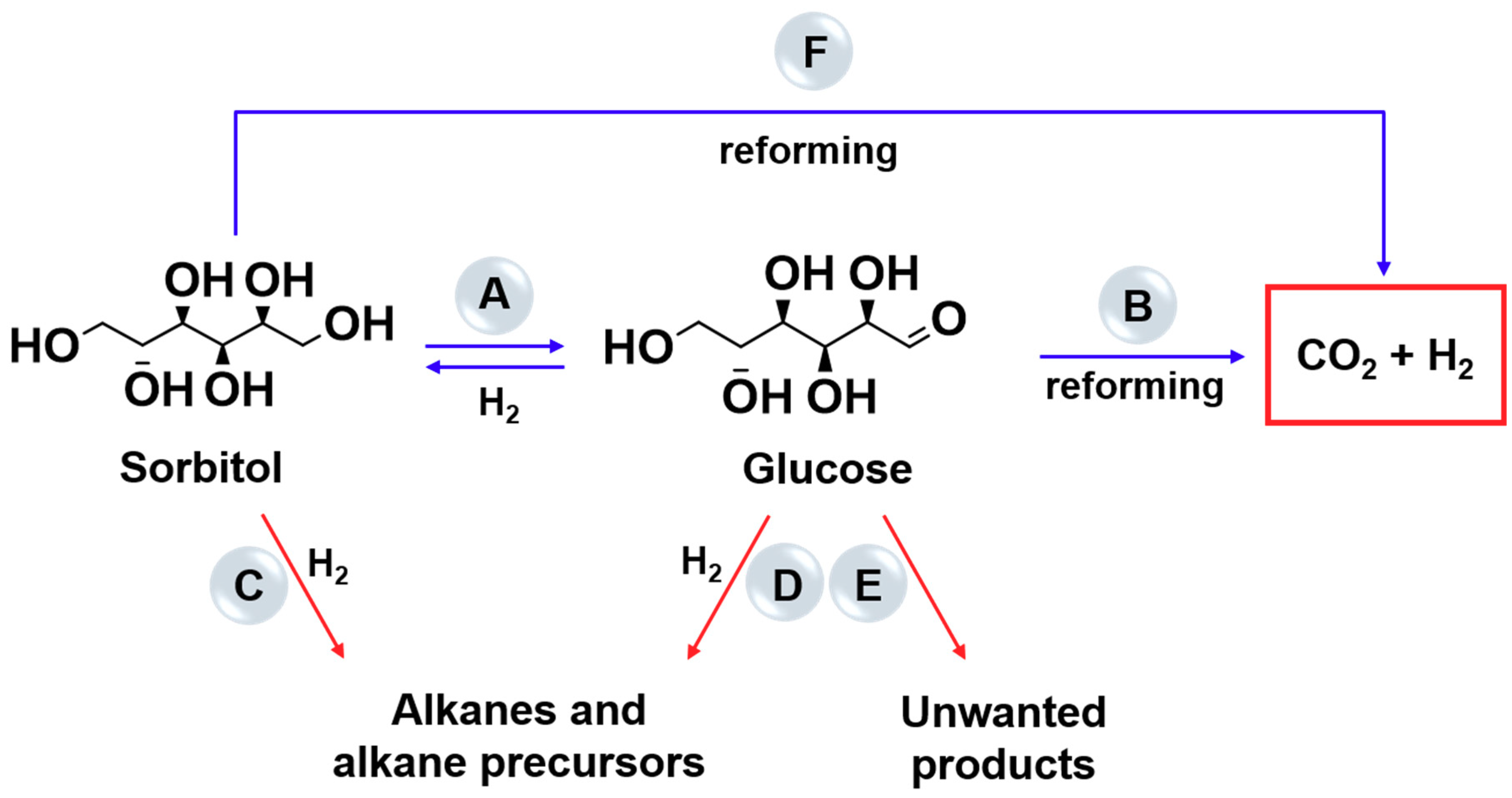
2.2. Aqueous Phase Reforming of Sugars
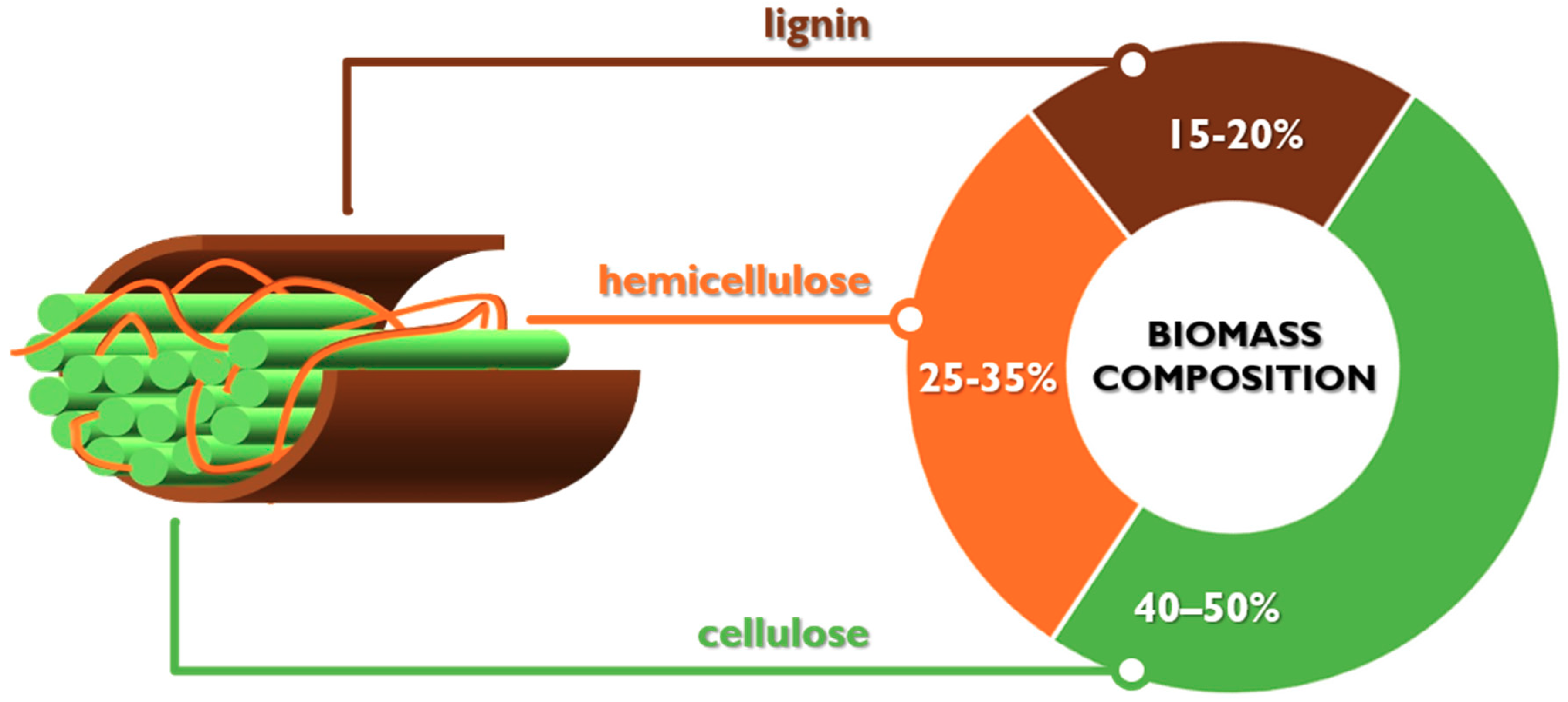
2.3. Direct Production of H2 from Cellulose and Lignocellulosic Biomasses Via APR
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Swarr, T.E.; Cucciniello, R.; Cespi, D. Environmental certifications and programs roadmap for a sustainable chemical industry. Green Chem. 2019, 21, 375–380. [Google Scholar] [CrossRef]
- Ricciardi, M.; Cespi, D.; Celentano, M.; Genga, A.; Malitesta, C.; Proto, A.; Capacchione, C.; Cucciniello, R. Bio-propylene glycol as value-added product from Epicerol process. Sustain. Chem. Pharm. 2017, 6, 10–13. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Gong, J. Catalytic reforming of oxygenates: State of the art and future prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef]
- Du, C.; Mo, J.; Li, H. Renewable hydrogen production by alcohols reforming using plasma and plasma-catalytic technologies: Challenges and opportunities. Chem. Rev. 2014, 115, 1503–1542. [Google Scholar] [CrossRef]
- Anastas, P. JC Warner Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A roadmap towards green packaging: The current status and future outlook for polyesters in the packaging industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Home – Avantium. Available online: https://www.avantium.com/ (accessed on 2 October 2019).
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef]
- Tabanelli, T.; Giliberti, C.; Mazzoni, R.; Cucciniello, R.; Cavani, F. An innovative synthesis pathway to benzodioxanes: The peculiar reactivity of glycerol carbonate and catechol. Green Chem. 2019, 21, 329–338. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fang, Z.; Smith, R.L.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Sun, N.; Rodriguez, H.; Rahman, M.; Rogers, R.D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem. Commun. 2011, 47, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Hanika, J.; Lederer, J.; Tukač, V.; Veselý, V.; Kováč, D. Hydrogen production via synthetic gas by biomass/oil partial oxidation. Chem. Eng. J. 2011, 176–177, 286–290. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, T.; Paone, E.; Blair Vásquez, P.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer Hydrogenation of Methyl and Ethyl Levulinate Promoted by a ZrO2 Catalyst: Comparison of Batch vs Continuous Gas-Flow Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic transfer hydrogenolysis as an effective tool for the reductive upgrading of cellulose, hemicellulose, lignin, and their derived molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef]
- Mauriello, F.; Vinci, A.; Espro, C.; Gumina, B.; Musolino, M.G.; Pietropaolo, R. Hydrogenolysis vs. aqueous phase reforming (APR) of glycerol promoted by a heterogeneous Pd/Fe catalyst. Catal. Sci. Technol. 2015, 5, 4466–4473. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar]
- Vaidya, P.D.; Rodrigues, A.E. Glycerol Reforming for Hydrogen Production: A Review. Chem. Eng. Technol. 2009, 32, 1463–1469. [Google Scholar] [CrossRef]
- Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Renewable Alkanes by Aqueous-Phase Reforming of Biomass-Derived Oxygenates. Angew. Chem. Int. Ed. 2004, 43, 1549–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabaker, J.W.; Dumesic, J.A. Kinetics of Aqueous-Phase Reforming of Oxygenated Hydrocarbons: Pt/Al2O3 and Sn-Modified Ni Catalysts. Ind. Eng. Chem. Res. 2004, 43, 3105–3112. [Google Scholar] [CrossRef]
- Coronado, I.; Stekrova, M.; Reinikainen, M.; Simell, P.; Lefferts, L.; Lehtonen, J. A review of catalytic aqueous-phase reforming of oxygenated hydrocarbons derived from biorefinery water fractions. Int. J. Hydrogen Energy 2016, 41, 11003–11032. [Google Scholar] [CrossRef]
- Kandoi, S.; Greeley, J.; Simonetti, D.; Shabaker, J.; Dumesic, J.A.; Mavrikakis, M. Reaction Kinetics of Ethylene Glycol Reforming over Platinum in the Vapor versus Aqueous Phases. J. Phys. Chem. C 2011, 115, 961–971. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Garcia, S.; Russo, V.; Kilpiö, T.; Godina, L.I.; Tokarev, A.V.; Kirilin, A.V.; Simakova, I.L.; Poulston, S.; Sladkovskiy, D.A.; et al. Kinetics, Modeling, and Process Design of Hydrogen Production by Aqueous Phase Reforming of Xylitol. Ind. Eng. Chem. Res. 2017, 56, 13240–13253. [Google Scholar] [CrossRef]
- Duarte, H.A.; Sad, M.E.; Apesteguía, C.R. Bio-hydrogen production by APR of C2 -C6 polyols on Pt/Al2O3: Dependence of H 2 productivity on metal content. Catal. Today 2017, 296, 59–65. [Google Scholar] [CrossRef]
- Godina, L.I.; Kirilin, A.V.; Tokarev, A.V.; Simakova, I.L.; Murzin, D.Y. Sibunit-Supported Mono- and Bimetallic Catalysts Used in Aqueous-Phase Reforming of Xylitol. Ind. Eng. Chem. Res. 2018, 57, 2050–2067. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, H.-D.; Kim, T.-W.; Jeong, K.-E.; Chae, H.-J.; Jeong, S.-Y.; Chung, Y.-M.; Park, Y.-K.; Kim, C.-U. Production of Biohydrogen by Aqueous Phase Reforming of Polyols over Platinum Catalysts Supported on Three-Dimensionally Bimodal Mesoporous Carbon. ChemSusChem 2012, 5, 629–633. [Google Scholar] [CrossRef]
- Duarte, H.A.; Sad, M.E.; Apesteguía, C.R. Production of bio-hydrogen by liquid processing of xylitol on Pt/Al2O3 catalysts: Effect of the metal loading. Int. J. Hydrogen Energy 2017, 42, 4051–4060. [Google Scholar] [CrossRef]
- Duarte, H.A.; Sad, M.E.; Apesteguía, C.R. Aqueous phase reforming of sorbitol on Pt/Al2O3: Effect of metal loading and reaction conditions on H2 productivity. Int. J. Hydrogen Energy 2016, 41, 17290–17296. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kim, T.-W.; Kim, H.J.; Kim, C.-U.; Bae, J.W. Aqueous phase reforming of polyols for hydrogen production using supported PtFe bimetallic catalysts. Renew. Energy 2016, 95, 396–403. [Google Scholar] [CrossRef]
- Kirilin, A.V.; Tokarev, A.V.; Manyar, H.; Hardacre, C.; Salmi, T.; Mikkola, J.-P.; Murzin, D.Y. Aqueous phase reforming of xylitol over Pt-Re bimetallic catalyst: Effect of the Re addition. Catal. Today 2014, 223, 97–107. [Google Scholar] [CrossRef]
- Dosso, L.A.; Vera, C.R.; Grau, J.M. Aqueous phase reforming of polyols from glucose degradation by reaction over Pt/alumina catalysts modified by Ni or Co. Int. J. Hydrogen Energy 2017, 42, 18853–18864. [Google Scholar] [CrossRef]
- Kirilin, A.V.; Tokarev, A.V.; Kustov, L.M.; Salmi, T.; Mikkola, J.-P.; Murzin, D.Y. Aqueous phase reforming of xylitol and sorbitol: Comparison and influence of substrate structure. Appl. Catal. A Gen. 2012, 435–436, 172–180. [Google Scholar] [CrossRef]
- Aiouache, F.; McAleer, L.; Gan, Q.; Al-Muhtaseb, A.H.; Ahmad, M.N. Path lumping kinetic model for aqueous phase reforming of sorbitol. Appl. Catal. A Gen. 2013, 466, 240–255. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, T.; Ma, L.; Li, Y.; Zhang, Q.; Zhang, X. Investigation on the xylitol aqueous-phase reforming performance for pentane production over Pt/HZSM-5 and Ni/HZSM-5 catalysts. Appl. Energy 2012, 90, 51–57. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Q.; Wang, T.-J.; Zhang, Q.; Ma, L.-L. High yield of pentane production by aqueous-phase reforming of xylitol over Ni/HZSM-5 and Ni/MCM22 catalysts. Energy Convers. Manag. 2012, 59, 58–65. [Google Scholar] [CrossRef]
- Sladkovskiy, D.A.; Godina, L.I.; Semikin, K.V.; Sladkovskaya, E.V.; Smirnova, D.A.; Murzin, D.Y. Process design and techno-economical analysis of hydrogen production by aqueous phase reforming of sorbitol. Chem. Eng. Res. Des. 2018, 134, 104–116. [Google Scholar] [CrossRef]
- Abdollahi, M.; Yu, J.; Liu, P.K.T.; Ciora, R.; Sahimi, M.; Tsotsis, T.T. Ultra-pure hydrogen production from reformate mixtures using a palladium membrane reactor system. J. Membr. Sci. 2012, 390–391, 32–42. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process. Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Fasolini, A.; Abate, S.; Barbera, D.; Centi, G.; Basile, F. Pure H2 production by methane oxy-reforming over Rh-Mg-Al hydrotalcite-derived catalysts coupled with a Pd membrane. Appl. Catal. A Gen. 2019, 581, 91–102. [Google Scholar] [CrossRef]
- Godina, L.I.; Heeres, H.; Garcia, S.; Bennett, S.; Poulston, S.; Murzin, D.Y. Hydrogen production from sucrose via aqueous-phase reforming. Int. J. Hydrogen Energy 2019, 44, 14605–14623. [Google Scholar] [CrossRef]
- Davda, R.R.; Dumesic, J.A. Renewable hydrogen by aqueous-phase reforming of glucose. Chem. Commun. 2004, 10, 36–37. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Garcia, A. Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ. Sci. 2012, 5, 7393–7420. [Google Scholar] [CrossRef]
- Tsao, G.T.; Ladisch, M.R.; Bose, A. Acid Hydrolysis of Cellulose to Yield Glucose. US Patent 4,174,976, 20 November 1979. [Google Scholar]
- Ranoux, A.; Djanashvili, K.; Arends, I.W.C.E.; Hanefeld, U. 5-Hydroxymethylfurfural Synthesis from Hexoses Is Autocatalytic. ACS Catal. 2013, 3, 760–763. [Google Scholar] [CrossRef]
- Shabaker, J.; Davda, R.; Huber, G.; Cortright, R.; Dumesic, J. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts. J. Catal. 2003, 215, 344–352. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Glucose and Fructose Decomposition in Subcritical and Supercritical Water: Detailed Reaction Pathway, Mechanisms, and Kinetics. Ind. Eng. Chem. Res. 1999, 38, 2888–2895. [Google Scholar] [CrossRef]
- Gursahani, K.I.; Alcalá, R.; Cortright, R.D.; Dumesic, J.A. Reaction kinetics measurements and analysis of reaction pathways for conversions of acetic acid, ethanol, and ethyl acetate over silica-supported Pt. Appl. Catal. A Gen. 2001, 222, 369–392. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Xu, Z.; Tian, Z. Characterization and Catalytic Properties of the Ni/Al2O3 Catalysts for Aqueous-phase Reforming of Glucose. Catal. Lett. 2009, 129, 250–257. [Google Scholar] [CrossRef]
- Taccardi, N.; Assenbaum, D.; Berger, M.E.M.; Bösmann, A.; Enzenberger, F.; Wölfel, R.; Neuendorf, S.; Goeke, V.; Schödel, N.; Maass, H.-J.; et al. Catalytic production of hydrogen from glucose and other carbohydrates under exceptionally mild reaction conditions. Green Chem. 2010, 12, 1150–1156. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.Q. Reaction Mechanisms for Renewable Hydrogen from Liquid Phase Reforming of Sugar Compounds. Dev. Chem. Eng. Miner. Process. 2006, 14, 9–18. [Google Scholar] [CrossRef]
- Tanksale, A.; Wong, Y.; Beltramini, J.; Lu, G. Hydrogen generation from liquid phase catalytic reforming of sugar solutions using metal-supported catalysts. Int. J. Hydrogen Energy 2007, 32, 717–724. [Google Scholar] [CrossRef]
- Gumina, B.; Mauriello, F.; Pietropaolo, R.; Galvagno, S.; Espro, C. Hydrogenolysis of sorbitol into valuable C3-C2 alcohols at low H2 pressure promoted by the heterogeneous Pd/Fe3O4 catalyst. Mol. Catal. 2018, 446, 152–160. [Google Scholar] [CrossRef]
- Meryemoglu, B.; Irmak, S.; Hasanoglu, A.; Erbatur, O.; Kaya, B. Influence of particle size of support on reforming activity and selectivity of activated carbon supported platinum catalyst in APR. Fuel 2014, 134, 354–357. [Google Scholar] [CrossRef]
- Koklin, A.; Klimenko, T.; Kondratyuk, A.; Lunin, V.; Bogdan, V. Transformation of aqueous solutions of glucose over the Pt/C catalyst. Kinet. Catal. 2015, 56, 84–88. [Google Scholar] [CrossRef]
- Tanksale, A.; Wong, Y.; Beltramini, J.; Lu, G.M. Effect of Promoter on Mesoporous Supports for Increased H2 Production from Sugar Reforming. In Proceedings of the 2006 International Conference on Nanoscience and Nanotechnology, Brisbane, Australia, 3–7 July 2006. [Google Scholar]
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A review of catalytic hydrogen production processes from biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Hoffer, B.; Crezee, E.; Devred, F.; Mooijman, P.; Sloof, W.; Kooyman, P.; Van Langeveld, A.; Kapteijn, F.; Moulijn, J. The role of the active phase of Raney-type Ni catalysts in the selective hydrogenation of D-glucose to D-sorbitol. Appl. Catal. A Gen. 2003, 253, 437–452. [Google Scholar] [CrossRef]
- Crezee, E.; Hoffer, B.W.; Berger, R.J.; Makkee, M.; Kapteijn, F.; Moulijn, J.A. Three-phase hydrogenation of D-glucose over a carbon supported ruthenium catalyst—Mass transfer and kinetics. Appl. Catal. A Gen. 2003, 251, 1–17. [Google Scholar] [CrossRef]
- Van Gorp, K.; Boerman, E.; Cavenaghi, C.; Berben, P. Catalytic hydrogenation of fine chemicals: Sorbitol production. Catal. Today 1999, 52, 349–361. [Google Scholar] [CrossRef]
- Wisnlak, J.; Simon, R. Hydrogenation of glucose, fructose, and their mixtures. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 50–57. [Google Scholar] [CrossRef]
- Wen, J.; Wang, C.; Liu, Y. Preparation of sorbitol from D-glucose hydrogenation in gas–liquid–solid three-phase flow airlift loop reactor. J. Chem. Technol. Biotechnol. 2004, 79, 403–406. [Google Scholar] [CrossRef]
- Martin, D.; Duprez, D. Evaluation of the acid-base surface properties of several oxides and supported metal catalysts by means of model reactions. J. Mol. Catal. A Chem. 1997, 118, 113–128. [Google Scholar] [CrossRef]
- Flego, C.; Carati, A.; Perego, C. Methanol interaction with mesoporous silica–aluminas. Microporous Mesoporous Mater. 2001, 44, 733–744. [Google Scholar] [CrossRef]
- Valenzuela, M.B.; Jones, C.W.; Agrawal, P.K. Batch Aqueous-Phase Reforming of Woody Biomass. Energy Fuels 2006, 20, 1744–1752. [Google Scholar] [CrossRef]
- Zakzeski, J.; Weckhuysen, B.M. Lignin Solubilization and Aqueous Phase Reforming for the Production of Aromatic Chemicals and Hydrogen. ChemSusChem 2011, 4, 369–378. [Google Scholar] [CrossRef]
- Meryemoglu, B.; Hesenov, A.; Irmak, S.; Atanur, O.M.; Erbatur, O. Aqueous-phase reforming of biomass using various types of supported precious metal and raney-nickel catalysts for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 12580–12587. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Xu, Z.; Tian, Z. Direct conversion of cellulose into hydrogen by aqueous-phase reforming process. Catal. Commun. 2010, 11, 522–526. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Liu, Q.; Wang, C.; Liu, H.; Tian, Z. Preparation of Ce-modified Raney Ni Catalysts and Their Application in Aqueous-Phase Reforming of Cellulose. Catal. Lett. 2011, 141, 1851–1858. [Google Scholar] [CrossRef]
- Chang, A.C.-C.; Louh, R.F.; Wong, D.; Tseng, J.; Lee, Y.S. Hydrogen production by aqueous-phase biomass reforming over carbon textile supported Pt–Ru bimetallic catalysts. Int. J. Hydrogen Energy 2011, 36, 8794–8799. [Google Scholar] [CrossRef]
- Minowa, T.; Ogi, T. Hydrogen production from cellulose using a reduced nickel catalyst. Catal. Today 1998, 45, 411–416. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, W.; An, Z.; Song, H.; He, J. Interface–Promoted Dehydrogenation and Water–Gas Shift toward High-Efficient H2 Production from Aqueous Phase Reforming of Cellulose. ACS Sustain. Chem. Eng. 2018, 6, 7313–7324. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992–5002. [Google Scholar] [CrossRef]
- Gumina, B.; Espro, C.; Galvagno, S.; Pietropaolo, R.; Mauriello, F. Bioethanol Production from Unpretreated Cellulose under Neutral Selfsustainable Hydrolysis/Hydrogenolysis Conditions Promoted by the Heterogeneous Pd/Fe3O4 Catalyst. ACS Omega 2019, 4, 352–357. [Google Scholar] [CrossRef]




| Polyols | Cat. | T (°C) | P (bar) | Conv. | H2 Yield (%) | Ref. | Notes |
|---|---|---|---|---|---|---|---|
| Sorbitol * | 7 wt.% Pt/3D-BMC-12 | 250 | 45 | - | 28 | [31] | Continuous flow, WHSV = 2 h−1. |
| Sorbitol * | 7 wt.% Pt/2D-CMK-3 | 250 | 45 | - | 9 | [31] | Continuous flow, WHSV = 2 h−1 |
| Sorbitol * | 6 wt.% Pt-Fe (1:3)/CMK-9 | 250 | 45 | - | 23 | [34] | Continuous flow, WHSV = 2 h−1. |
| Sorbitol ** | 2.8%Pt/Al2O3 | 225 | 29 | 61 | 21 | [29] | Continuous flow, WHSV = 1.2 h−1. |
| Xylitol * | 6 wt.%Pt-Fe (1:3)/CMK-9 | 250 | 45 | - | 30 | [34] | Continuous flow, WHSV = 2 h−1. |
| Xylitol * | 2.5%Pt/C | 225 | 29 | 80 | [28] | Continuous flow, WHSV = 2 h−1. | |
| Xylitol * | 2.5%Pt-Re/C | 225 | 29 | 100 | [30] | Continuous flow, WHSV = 2 h−1. | |
| Xylitol * | 8%Pt-Re/TiO2 | 225 | 29 | 91 | [35] | Continuous flow, WHSV = 3.6 h−1. | |
| Xylitol ** | 2.8%Pt/Al2O3 | 225 | 29 | 79 | 26 | [29] | Continuous flow, WHSV = 1.2 h−1. |
| Xilitol* | 5%Pt/Al2O3 | 225 | 29 | 66 | 33 | [37] | Continuous flow, WHSV = 1.8 h−1. |
| Sugar | Catalyst | T (°C) | Conv. (%) | Sel. H2 (%) | Ref. | Notes |
|---|---|---|---|---|---|---|
| Glucose | Ni48%wt/Al2O3 | 260 | 95 | 46 | [54] | Conv. to gas = 33% ** |
| Fructose | Pt3%/Al2O3 | 200 | 2.3 * | [56] | - | |
| Glucose | Pt3%/Al2O3 | 200 | 0.4 * | [56] | - | |
| Sucrose | Pt3%/Al2O3 | 200 | 0.6 * | [56] | - | |
| Glucose-Fructose mix. | Pt3%/Al2O3 | 200 | 1.3 * | [56] | - | |
| Glucose | Pt3%/C | 250 | 83 | [60] | Conv. to gas = 9% ** | |
| Glucose | Ni/Al2O3 | 200 | 0.2 * | [61] | after 3 h | |
| Glucose | Ni/Zr2O | 200 | 0.3 * | [61] | after 3 h | |
| Glucose | Ni/Al2O3 | 200 | 0.2 * | [61] | after 9 h | |
| Glucose | Ni/Zr2O | 200 | 0.1 * | [61] | after 9 h | |
| Glucose | Pt/Al2O3 | 310 | 93 | 63 | [47] | Dual reactor |
| Glucose | Pt/Al2O3 | 310 | 96 | 11 | [47] | 1 reactor with H2 atm |
| Glucose | Pt/Al2O3 | 310 | 93 | 13 | [47] | 1 reactor with N2 atm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts 2019, 9, 917. https://doi.org/10.3390/catal9110917
Fasolini A, Cucciniello R, Paone E, Mauriello F, Tabanelli T. A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts. 2019; 9(11):917. https://doi.org/10.3390/catal9110917
Chicago/Turabian StyleFasolini, Andrea, Raffaele Cucciniello, Emilia Paone, Francesco Mauriello, and Tommaso Tabanelli. 2019. "A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols" Catalysts 9, no. 11: 917. https://doi.org/10.3390/catal9110917
APA StyleFasolini, A., Cucciniello, R., Paone, E., Mauriello, F., & Tabanelli, T. (2019). A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts, 9(11), 917. https://doi.org/10.3390/catal9110917