Solid Acids for the Reaction of Bioderived Alcohols into Ethers for Fuel Applications
Abstract
:1. Introduction
2. The Use of Solid Acids in MTBE Synthesis
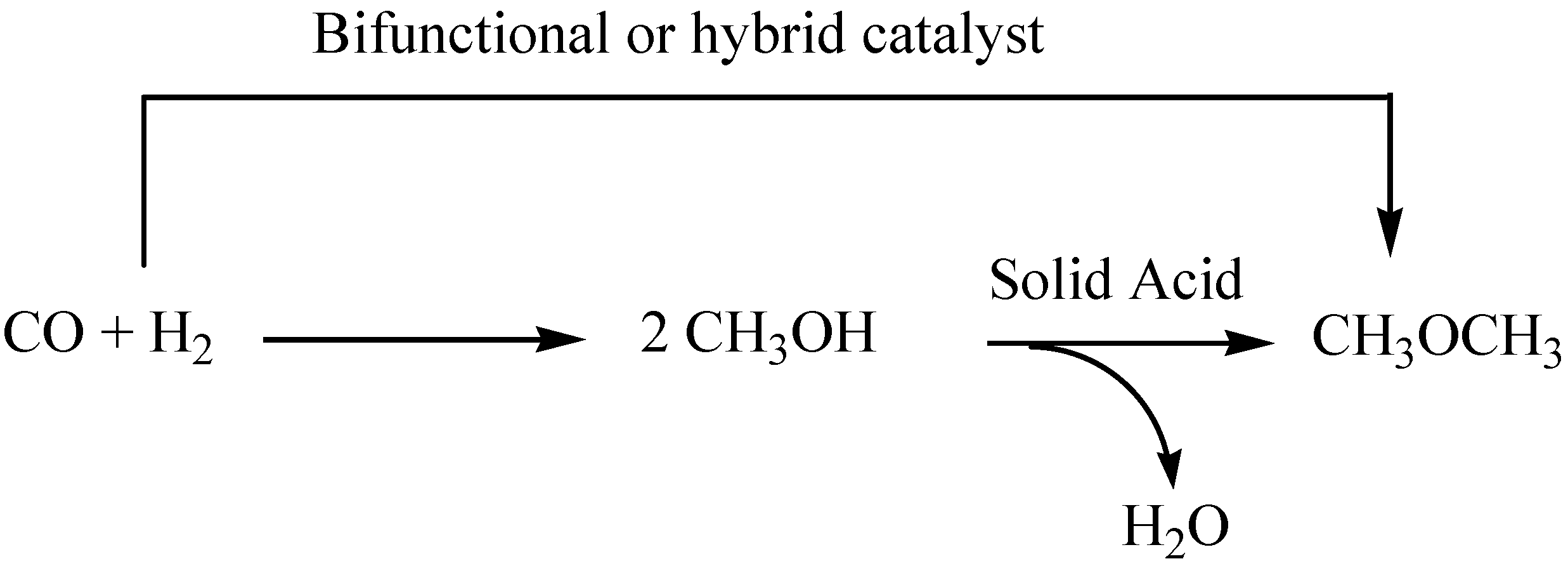
3. The Use of Solid Acids in DME synthesis
4. The Use of Solid Acid in Glycerol Ethers Synthesis
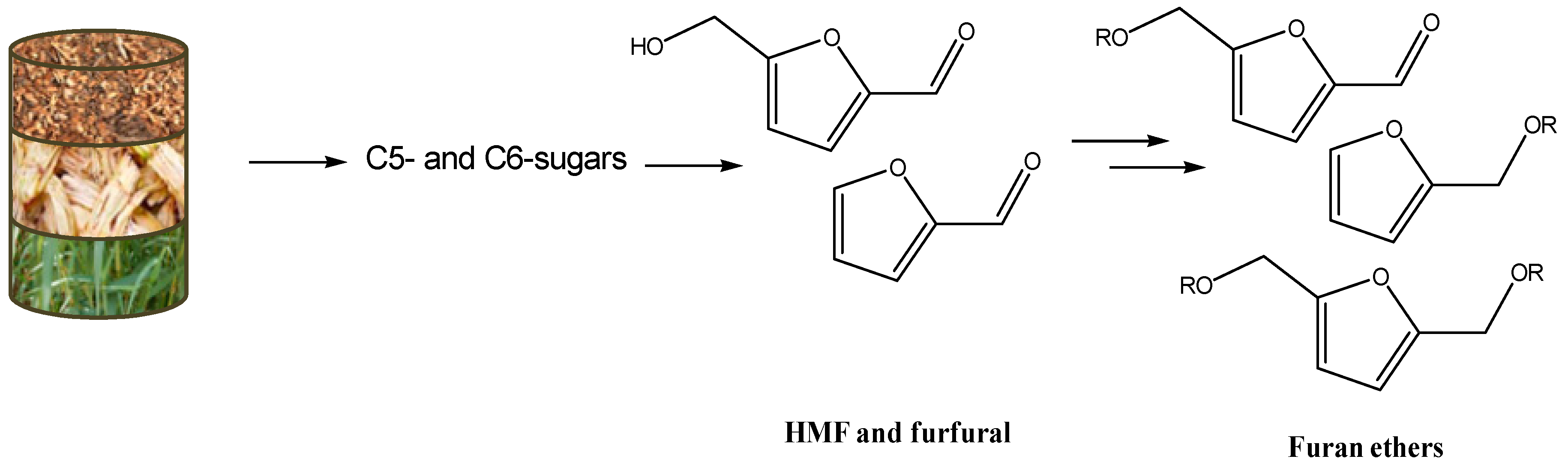
5. The Use of Solid Acids in Furanic Ethers Synthesis
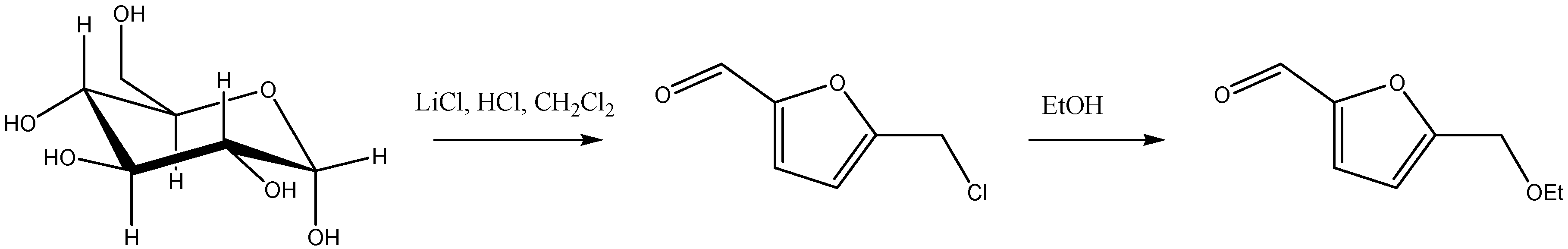
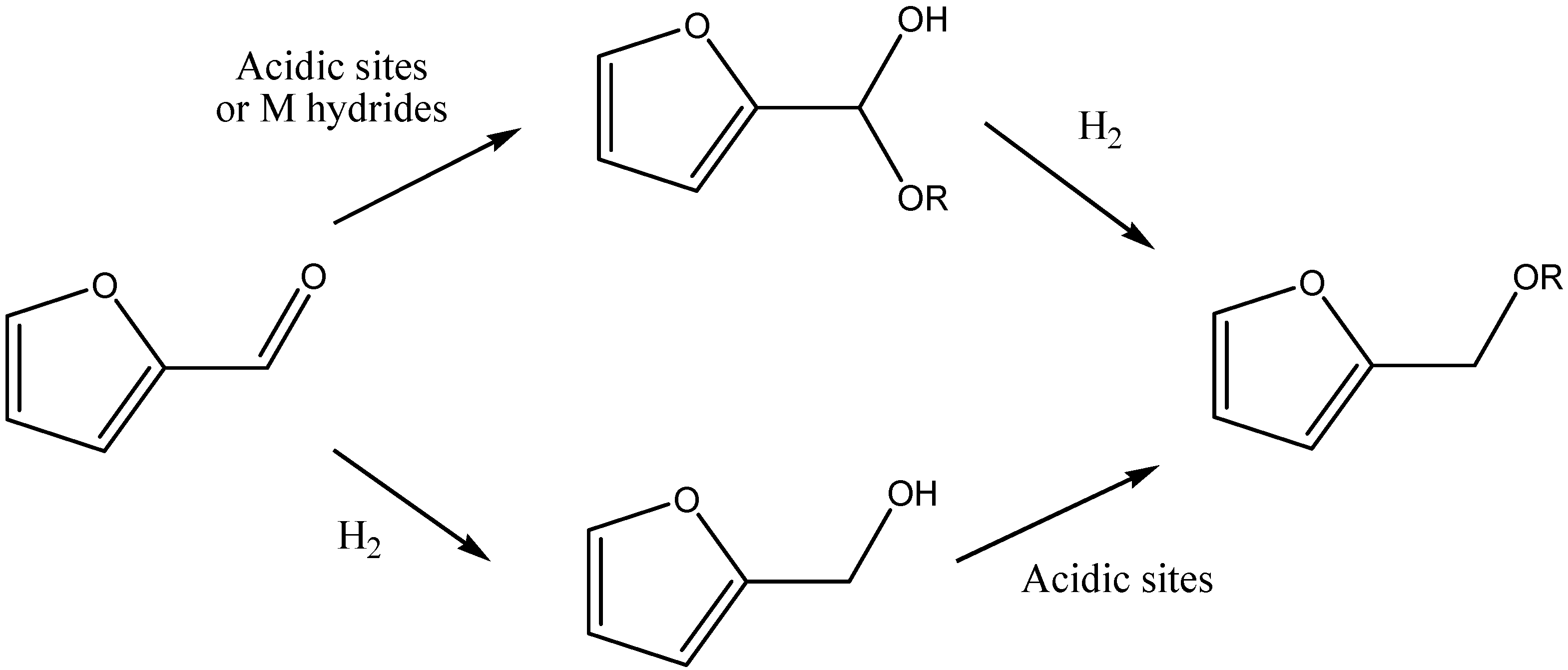
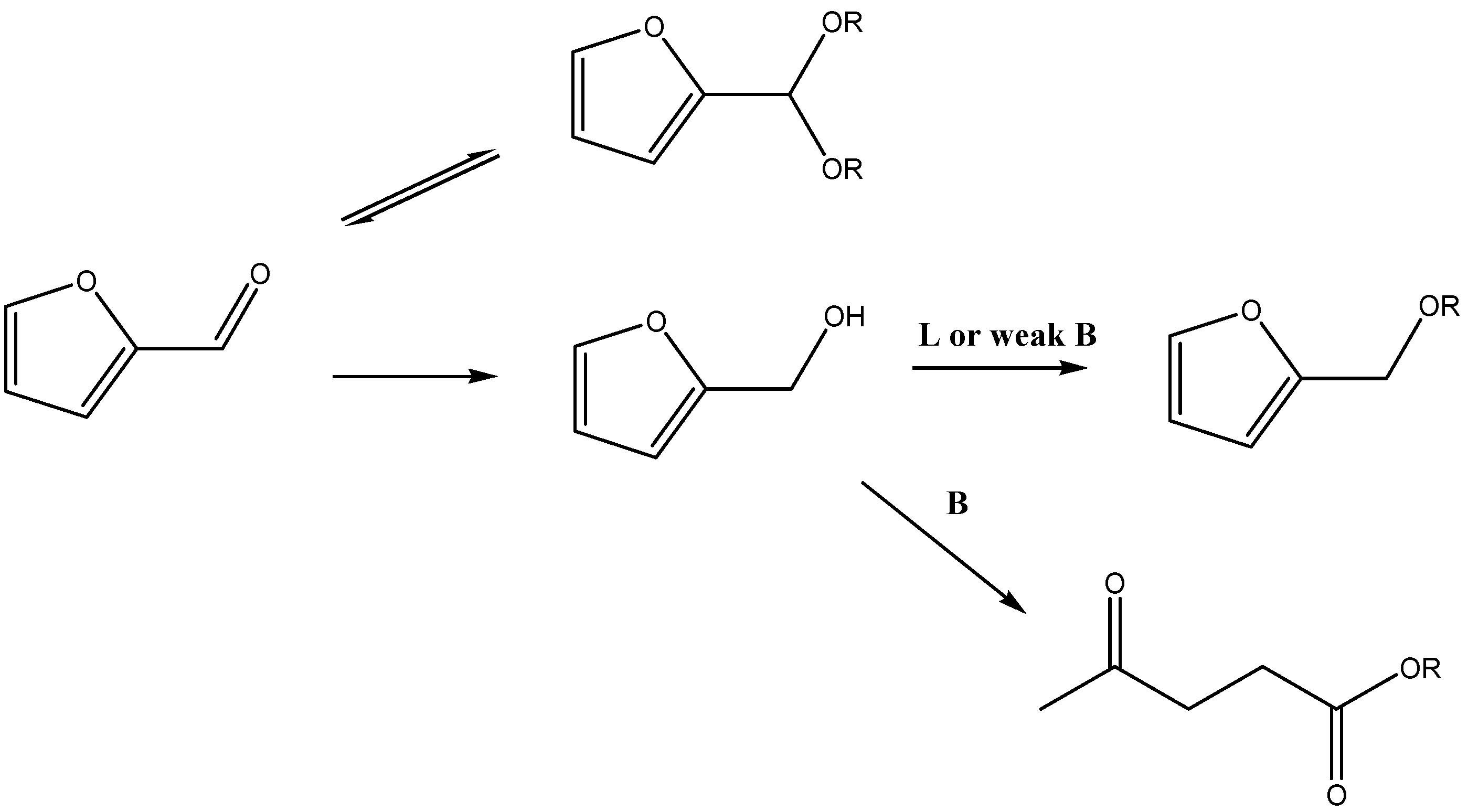
5.1. Direct Etherification of HMF and Furfural
5.2. Cascade Processes from HMF and Furfural
5.3. Cascade Processes from C6 and C5 Sugars
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Awad, O.I.; Mamat, R.; Ibrahim, T.K.; Hammid, A.T.; Yusri, I.M.; Hamidi, M.A.; Humada, A.M.; Yusop, A.F. Overview of the oxygenated fuels in spark ignition engine: Environmental and performance. Renew. Sustain. Energy Rev. 2018, 91, 394–408. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ali, O.M.; Sidik, N.A.C.; Yusaf, T.; Kadirgama, K.; Kettner, M. Alcohol and ether as alternative fuels in spark ignition engine: A review. Renew. Sustain. Energy Rev. 2018, 82, 2586–2605. [Google Scholar] [CrossRef]
- Mahdi, H.I.; Muraza, O. Conversion of isobutylene to octane-booster compounds after methyl tert-butyl ether phaseout: The role of heterogeneous catalysis. Ind. Eng. Chem. Res. 2016, 55, 11193–11210. [Google Scholar] [CrossRef]
- Pal, D.B.; Lavania, R.; Srivastava, P.; Singh, P.; Srivastava, K.R.; Madhav, S.; Mishra, P.K. Photo-catalytic degradation of methyl tertiary butyl ether from wastewater using CuO/CeO2 composite nanofiber catalyst. J. Environ. Chem. Eng. 2018, 6, 2577–2587. [Google Scholar] [CrossRef]
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl. Catal. B: Environ. 2017, 217, 494–522. [Google Scholar] [CrossRef]
- Renewable Fuels Associations, Ethanol Market. Available online: https://ethanolrfa.org/resources/industry/statistics/#1537811482060-17cad4ed-d2ca (accessed on 11 February 2019).
- Gavahian, M.; Munekata, P.E.S.; Eş, I.; Lorenzo, J.M.; Khaneghah, A.M.; Barba, F.J. Emerging techniques in bioethanol production: From distillation to waste valorization. Green Chem. 2019. [Google Scholar] [CrossRef]
- Bankar, S.B.; Survase, S.A.; Ojamo, H.; Granström, T. Biobutanol: The outlook of an academic and industrialist. RSC Adv. 2013, 3, 24734. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Kumar, R.P.; Elavazhagan, S.; Barathiraja, B.; Jayakumar, M.; Varjani, S.J. Utilization of Crude Glycerol from Biodiesel Industry for the Production of Value-Added Bioproducts. In Waste to Wealth; Springer: Singapore, 2017; pp. 65–82. [Google Scholar]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Javier Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Leitner, W.; Klankermayer, J.; Pischinger, S.; Pitsch, H.; Kohse-Höinghaus, K. Advanced biofuels and beyond: Chemistry solutions for propulsion and production. Angew. Chem. Int. Ed. 2017, 56, 5412–5452. [Google Scholar] [CrossRef]
- Bhanja, P.; Bhaumik, A. Porous nanomaterials as green catalyst for the conversion of biomass to bioenergy. Fuel 2016, 185, 432–441. [Google Scholar] [CrossRef]
- Safari, M.; Nikazar, M.; Dadvar, M. Photocatalytic degradation of methyl tert-butyl ether (MTBE) by Fe-TiO2 nanoparticles. J. Ind. Eng. Chem. 2013, 19, 1697–1702. [Google Scholar] [CrossRef]
- Horvath, T.; Seiler, M.; Hunger, M. A comparative study of methyl-tert-butyl ether synthesis on zeolites HY, HBeta, HBeta/F and HZSM-5 by in situ MAS NMR spectroscopy under flow conditions and on-line gas chromatography. Appl. Catal. A Gen. 2000, 193, 227–236. [Google Scholar] [CrossRef]
- Goodwin, J.G., Jr.; Natesakhawat, S.; Nikolopoulos, A.A.; Kim, S.Y. Etherification on Zeolites: MTBE synthesis. Catal. Rev. 2002, 44, 287–320. [Google Scholar] [CrossRef]
- Nikolopoulos, A.A.; Kegelbauer, A.; Goodwin, J.G., Jr.; Marcelin, G. Effect of dealumination on the catalytic activity of acid zeolites for the gas phase synthesis of MTBE. Appl. Catal. A: Gen. 1994, 119, 69–81. [Google Scholar] [CrossRef]
- Xu, B.; Bordiga, S.; Prins, R.; van Bokhoven, J.A. Effect of framework Si/Al ratio and extra-framework aluminum on the catalytic activity of Y zeolite. Appl. Catal. A: Gen. 2007, 333, 245–253. [Google Scholar] [CrossRef]
- Nikolopoulos, A.A.; Kegelbauer, A.; Goodwin, J.G., Jr.; Marcelin, G. Gas phase synthesis of MTBE on triflic acid modified zeolites. J. Catal. 1996, 158, 76–82. [Google Scholar] [CrossRef]
- Nikolopoulos, A.A.; Kegelbauer, A.; Goodwin, J.G., Jr.; Marcelin, G. Gas phase synthesis of MTBE on fluoride-modified zeolites. Catal. Lett. 1996, 39, 173–178. [Google Scholar] [CrossRef]
- Collignon, F.; Mariani, M.; Moreno, S.; Remy, M.; Poncelet, G. Gas Phase Synthesis of MTBE from Methanol and Isobutene over Dealuminated Zeolites. J. Catal. 1997, 166, 53–66. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, Q.-H.; Lu, X.-H.; Ma, X.-T.; Su, K.-X. Gas-phase catalytic synthesis of MTBE from MeOH and ButOH over various microporous H-zeolites. Indian J. Chem. 2009, 48, 788–792. [Google Scholar]
- Xia, Q.-H.; Hidajat, K.; Kawi, S. Structure, Acidity, and Catalytic Activity of Mesoporous Acid Catalysts for the Gas-Phase Synthesis of MTBE from MeOH and ButOH. J. Catal. 2002, 209, 433–444. [Google Scholar] [CrossRef]
- Yee, K.F.; Mohamed, A.R.; Tan, S.H. A review on the evolution of ethyl tert-butyl ether (ETBE) and its future prospects. Renew. Sustain. Energy Rev. 2013, 22, 604–620. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Ezhova, N.N.; Yashina, O.V. Catalysis in the Dispersed Phase: Slurry Technology in the Synthesis of Dimethyl Ether. Petroleum Chem. 2017, 57, 553–570. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Surya Prakash, G.K. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Catalysis Chemistry of dimethyl ether synthesis. ACS Catal. 2014, 4, 3346–3356. [Google Scholar] [CrossRef]
- Kang, M.; Bhen, A. Kinetics and mechanisms of alcohol dehydration pathways on alumina materials. Catal. Sci. Technol. 2016, 6, 6667–6678. [Google Scholar] [CrossRef]
- Larmier, K.; Nicolle, A.; Chizallet, C.; Cadran, N.; Maury, S.; Lamic-Humblot, A.-F.; Marceau, E.; Lauron-Pernot, H. Influence of Coadsorbed Water and Alcohol Molecules on Isopropyl Alcohol Dehydration on γ-Alumina: Multiscale Modeling of Experimental Kinetic Profiles. ACS Catal. 2016, 6, 1905–1920. [Google Scholar] [CrossRef]
- Seo, C.W.; Jung, K.D.; Lee, K.Y.; Yoo, K.S. Influence of Structure Type of Al2O3 on Dehydration of Methanol for Dimethyl Ether Synthesis. Ind. Eng. Chem. Res. 2008, 47, 6573–6578. [Google Scholar] [CrossRef]
- Khom-in, J.; Praserthdam, P.; Panpranot, J.; Mekasuwandumrong, O. Dehydration of methanol to dimethyl ether over nanocrystalline Al2O3 with mixed γ- and χ-crystalline phases. Catal. Commun. 2008, 9, 1955–1958. [Google Scholar] [CrossRef]
- Ramos, F.S.; Duarte de Farias, A.M.; Borges, L.E.P.; Monteiro, J.L.; Fraga, M.A.; Sousa-Aguiar, E.F.; Appel, L.G. Role of dehydration catalyst acid properties on one-step DME synthesis over physical mixtures. Catal. Today 2005, 101, 39–44. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Jun, K.-W.; Kim, J.-W.; Roh, H.-S. Vapour phase dehydration of crude methanol to dimethyl ether over Na-modified H-ZSM-5 catalysts. Appl. Catal. A: Gen. 2004, 276, 251–255. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, B.; Hou, Z.; Fei, J.; Lou, H.; Zheng, X. Dimethyl ether synthesis via methanol and syngas over rare earthmetals modified zeolite Y and dual Cu-Mn-Zn catalysts. Fuel 2007, 86, 2707–2713. [Google Scholar] [CrossRef]
- Sutter, M.; Da Silva, E.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies. Chem. Rev. 2015, 115, 8609–8651. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Song, H.; Chen, J. Propylsulfonic Acid Functionalized SBA-15 Mesoporous silica as efficient catalysts for the acetalization of glycerol. Catalysts 2018, 8, 297. [Google Scholar] [CrossRef]
- Garcìa, E.; Laca, M.; Perez, E.; Garrido, A.; Peinado, J. New Class of Acetal Derived from Glycerin as a Biodiesel Fuel Component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Bajus, M. tert-Butylation of glycerol catalysed by ion-exchange resins. Appl. Catal. A: Gen. 2003, 45, 54–57. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Kaszonyi, A.; Bajus, M. Etherification of glycerol and ethylene glycol by isobutylene. Appl. Catal. A: Gen. 2007, 328, 1–13. [Google Scholar] [CrossRef]
- Karinen, R.S.; Krause, A.O.I. New biocomponents from glycerol. Appl. Catal. A: Gen. 2006, 306, 128–133. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; Di Blasi, O. Catalytic etherification of glycerol by tert-butyl alcohol to produce oxygenated additives for diesel fuel. Appl. Catal. A: Gen. 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Estevez, R.; López, M.I.; Jiménez-Sanchidrián, C.; Luna, D.; Romero-Salguero, F.J. Etherification of glycerol with tert-butyl alcohol over sulfonated hybrid silicas. Appl. Catal. A: Gen. 2016, 526, 155–166. [Google Scholar] [CrossRef]
- Manjunathan, P.; Kumar, M.; Churipard, S.R.; Sivasankaran, S.; Shanbhag, G.V.; Maradur, S.P. Catalytic etherification of glycerol to tert-butyl glycerol ethers using tert-butanol over sulfonic acid functionalized mesoporous polymer. RSC Adv. 2016, 6, 82654–82660. [Google Scholar] [CrossRef]
- Magar, S.; Kamble, S.; Mahanraj, G.T.; Jana, S.K.; Rode, C. Solid-Acid-Catalysed etherification of glycerol to potential fuel additives. Energy Fuel 2017, 31, 12272–12277. [Google Scholar] [CrossRef]
- Pariente, S.; Tanchoux, N.; Fajula, F. Etherification of glycerol with ethanol over solid acid catalysts. Green Chem. 2008, 11, 1256–1261. [Google Scholar] [CrossRef]
- Mravec, S.; Turana, T.; Filková, A.; Mikesková, N.; Volkovicsová, E.; Onyestyák, G.; Harnos, S.; Lónyi, F.; Valyon, J.; Kaszonyi, A. Catalytic etherification of bioglycerol with bioethanol over H-Beta, H-Y and H-MOR zeolites. Fuel Process. Technol. 2017, 59, 111–117. [Google Scholar] [CrossRef]
- Pinto, B.P.; de Lyra, J.T.; Nascimento, J.A.C.; Mota, C.J.A. Ethers of glycerol and ethanol as bioadditives for biodiesel. Fuel 2016, 168, 76–80. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Paniagua, M.; Morales, G.; Muñoz, P. Etherification of biodiesel-derived glycerol with ethanol for fuel formulation over sulfonic modified catalysts. Bioresour. Technol. 2012, 103, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Etherification of Biodiesel- based glycerol with bioethanol over tungstophosphoric acid to synthesize glyceryl ethers. Energy Fuels 2011, 25, 3186–3191. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Batch reactor coupled with water permselective membrane: Study of glycerol etherification reaction with butanol. Chem. Eng. J. 2015, 282, 187–193. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kadlec, D.; Velvarská, R.; Kubička, D. Using Mg-Al Mixed Oxide and Reconstructed Hydrotalcite as Basic Catalysts for Aldol Condensation of Furfural and Cyclohexanone. ChemCatChem 2018, 10, 1464–1475. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Bulánek, R.; Frolich, K.; Čejka, J.; Kubička, D. Aldol condensation of furfural with acetone over ion-exchanged and impregnated potassium BEA zeolites. J. Mol. Catal. A: Gen. 2016, 424, 358–368. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Bell, A.T. Syntheses of Biodiesel Precursors: Sulfonic Acid Catalysts for Condensation of Biomass-Derived Platform Molecules. ChemSusChem 2014, 7, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of γ-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Gruter, G.J.M.; Dautzenberg, F. Method for the Synthesis of 5-Alkoxymethylfurfural Ethers and Their Use. EP 2007 1834950 A1; Furanix Technologies BV [NL], 19 September 2007. [Google Scholar]
- Gruter, G.-J.; de Jong, E. Furanics: Novel fuel options from carbohydrates. Biofuels Technol. 2009, 1, 11–17. [Google Scholar]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural-A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. Direct, High-yield conversion of cellulose into Biofuel. Angew. Chem. Int. Ed. 2008, 47, 7924–7926. [Google Scholar] [CrossRef] [PubMed]
- Mascal, M.; Nikitin, E.B. Towards the efficient, total glycan utilization of biomass. ChemSusChem 2009, 2, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Centi, G.; Macario, A.; Aloise, A.; Giordano, G. Etherification of 5-hydroxymethyl-2-furfural (HMF) with ethanol to biodiesel components using mesoporous solid acid catalysts. Catal. Today 2011, 175, 435–441. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Bell, A. Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential biodiesel candidates. Green Chem. 2012, 14, 1626–1634. [Google Scholar] [CrossRef]
- Johnson, W.S.; Werthemann, L.; Bartlett, W.R.; Brockson, T.J.; Li, T.; Faulkner, D.J.; Petersen, M.R. Simple Stereoselective Version of the Claisen Rearrangement Leading to trans-Trisubstituted Olefinic Bonds. Synthesis of Squalene. J. Am. Chem. Soc. 1970, 92, 741. [Google Scholar] [CrossRef]
- Sampath Kumar, H.M.; Joyasawal, S.; Reddy, B.V.S.; Chakravarthy, P.; Krishna, A.D.; Yadav, J.S. Reaction of orthoesters with alcohols in the presence of acidic catalysts: A study. Indian J. Chem. 2005, 44B, 1686–1692. [Google Scholar]
- Chaffey, D.R.; Davies, T.E.; Taylor, S.H.; Graham, A.E. Etherification Reactions of Furfuryl Alcohol in the Presence of Orthoesters and Ketals: Application to the Synthesis of Furfuryl Ether. ACS Sustain. Chem. Eng. 2018, 6, 4996–5002. [Google Scholar] [CrossRef]
- Li, X.-L.; Zhang, K.; Chen, A.-Y.; Li, C.; Li, F.; Xu, H.-J.; Fu, Y. A cobalt catalyst for reductive etherification of 5-hydroxymethyl-furfural to 2,5-bis(methoxymethyl)furan under mild conditions. Green Chem. 2018, 20, 1095–1105. [Google Scholar] [CrossRef]
- Pizzi, R.; van Putten, R.-J.; Brust, H.; Perathoner, S.; Centi, G.; van der Waal, J.C. High—Throughput Screening of Heterogeneous Catalysts for the Conversion of Furfural to Bio-Based Fuel Components. Catalysts 2015, 5, 2244–2257. [Google Scholar] [CrossRef]
- Bethmont, V.; Montassier, C.; Marecot, P. Ether synthesis from alcohol and aldehydes in the presence of hydrogen and palladium deposited on charcoal. J. Mol. Catal. A 2000, 152, 133–140. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Q.; Guan, Y.; Wu, P. Facile synthesis of furfuryl ethyl ether in high yield via reductive etherification of furfurol in ethanol over Pd/C under mild conditions. Green Chem. 2018, 20, 2110–2117. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Liu, X.; Zhang, Y.; Fu, Y. Hydrogenation of biomass-derived furfurol to tetrahydrofurfuryl alcohol over hydroxyapatite-supported Pd catalyst under mild conditions. Ind. Eng. Chem. Res. 2017, 56, 8843–8849. [Google Scholar] [CrossRef]
- Boronat, R.M.; Corma, A.; Renz, M. Mechanism of the Meerwein-Ponndorf-Verley-Oppenauer (MPVO) Redox Equilibrium on Sn- and Zr-Beta Zeolite Catalysts. J. Phys. Chem. B 2006, 110, 21168–21174. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Valencia, S. Water-resistant solid Lewis acid catalysts: Meerwein-Ponndorf-Verley and Oppenauer reactions catalyzed by tin-beta zeolite. J. Catal. 2003, 215, 294–304. [Google Scholar] [CrossRef]
- Corma, A.; Renz, M. A General Method for the Preparation of Ethers Using Water-Resistant Solid Lewis Acids. Angew. Chem. Int. Ed. 2007, 46, 298–300. [Google Scholar] [CrossRef]
- Jae, J.; Mahmoud, E.; Lobo, R.F.; Vlachos, D.G. Cascade of liquid-phase catalytic transfer hydrogenation and etherification of 5-hydroxymethylfurfural to potential biodiesel components over Lewis acid zeolites. ChemCatChem 2014, 6, 508–513. [Google Scholar] [CrossRef]
- Luo, J.; Yu, J.; Gorte, R.J.; Mahmoud, E.; Vlachos, D.G.; Smith, M.A. The effect of oxide acidity on HMF etherification. Catal. Sci. Technol. 2014, 4, 3074–3081. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Moreno, J.; Segura, Y.; Paniagua, M.; Cambra, A.; Hernandez, B. Zr-SBA-15 Lewis Acid Calayst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts 2015, 5, 1911–1927. [Google Scholar] [CrossRef]
- Nguyen, H.; Xiao, N.; Daniels, S.; Marcella, N.; Timoshenko, J.; Frenkel, A.; Vlachos, D.G. Role of Lewis and Bronsted acidity in metal chloride catalysis in organic media: Reductive etherification of furanics. ACS Catal. 2017, 7, 7363–7370. [Google Scholar] [CrossRef]
- Shinde, S.; Rode, C. Cascade reductive etherification of bioderived aldehydes over Zr-based catalyst. ChemSusChem 2017, 10, 4090–4101. [Google Scholar] [CrossRef] [PubMed]
- Scotti, N.; Zaccheria, F.; Bisio, C.; Vittoni, C.; Ravasio, N. Switching Selectivity in the Hydrogen Transfer Reduction. ChemistrySelect 2018, 3, 8344–8348. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the Role of Low Coordination Sites in a Cu Metal Nanoparticle: A Step toward the Selective Synthesis of Second Generation Biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Zaccheria, F.; Psaro, R.; Ravasio, N. Bifunctional copper catalysts for an atom efficient ether synthesis. Tetrahedron Lett. 2009, 50, 5221–5224. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudolignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Hu, X.; Westerhof, R.J.M.; Wu, L.; Dong, D.; Li, C.-Z. Upgrading biomass-derived furans via acid-catalysis/hydrogenation: The remarkable difference between water and methanol as the solvent. Green Chem. 2015, 17, 219–224. [Google Scholar] [CrossRef]
- Barbera, K.; Lanzafame, P.; Pistone, A.; Millesi, S.; Malandrino, G.; Gulino, A.; Perathoner, S.; Centi, G. The role of oxide location in HMF etherification with ethanol over sulfated ZrO2 supported on SBA-15. J. Catal. 2015, 323, 19–32. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, L.; Yang, M.; Chen, H.; Holdren, S.; Zhu, G.; Tran, D.T.; Yao, C.; Sun, R.; Pan, Y.; et al. Three-step cascade over a single catalyst: Synthesis of 5-(ethoxymethyl)furfural from glucose over a hierarchical lamellar multi-functional zeolite catalyst. J. Mater. Chem. A 2018, 6, 7693–7705. [Google Scholar] [CrossRef]
- Xiang, B.; Wang, Y.; Qi, T.; Yang, H.-Q.; Hu, C.-W. Promotion catalytic role on Bronsted acid for the sequential dehydration-etherification of fructose to 5-ethoxymethylfurfural. J. Catal. 2017, 352, 586–598. [Google Scholar] [CrossRef]
- Zaccheria, F.; Scotti, N.; Ravasio, N. The Role of Copper in the Upgrading of Bioalcohols. ChemCatChem 2018, 10, 1526–1535. [Google Scholar] [CrossRef]
- Seemala, B.; Kumar, R.; Cai, C.M.; Wyman, C.E.; Christopher, P. Single-step catalytic conversion of furfural to 2-pentanol over bimetallic Co-Cu catalysts. React. Chem. Eng. 2019, 4, 261–267. [Google Scholar] [CrossRef]







| Ether | Structure | Bio-moiety | Fuel sector |
|---|---|---|---|
| Dimethyl ether DME | 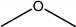 | Methanol | Gasoline and diesel blend |
| Diethyl ether DEE |  | Ethanol | Gasoline and diesel blend |
| Dibutyl ether DBE | 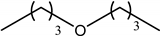 | Butanol | Gasoline and diesel blend |
| Methyl tert-butyl ether MTBE |  | methanol | Octane booster for gasoline |
| Ethyl tert-butyl ether ETBE |  | ethanol | Octane booster for gasoline |
| Tert-amyl methylether TAME | 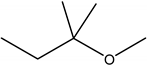 | nethanol | Octane booster for gasoline |
| Tert-amyl ethyl ether TAEE |  | ethanol | Octane booster for gasoline |
| di- and tri-tert-butyl glycerol ether DTBG and TTBG |  | Glycerol | Diesel additives |
| di- and tri-ethyl glycerol ether DEG and TEG | 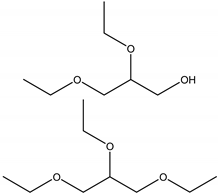 | Glycerol ethanol | Diesel additives |
| Alkoxymethyl furan | 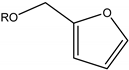 | Furfural alcohol | Diesel blend |
| Alkoxymethyl furfural | 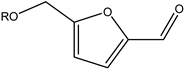 | HMF alcohol | Diesel blend |
| 2,5-bis(alkoxymethyl)furan | 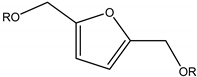 | HMF alcohol | Diesel blend |
| Entry | Catalyst | Alcohol | T (°C) | Product | Conversion (%) | Selectivity (%) a | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Amberlyst A35 | ethanol | 150 | Monoethyl glycerol ether | 52 | 90(10) | [44] |
| 2 | BEA 25 | ethanol | 200 | Monoethyl glycerol ether | 57 | 75(25) | [44] |
| 3 | Amberlyst-15 | ethanol | 180 | - | 96 | 65(19; 6) | [46] |
| 4 | H-Beta | ethanol | 180 | - | 92 | 71(17; 12) | [46] |
| 5 | Ar-SBA-15 | ethanol | 200 | Monoethyl glycerol ether | 73 | 54 (14) | [47] |
| 6 | H3PW12O40 (HPW) | ethanol | 160 | Monoethyl glycerol ether | 97 | 62(28;10) | [48] |
| 7 | HPW/SiO2 | ethanol | 160 | Monoethyl glycerol ether | 91 | 67 (23; 9) | [48] |
| Entry | substrate | Catalyst | Reagent | T (°C) | Conv | Yield % | Ref |
|---|---|---|---|---|---|---|---|
| 1 | HMF | Zr-SBA-15 | ethanol | 140 | 100 | 76 | [60] |
| 2 | HMF | Amberlyst | ethanol | 110 | 100 | 71 | [61] |
| 3 | Furfuryl alcohol | ZSM-5 | ethanol | 125 | 80 | 40 | [57] |
| 4 | Furfuryl alcohol | ZSM-5 | CH(OMe)3 | 40 | 92 | 73 | [64] |
| 5 | Furfuryl alcohol | ZSM-5 | CH(OEt)3 | 40 | 57 | 42 | [64] |
| 6 | Furfuryl alcohol | ZSM-5 | CH(OPr)3 a | 40 | 54 | 43 | [64] |
| 7 | Furfuryl alcohol | ZSM-5 | CH(OBu)3 a | 40 | 49 | 37 | [64] |
| Catalyst | Reagent | Reduction Condtions | Alcohol | Product | T (°C) | Conv % | Yield % | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PtSn/Al2O3 + Amberlyst15 | HMF | H2 1.4 MPa | ethanol |  | 60 | - | 64 | [61] |
| 2 | - | HMF | H2 1.4 MPa | n-butanol | 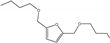 | 60 | - | 47 | [61] |
| 3 | Reduced Co | HMF | H2 2 MPa | Methanol | 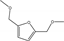 | 140 | 100 | 98.5 | [65] |
| 4 | Pd/charcoal | furfural | H2 5 MPa | methanol | 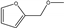 | 80 –120 | - | 77 a | [66] |
| 5 | Pd/C | furfural | H2 0.3 MPa | ethanol | 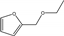 | 60 | 98 | 81 | [68] |
| 6 | Pd-HAP | furfural | H2 1 MPa | 2-propanol | 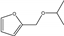 | 40 | 98 | 60 | [69] |
| 7 | Sn-Beta | HMF | HT | 2-propanol |  | 180 | 70 | 87 | [74] |
| 8 | Zr-Mont + ZrO(OH)2 | HMF | HT | 2-propanol | 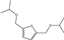 | 160 | 100 | 95 | [77] |
| 9 | Zr-Mont + ZrO(OH)2 | HMF | HT | 2-butanol |  | 150 | 100 | 96 | [77] |
| 10 | Zr-Mont + ZrO(OH)2 | HMF | HT | n-butanol | 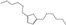 | 150 | 100 | 49 | [77] |
| 11 | Zr-Mont + ZrO(OH)2 | HMF | HT | ethanol |  | 150 | 100 | 0 | [77] |
| 12 | Zr-Mont + ZrO(OH)2 | furfural | HT | 2-propanol | 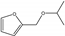 | 100 | 100 | 67 | [77] |
| 13 | Zr-Mont + ZrO(OH)2 | furfural | HT | 2-butanol | 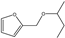 | 100 | 100 | 69 | [77] |
| 14 | Zr-SBA-15 | furfural | HT | 2-propanol | 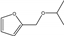 | 130 | 95 | 84 | [75] |
| 15 | SiO2-ZrO2 | furfural | HT | 2-butanol | 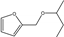 | 140 | 80 | 81 | [78] |
| 16 | Cu/SiO2 | furfural | HT | 2-butanol | 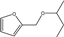 | 180 | 72 | 50 | [78] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccheria, F.; Scotti, N.; Ravasio, N. Solid Acids for the Reaction of Bioderived Alcohols into Ethers for Fuel Applications. Catalysts 2019, 9, 172. https://doi.org/10.3390/catal9020172
Zaccheria F, Scotti N, Ravasio N. Solid Acids for the Reaction of Bioderived Alcohols into Ethers for Fuel Applications. Catalysts. 2019; 9(2):172. https://doi.org/10.3390/catal9020172
Chicago/Turabian StyleZaccheria, Federica, Nicola Scotti, and Nicoletta Ravasio. 2019. "Solid Acids for the Reaction of Bioderived Alcohols into Ethers for Fuel Applications" Catalysts 9, no. 2: 172. https://doi.org/10.3390/catal9020172
APA StyleZaccheria, F., Scotti, N., & Ravasio, N. (2019). Solid Acids for the Reaction of Bioderived Alcohols into Ethers for Fuel Applications. Catalysts, 9(2), 172. https://doi.org/10.3390/catal9020172






