1. Introduction
At present, the problem of environmental pollution becomes more and more serious, leading to poor living conditions for the people all over the world. Effectively reducing environmental pollution is an urgent issue for the scientific community [
1,
2,
3]. One of the efficient ways is the catalytic method, treating pollutants as the reaction substrates and catalytically converting them into harmless ones under certain conditions [
4,
5], achieving the purpose of environment protection. Therefore, preparing highly efficient and suitable catalysts for industrial productions and applications has received extensive attention in the field of chemical research.
There is an important problem in industrial applications though lots of catalysts were prepared. Most catalytic materials are in the form of powder or granules, which are far less useful due to the high pressure drop, the catalyst running off, and the poor recyclability [
6,
7,
8]. The catalysts combined with monolithic carriers finely solve the above problems and greatly improve the practical applications. Furthermore, the stability of such monolithic catalysts would be significantly enhanced by decreasing the agglomeration owing to the dispersion and adhesion of the binder and/or carriers during a long period of reaction. Monolithic catalysts become an important subject in the field of catalysis, and a prospective direction with industrial application.
Extrusion molding, one of the most commonly used monolithic integration technologies, forces muddy material (catalysts, binders, and pore-forming agents) extruded from a specific mold, and cuts them into aimed shapes [
9,
10]. However, the catalysts exist both on the surface and inside the bulk directly leading to a low utilization rate of the catalysts. Moreover, the catalysts have a great effect on the fluidity and viscosity of the muddy material which results in a lack of universality for this method. Deposition of active phases on the monolithic carriers dramatically increases their utilization rate, which has been attracted continuous attention. Additionally, environmental friendliness, safety, efficient production, and low energy consumption are also very important for green integration considering sustainable development.
In this paper, we reviewed the traditional (impregnation, coating, and spraying) and novel (hydrothermal and electrodeposition) strategies of surface deposition integration, analyzed the advantages and disadvantages of them, and then prospected the possible directions for future development of integration technologies.
2. Traditional Preparation Methods
The monolithic catalysts prepared by traditional method (impregnation, coating, and spraying) usually consist of three parts: the monolithic carrier, the secondary layer, and the active species [
11,
12]. The carrier is the basic skeleton of the monolithic catalyst with a large number of channels as well as inner pores. The channels and pores provide a good mass and heat transfer, a fine stability in thermal and mechanical as well as a low pressure drop. The secondary layer always has larger specific surface area to help disperse the active species. The active species are deposited on the secondary layer by impregnation or coating and calcination of corresponding precursors.
2.1. Monolithic Carriers
Ceramic and metallic monoliths are the two most common types of carriers. The former has more pores, providing better heat stability and coating adherence than the metallic monoliths. Although expensive, metal monoliths have better mechanical strength and heat conductivity, allowing them have thinner walls, higher cell densities and lower pressure drops [
13]. Nevertheless, the low adhesion of coatings is the main disadvantage.
Ciambelli et al. [
14] prepared monolithic Ni-based catalysts using honeycomb and foam cordierite as carriers. The support structure had a direct effect on the activity of the monolithic catalyst in CH
4 auto-thermal reforming. The catalytic activity tests showed foam-structured Ni catalysts had better performance than honeycomb monolith structured ones, since the foam-structured Ni catalysts had a higher available amount of Ni species with respect to honeycomb monolith catalyst (0.014 vs. 0.028 g∙cm
−3). More importantly, the Ni species were finely dispersed on the foam monolith since the XRD could not detect any peak from them [
15], while there were observable diffraction peaks for NiO on cordierite honeycomb monolith.
Li et al. [
16] used Fe–Ni alloy (30%Ni–70%Fe) foam with unique three-dimensional non-regular channels as a monolithic carrier. After supporting a thin coating layer of γ-Al
2O
3, Pd was deposited on by an impregnation method. The obtained monolithic catalyst had an excellent performance in methane combustion with a conversion up to 99% at 550 °C in a CH
4–air ratio range from 2% to 5%.
Nowadays, fiber-based membranes, having good mass transfer as well as relative low pressure drop, attract more and more attention as novel monolithic carriers [
17]. Cuo et al. [
18] prepared porous ceramic membranes by using mullite fibers as a raw material, and kaolin and feldspar as binders. The uniform interconnected pore structure not only facilitated the dispersion of Mn-Ce particles but also kept a relative low pressure drop. The MnO
x/CeO
2 on ceramic membranes with a mole ratio of 3:1 catalyst even achieved 90% conversion at 244 °C in benzene combustion.
Metal oxide arrays with high aspect ratio guaranteed large outer surface area are also exploited as emerging monolithic carriers [
19]. Chen et al. [
20] modified highly-ordered pore-through TiO
2 nanotube arrays, the carrier, with MnO
2 by dipping in Mn(NO
3)
2·4H
2O solution. An optimized amount of MnO
2 could decrease the loading of Pt and enrich chemisorbed oxygen species on the surface of catalyst. The monolithic catalysts achieved and maintained 95% conversion of HCHO for more than 100 h at 30 °C, exhibiting stability performance. The confinement effect of the regular TiO
2 nanotube was beneficial for the enrichment of surface Pt and storage of HCHO. In addition, the Pt nanoparticles confined in nanotube and the specific regular structure provided the sintering resistance ability and good durability. Yu et al. [
21] used CuO array as a carrier and CeO
2 as an active species to catalyze diesel soot combustion under simulated practical conditions. The lawn-like CuO nanorods array significantly increased the contact chance between the catalyst and the soot. The contact chance was 25 times higher compared with that on particle-like catalyst. The temperature for the maximal rate of soot combustion on nanorods array was 17 °C lower than that on particle-like catalyst.
Here a brief conclusion could be made. The honeycomb monolithic carriers with regular passages and holes usually possess low pressure drop and high mechanical strength, while they have relatively poor capability for dispersion of active species and mass transfer. The foams and membranes with three-dimensional non-regular channels are opposite. There should be one but not the only one balance between them in practical application, which totally depends on the special working conditions. Metal oxide arrays perform well in some applications, e.g., soot combustion. The highly ordered pore-through or nanorod arrays usually need some substrate to grow on, and the productions are complicated, so they suit the situations wherein the monolithic catalysts do not required much usage.
2.2. Secondary Layer Formation
Normally, all monolithic carriers are extremely limited with a low specific surface area (e.g., 0.7 m2∙g−1 for cordierite; 0.5–2.0 m2∙g−1 for nickel foams), so the washcoating is introduced as the secondary layer. The secondary layers are usually oxides with high surface area to provide a better adherence and a higher dispersion of the active species.
The dip-coating method is the most prevailing technique to coat the secondary layer on monolithic carriers. The typical procedures are (1) dipping the monolithic carriers into the slurry; (2) blowing to remove the excess slurry and; and (3) drying and calcination. After several such cycles, a high mass loading of uniform coating could be obtained. Lu et al. [
22] prepared 8%–12% of γ-Al
2O
3 as a secondary layer on cordierite honeycomb in this way with 3–4 cycles. Yuan et al. [
23] developed a facile method for dip coating, which achieved 30% γ-Al
2O
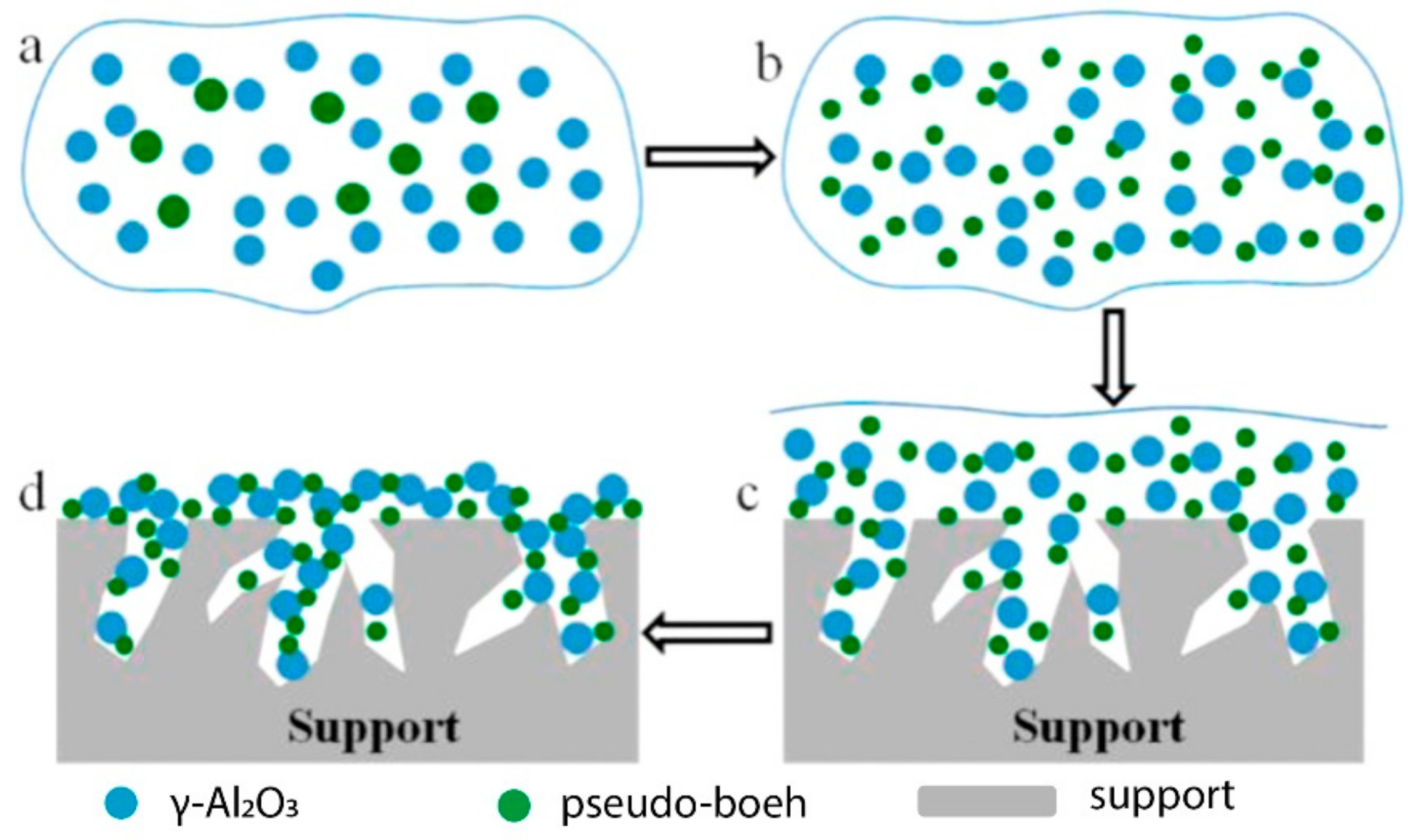
3 loading by one step, and the weight loss was less than 1% after 3 h ultrasonic treatment. They pointed out that the “nail-like” interaction between the coating layer and the carrier played a key role (
Figure 1). The palladium-copper catalyst dispersed well on γ-Al
2O
3 and gave an initial CO conversion of 98% at 30 °C in a feed gas of 17.5 ppm CO balanced with air and 10,000 h
−1 GHSV(gas hourly space velocity). The catalysts showed an excellent stability with the conversion decreased only 6% after 192 h test.
Pd/Al
2O
3 monolithic catalysts are active for methane combustion at low temperature and high oxygen/fuel ratios. However, under low oxygen/fuel ratios, there would be a periodic PdO/Pd ratio change, the so-called “oscillatory behavior”. This characteristic limits the application of Pd/Al
2O
3 monolithic catalysts. Fortunately, adjusting the components of the secondary layer could slow down the decompositon and sintering of the active metals. Jin et al. [
24] used Zr and Ce as the secondary layer for Pd-based monolithic catalysts. In methane de-oxygenation processes, Zr served as the oxygen storage material on the interface between Zr and Pd, while Ce prevented the catalysts from the deactivation. With a low palladium loading of 0.3 wt%, the catalyst was reported to show excellent catalytic activity in a feed stream containing 50% of CH
4, 3% of O
2 and Ar. For the catalyst, the oxygen was completely consumpted below 350 °C, and the activity was still quite stable after a running of 500 h.
Morales-Torres et al. [
25] coated cordierite with carbon nanofibers (CNF) and/or γ-Al
2O
3 as a secondary layer and prepared a series of Pt-based monolithic catalysts. The Pt-CNF/γ-Al
2O
3 monolithic catalyst had better water resistance than Pt-γ-Al
2O
3. In benzene combustion, the activity of the Pt-CNF/γ-Al
2O
3 monolith decreased less than 8% in the presence of water stream (22,000 ppm), while that of the Pt-γ-Al
2O
3 monolith decreased around 25%. The main reason was that the CNF surface had a better hydrophobic property than that of γ-Al
2O
3. Graphene was found to have a similar effect. Li et al. [
26] compared the catalytic performances of Pd/cordierite (Pd/Cor) and Pd/graphene/cordierite (Pd/Gr/Cor) catalysts for toluene combustion in both dry and wet conditions. Compared with the Pd/Cor, complete combustion temperature of Pd/Gr/Cor was 40 °C lower in a dry condition. The activities of both catalysts were decreased in relative humidity of 81%. However, the decrease degrees were different. The complete combustion temperature of Pd/Gr/Cor increased from 260 to 360 °C, while that of Pd/Cor increased from 300 to 430 °C. The better dispersion of Pd on graphene layer contributed to the improved activity and thus better affinity to toluene. The enhanced water resistance should be due to the hydrophobicity of grapheme [
27].
In conclusion, the most basic function of the secondary layer is to increase the surface of monolithic carriers which further increase the dispersion of active species. With the development of the science, multifunctional coatings ocurred. Secondary layers could contribute to increase the synergy between the coatings and the active components. In the coatings, reaserchers tend to add some component(s) that could improve both the performance and the stability of the active components (Ce/Zr as a cocatalyst to be incorporated into the coatings). Additionally, the secondary layer could help to meet the practical application requirements (carbon nanofibers or graphene enhance the moisture resistance of the catalysts).
2.3. Active Phase Deposition
The active species, the key players during the catalytic process, are loaded onto the surface of the secondary layer. As well as powder catalysts, the physicochemical states of active species directly affect the final performances of the monolithic catalysts.
High dispersion of active species often leads to good performances of the catalyst. As mentioned above, Pd–Cu well dispersed on cordierite coated with γ-Al
2O
3 was very active and stable in CO oxidation with a more than 92% CO conversion at 30 °C during a 192 h test [
23]. Pd/Gr/Cor with an improved dispersion exhibited a higher activity for toluene combustion compared with Pd/Cor. The complete combustion temperatures of Pd/Gr/Cor were 40 and 70 °C lower than that of Pd/Cor in dry and wet condition, respectively [
26].
The ratio of different chemical states of active species and the synergistic effect between active species and coating also gave the performance a substantial enhancement. Cuo et al. [
18] prepared MnO
x/CeO
2 on ceramic membranes with mole ratio from 1 to 4. The MnO
x/CeO
2 catalyst with the Mn/Ce ratio of 3 was best for benzene combustion, having a T
10 temperature of 103 °C which was 9–38 °C lower than that of those with other ratios. The characterization results suggested that the high amounts of Ce
3+ and Mn
4+, due to the synergistic effect of Mn and Ce with suitable ratio, promoted the electron or oxygen transfer, which further contributed twoard the high activity.
The crystalline structures of active species also play an important role to obtain excellent performances. Wang et al. [
28] compared the turnover frequency (TOF) for HCHO oxide on amorphous MnO
x (A-MnO
x), partially crystallized MnO
x (PC-MnO
x), and crystallized MnO
2 (C-MnO
2) at room temperature within 50 min. The PC-MnO
x catalyst showed obvious advantages, and the TOF value of it was 2.13 and 1.68 times of that on A-MnO
x and C-MnO
2. Improving the preparation method has been considered an efficient way to change the physicochemical states of active species.
2.3.1. Impregnation Method
Impregnation is a simple method to load the active species on monolithic carriers coated with secondary layer. In this method, coated monolithic carriers are immersed in the precursor solution for a certain period of time. Then they are dried and calcined at proper temperatures [
29]. The precursor finally transforms to activity species. Sometimes a prepared monolithic catalyst needs 3–7 cycles of impregnation [
22].
Loading sequence is important while there are more than one active materials. Stefanov et al. [
30] varied the sequence of Pd and Co loading onto γ-Al
2O
3. The result showed that cobalt deposited first or cobalt–palladium co-deposited had lower “light-off” temperatures (T
10) and longer stabilization periods than that of palladium deposited first in methane combustion. It was argued that PdO clusters were the main active species, and the surface cobalt oxide phase stabilized the PdO and served as a reservoir of the oxygen species. The Pd–Co monolithic catalysts used anodic alumina and Fe–Cr–Al-alloy as carriers were prepared by the optimized deposition sequence. They were active for methane combustion with a “light-off” temperature at 350 and 310 °C respectively.
Different metal precursors also had an effect on the performances of monolithic catalysts. Neyertz et al. [
31] prepared K/CeO
2/cordierite catalysts with different potassium precursors (KNO
3, K
2CO
3, and KOH). For catalysts prepared with KNO
3 and K
2CO
3, the weight losses after ultrasonic treatment were lower than 0.4%. While for catalyst prepared with KOH, the weight loss was 2.8%. This indicated that the precursor affected the mechanical stability of catalyst. Additionally, KOH, with a higher basicity, decreased the adsorption and dissociation of gaseous oxygen and, thus, decreased the reactivity of the lattice oxygen [
32]. The three catalysts impregnated with soot were analyzed by temperature programmed oxidation (TPO). The initial temperature (T
i) presented the activity of the catalyst for soot combustion. The T
i of the monolithic catalyst prepared with KNO
3 was 330 °C, while that of catalysts prepared with K
2CO
3 and KOH were 355 °C and 385 °C, respectively. Therefore, the catalysts increased in the following order: KNO
3 > K
2CO
3 > KOH [
31]. Liao et al. [
33] reported that both of loading route and precursor of Pd significantly affected the surface Pd species and thus the catalytic activity. Although sol-dipping method led to lower Pd loading which was only 1/2 or even 1/3 of that obtained with aqueous solution impregnation, the sol-dipping produced Pd and PdO that guaranteed the action of self-catalytic reaction between them, resulting in a good performance. The monolithic catalyst prepared by sol-dipping combined with citrate acid and Pd(NO
3)
2 precursor had a T
10 value of 290 °C for methane catalytic combustion, showing the best performance among the prepared catalysts.
The impregnation step is simple to operate. And some monolithic catalysts could be obtained by impregnation method. However, prior to impregnation, there is often the need for a secondary layer to increase the surface area of monolithic carrier, which sometimes needs 3–4 cycles of dip coating [
22]. Considering that the metal precursors and loading sequence have a significant influence on the performances of the obtained catalyst, step impregnating may be used for multi-component catalysts. That makes whole impregnation method complicated and time-consuming. It consumes a great deal of energy, too, since there are two calcination steps needed during secondary layer and active species deposition. Additionally, toxic gases will be released during calcination when the precursors containing NO
3− and Cl
−. Thus, the disadvantages of this method are not negligible, and the development is in need and urgent.
2.3.2. Coating Method
Coating is another frequently-used method to deposit the active species on surface of monolithic catalysts [
33]. Using this method, the precursors or the ready-made powder catalysts were added into the slurry of the secondary layer directly. The monolithic carriers were immersed and coated by the slurries. Then several immersions were needed to obtain efficient loadings. Finally, calcination at high temperatures is always needed to enhance the interaction between the active species and carriers.
Aguero et al. [
34] prepared the MnO
x/Al
2O
3/FeCralloy monoliths by two kinds of coating methods: wash-coating with MnO
x/Al
2O
3 ready-made powder catalyst and wash-coating with θ–δ-Al
2O
3 suspended in Mn(CH
3COO)
2 solution. The addition of a binder was not necessary because the suspensions were stable enough by themselves after ball-milling for several hours. Repeated immersions might be needed with each slurry to obtain aimed loadings. After each immersion, they were dried at 120 °C and calcined at 500 °C for 2 h. The coating method with θ–δ-Al
2O
3 suspended directly in Mn(CH
3COO)
2 solution not only simplified the preparation process but also produced more active catalysts. The “light-off” temperatures of the prepared catalysts were about 30–50 °C lower than that of the catalyst prepared by wash-coating with MnO
x/Al
2O
3 ready-made powder catalyst in ethanol, ethyl acetate, and toluene combustion.
Long et al. [
35] prepared Mn-Ce-Zr monolithic catalyst by one-pot coating. The cordierite was coated by a slurry containing Mn(NO
3)
2, Ce(NO
3)
3, Zr(NO
3)
4 and hydroxyethyl cellulose (binder). Then the monolithic catalyst was dried at 120 °C and calcinated at 500 °C in air for 2 h. The Mn-Ce-Zr catalyst exhibited superior catalytic activity for combustion of chlorobenzene with T
90 at 390 °C. The larger the surface area, the higher the content of Mn
4+ and surface oxygen, and the greater oxygen mobility and manganese reducibility led to the superior activity.
Zhang and Wu [
36] prepared Mn-Ce-M (M = Cu, Ni and Co) catalysts on cordierite honeycomb for
o-xylene combustion. The corresponding nitrates and citric acid were dissolved in ethanol and continuously stirred at 50 °C to form a sol. The cordierite was immersed in the prepared sol, and then purged under a weak air flow. Repeated coating was needed to achieve a desired loading. After each coating, they were dried at 80 °C for 2 h. The monoliths were calcined at 450–700 °C for 2–5 h. The MnCeCu catalysts were more active than others. The optimized MnCeCu
0.4 monolith catalyst achieved 95% conversion of
o-xylene at 300 °C, which increased slowly to 98.4% for 500 min test in
o-xylene combustion.
Wu et al. [
37] prepared monolithic Cu–Mn mixed-oxide/γ-alumina/cordierite catalysts by three methods. Method A (two-step impregnation): Cu and Mn nitrates were deposited onto cordierite with a secondary layer. Method B (sol-gel coating): Cu, Mn nitrates, and alumina sol were mixed and coated repeatedly onto cordierite. Method C (one-step impregnation): Cu, Mn, Al nitrates, and urea were mixed and deposited onto cordierite without a secondary layer. The samples obtained by the three methods were denoted as sample A/B/C hereafter.
Figure 2 shows the distributions of active materials on the prepared samples. For sample C, the active phase prefered to accumulate at the outer part of the monolith, due to the movement of metal precursor salts during drying. For samples A and B, the homogeneous distribution of the active phase was obtained. There were relative more active phases in the channels at both sides of monolith sample A, mainly because of the inaccurate control of preparation. Sample A and B exhibited similar activity for
o-xylene combustion. However, the weight loss of sample A were 3~5 times higher than that of sample B, showing some inferiority in stability.
The coating method, especially adding the precursors into the slurry of the secondary layer directly, reduced the number of stages during the synthesis of monolithic catalysts and, thus, somehow decreased the fabrication costs. However, repeated coating cycles were often needed to achieve sufficient loading, making it a time-consuming production. Furthermore, the decomposition of the binders and precursors during the subsequent calcination may cause the emission of toxic gases.
2.3.3. Spraying Method
Spraying is a derivation method from coating. The nanoparticle dispersion liquid was prepared by the solvothermal method firstly. Then active species were deposited by spraying onto different supports to obtain monolithic catalysts.
Chen et al. [
38] prepared the Cu–Ce catalyst dispersion liquid through the solvothermal method and sprayed it on cordierite honeycomb. The obtained monolith catalysts showed 95% conversion of toluene at 300 °C. After this, the same group synthesized Pt/TiO
2/cordierite catalysts [
39] and Pt/FeCrAl fiber catalyst [
40] in the same way. Compared the Pt/TiO
2/cordierite prepared by traditional coating (Pt/TiO
2/CorT), the catalyst prepared by spraying (Pt/TiO
2/CorS) exhibited better adhesion and catalytic performance for toluene combustion. The weight loss was only 0.11% after ultrasonic treatment for 1 h. And the stability test at 240 °C showed that there was no observable deactivation within 120 h. The Pt/TiO
2/CorS had an improved activity showing a 20 °C decrease of T
10 (202 vs. 222 °C) compared with that of Pt/TiO
2/CorT. The Pt/FeCrAl fiber catalyst prepared by spraying also delivered 100% toluene conversion at 280 °C and stable for at least 10 h without deactivation for toluene combustion.
Spraying the liquid with active nanoparticles on different supports is a facile method. However, the preparation of “active” liquid by solvothermal method is complicated and expensive, which might be a restraint from application. Chen et al. [
26] reported that the deactivated catalysts could be reactivated through respraying the liquid, but the adhesion between active species and carriers may become weaker after repeated spraying, resulting in catalyst loss. This method is inappropriate to prepare large porous monolithic catalysts since the deposition mainly occurs at the outer surface of the carriers.
3. Novel Preparation Methods
The intrinsic characteristic of the traditional methods is that the active species were deposited on carriers by ex-situ method and thus the interaction between them was weak. The subsequent calcination to enhance the interaction force comes at the cost of high energy consumption. Additionally, the decomposition of the binders and precursors during the subsequent calcination may cause the emission of toxic gases. In recent years, novel synthesis, e.g., hydrothermal and electrodeposition methods, have been emerging to solve the issues mentioned above. The most distinguished characteristic is that the active species grow on the carriers in- situ and, thus, a strong interaction could be obtained.
3.1. Hydrothermal Process
The hydrothermal method as a conventional one to prepare powder catalysts, and it is viable to grow active species on cordierite honeycombs [
41] and metal monoliths [
42]. Cai et al. [
43] used the hydrothermal method to prepare Mn–Co oxides supported on Fe meshes via in situ growth. Typically, Fe meshes in the homogenous solution of Co(NO
3)
2, Mn(CH
3COO)
2 and CO(NH
2)
2 were transferred into a Teflon reactor and heated to 90 °C for the hydrothermal reaction. The procedures are shown in
Figure 3: firstly a layer of Mn and Co hydroxide precursors grew in situ on Fe mesh, then Mn and Co hydroxide seeds were generated in a polyhedron shape which further grew to form a spherical structure and, finally, a block-like structure. The block-like Mn and Co hydroxides were transformed to porous Co
2MnO
4 spinel oxides with the same structure after the calcination treatment. The hydrothermal process not only provided the strong interaction between Co
2MnO
4 and supports but also ensured the uniform distribution of active species.
Due to the universality of this method, Ni-Mn@Fe mesh [
44], Ni-Mn@Ni foam [
45], MnO
2@NiCo
2O
4@Ni foam [
46], Mn-Fe@Fe mesh [
47], and MnCo
xO
y@Ti mesh [
48] were prepared for selective catalytic reduction of NO with NH
3 (NH
3-SCR). Ni-Mn@Fe obtained ∼100% selectivity for N
2 and 80% NO conversion in the range of 250–370 °C. Ni-Mn@Ni foam used Ni foam as the source of Ni, exhibiting a wide temperature range (245–360 °C) for 80% NO conversion. The performance was slightly better than Ni-Mn@Fe even the precursor was manganese nitrate and ammonium chloride only. The process of preparing MnO
2@NiCo
2O
4@Ni foam was the most complicated. Ni–Co basic carbonate precursors was formed on the nickel foam substrate at 160 °C first, and then MnO
2 nanoparticles were precipitated on Ni-Co@Ni foam. The material was calcined at 500 °C finally. The MnO
2@NiCo
2O
4@Ni catalyst kept NO conversion above 80% in the wide temperature range (127–227 °C) at 20,000 h
−1 GHSV. The MnCo@Fe [
43], Mn-Fe@Fe mesh [
47] and MnCo
xO
y@Ti mesh [
48] also exhibited good NH
3-SCR performance. However, the active performance, benefited from the synergistic effect of Ni, Co, and Mn ternary oxides, was better than that of Ni–Mn, Co–Mn or Ni–Co.
Li et al. [
49] prepared monolithic CoMnAl film via in situ hydrothermal growing. CoMnAl-LDH precursor grew on Al substrate at 120 °C following by a calcination at 400 °C. This thin curved hexagonal platelet of CoMnAl film was full of edges of crystallites, which provided a large number of available active sites. The reaction rate of the toluene oxidation of monolith was five times higher than that of powders (1.32 vs. 0.24 mmol∙g
−1 h
−1 under T
99 = 260 °C).
Guan’s group prepared MnO
x [
50], MnO
2-Co
3O
4 [
51] on AISI304 stainless steel wire-mesh by the hydrothermal method, held at 160 °C. The T
50 of soot combustion on MnO
x monolithic catalysts was 382 °C while it was 354 °C on MnO
2-Co
3O
4 monolithic catalysts. The MnO
2-Co
3O
4 monolithic catalyst had a synergistic effect between Mn and Co, which weakened Mn–O bonds, improving the amount of surface oxygen species and redox property.
Chen et al. [
52] analyzed the differences between CuMn
2O
4@MnO
2@cordierite prepared by the hydrothermal method and CuMn
2O
4@Al
2O
3@cordierite prepared by traditional slurry coating method. The monolithic catalysts formed by hydrothermal method had less average weight (~600 g L
−1), less loading percentage (9%–15%), but higher specific surface area (35–43 m
2∙g
−1) than the wash-coated catalyst (~800 g∙L
−1, 31%–34%, 12–17 m
2∙g
−1). The 50% conversion temperature of the hydrothermal monolithic catalyst for C
3H
8 oxidation was 25 °C lower than that of the wash-coated monolithic catalyst. Furthermore, all procedures were conducted under atmospheric conditions, so these had a prospect of industrial manufacturing processes.
The hydrothermal methods allow the active species to grow in situ on the monolithic carrier. Meanwhile, the strong interaction between the active species and the carrier is generated simultaneously, which is beneficial for enhancing the catalytic performance. The results show that catalysts obtained by hydrothermal methods usually have a better performance than that of catalysts obtained by coating. However, hydrothermal processes are usually operated at high temperature, resulting in high energy consumption. Meanwhile, it is difficult to guarantee that the nucleation and growth only occurred on the surface of carriers. The nanoparticles generated in the solution increased the cost of production and wastes treatment.
3.2. Electrodepositing
Electrodeposition is an electrochemical process occurring at the interface between electrode and electrolyte, which has been applied in the field of surface structure modification for a long time [
53]. In an external electric field, the ions in the electrolyte migrated and deposited rapidly on the surface of the electrode materials, and the morphology could be controlled by adjusting the operation parameters. Electrodeposition, as an in situ method for monolithic catalysts preparation, has been continuously developing in recent years.
Zhang et al. [
54] reported a structured PdNi alloy via in situ electrodepositing Pd nanoparticles on the surface of Ni-foam followed by calcination at 450 °C. For the coal bed methane deoxygenation precession, the oscillatory behavior of Pd-based catalysts is a challenge in low-temperature catalytic CH
4 combustion. PdNi(111) alloy formed by electrodepositing and calcination, in which the Ni atoms diffused into Pd nanoparticles, was more active in promoting O
2 conversion to H
2O than Pd(111) and the catalysts achieved full O
2 conversion at 350 °C with no signs of oscillation and deactivation in 230 h running.
Tzaneva et al. [
55] prepared the Co/Al
2O
3 by galvanostatic deposition and traditional impregnation method. Then Pd was deposited on the above carriers via impregnation to obtain monolithic Pd-Co/Al
2O
3 catalysts.
Figure 4a depicts the cross section of Pd-Co/Al
2O
3 prepared based electrodepositing. There were three kinds of Co species, the non-uniform Co nanowires along the pores of Al
2O
3, the Co particles in the middle layer and the Co mixed with Pd oxides in outer layer. The catalysts prepared based electrodepositing had larger surface area and, thus, exhibited a reaction rate 7.6 times higher than that of the sample prepared by impregnation for methane combustion as shown in
Figure 4b.
Xiao et al. [
56] assembled nanoflower-like Co
3O
4/Ni-foam by the electrodeposition method with cobalt nitrate as the only reagent. The deposition time varied from 300 to 3600 s. There was more Co
2+ with a Co
2+/Co ratio of 48.7%, calculated by the reduction peak area I and II in
Figure 5a, on the Co-Ni-300s catalyst. Meanwhile, the Co-Ni-300s had the weakest Co-O bond than other catalysts since the peak of A
1g vibration shifted from 680 to 660 cm
−1 as the deposition period decreased according to
Figure 5b. The high content of Co
2+ and the weak Co-O bond were beneficial for propane combustion. The results showed that the catalytic activity per gram of Co
3O
4 at 220 °C for the Co-Ni-300s was almost three times higher than that of powder Co
3O
4.
Cimino et al. [
57] potentiostatic electrodeposited Pt particles on FeCralloy foams with different specific surface areas (100 vs. 35 cm
−1) for methanol combustion. The catalytic activities of Pt/FeCr alloy, having low correlation with specific surface areas, increased progressively with the increase of Pt loading from 0.8 to 13 mg∙cm
−3 for combustion of methanol. Verlato et al. [
58] prepared Pt nanoparticles covered with discontinuous CeO
2 layers on FeCralloy by pulsed cathodic electrodeposition Pt followed by CeO
2. The catalytic combustion of methanol over the obtained catalyst with 0.37 mg∙cm
−3 Pt loading started at ca. 100 °C and eventually maintained at a conversion of ca. 87.7% when the temperature above 250 °C. However, simultaneous electrodeposition of Pt and CeO
2, and electrodeposition CeO
2 followed by Pt could not realize the sufficient deposition of active species. The reason was that the inductivity of CeO
2 hindered the deposition of Pt. Ho et al. [
59] potentiostatic electrodeposited Pd–CeO
2 on FeCr alloy in one step. During the synthesis procedure, part of Pd
2+ was doped into CeO
2 lattice forming a Pd
xCe
1−xO
2−δ solid solution. Finally, the defective nano-CeO
2 coatings containing Pd
xCe
1−xO
2−δ solid solution and Pd
0 particles were electrodeposited. The monolithic catalysts showed stability keeping more than 90% CO conversion at the temperature range of 375–425 °C after 48 h time-on-stream and 12 thermal cycles in CO oxidation even at high GHSV of 4 × 10
6 h
−1.
The catalysts prepared by electrodeposition show similar high adhesion and activity as those obtained by hydrothermal methods. Additionally, under the effect of an external electric field, electrodeposition only occurs at the electrode/electrolyte interface and the whole process is very fast. Furthermore, electrodeposition technology can improve the function of electron transfer and enhance the redox ability. This is almost a “mission impossible” for the traditional methods. This method also has some restrictions, e.g., the carriers have to be electric conductive and the influence of operation parameters still needs systematic and in-depth investigations.
4. Summary
In conclusion, we reviewed the traditional (impregnation, coating, and spraying) and novel (hydrothermal and electrodeposition) strategies for monolithic catalysts preparation. For all the methods, surface deposition is integrated to realize a relatively high utilization ratio of active atoms.
The impregnation step is simple to operate. However, several repeated cycles of coating or immersion are needed to obtain a desired loading during the deposition of secondary layer and active species, respectively. It makes the whole impregnation method tedious and time-consuming. It consumes a great deal of energy, too, since there are two calcination steps needed for secondary layer and active species deposition. Additionally, toxic gases would emit during the calcination when the precursors contained NO3− and Cl−.
The coating method shows some progress that it reduces the number of stages during the synthesis and, thus, decreases the fabrication costs by adding the precursors into the slurry of the secondary layer directly. However, quite similar to the impregnation, it is also a time-consuming production since repeated cycles of coating are often needed to achieve target loading. Additionally, it might also cause emissions of toxic gases due to the decomposition of the binders and precursors during the subsequent calcination.
Spraying the active nanoparticle dispersion liquid on the substrates is a facile method. However, the preparation of “active” liquid by solvothermal method is complicated and expensive. The utmost problem is that the sprayed coating is not appropriate for substrates with large porosity since the deposition mainly occurred at the outer surface of the carriers.
Catalysts obtained by hydrothermal methods usually have a better performance than those of catalysts obtained by traditional methods. However, the hydrothermal method has its inherent drawbacks. The unavoidable nucleation and growth of nanoparticles in solution exhibit a high cost of production and waste treatment. Furthermore, the hydrothermal reaction usually requires a high temperature which produces another problem: the high consumption of energy.
The catalysts prepared by electrodeposition have superior characteristics (i.e., high adhesion and activity) similar to those obtained by hydrothermal methods. The electrodeposition only occurs at the electrode/electrolyte interface, different from the hydrothermal method, showing significant advantages. Furthermore, the whole process was very fast and the external electric field can improve the function of electron transfer and enhance the redox ability. Although there are certain electrical and applicable restrictions, it might be a promising method for future development.
In general, the traditional methods have been proven effective in the applications for both labs and industries, but also have certain disadvantages, such as tedious operation, repeated cycles, high temperature calcination, and even toxic gases. By contrast, the novel and green strategies having emerged in recent years solve some intrinsic issues of the traditional methods, but scaling-up should take much more time and in-depth investigations. For the researchers in this field, there are three prospective directions: (1) developing the traditional methods to make them more facile and environmentally friendly; (2) scaling-up the green and novel methods to meet the requirement of industrial applications; and (3) exploring brand new technologies. The second direction might become the most interesting and intriguing one, since it attracts the attention from both basic researchers and industrial practitioners.