Preparation of Amylose-Carboxymethyl Cellulose Conjugated Supramolecular Networks by Phosphorylase-Catalyzed Enzymatic Polymerization
Abstract
:1. Introduction
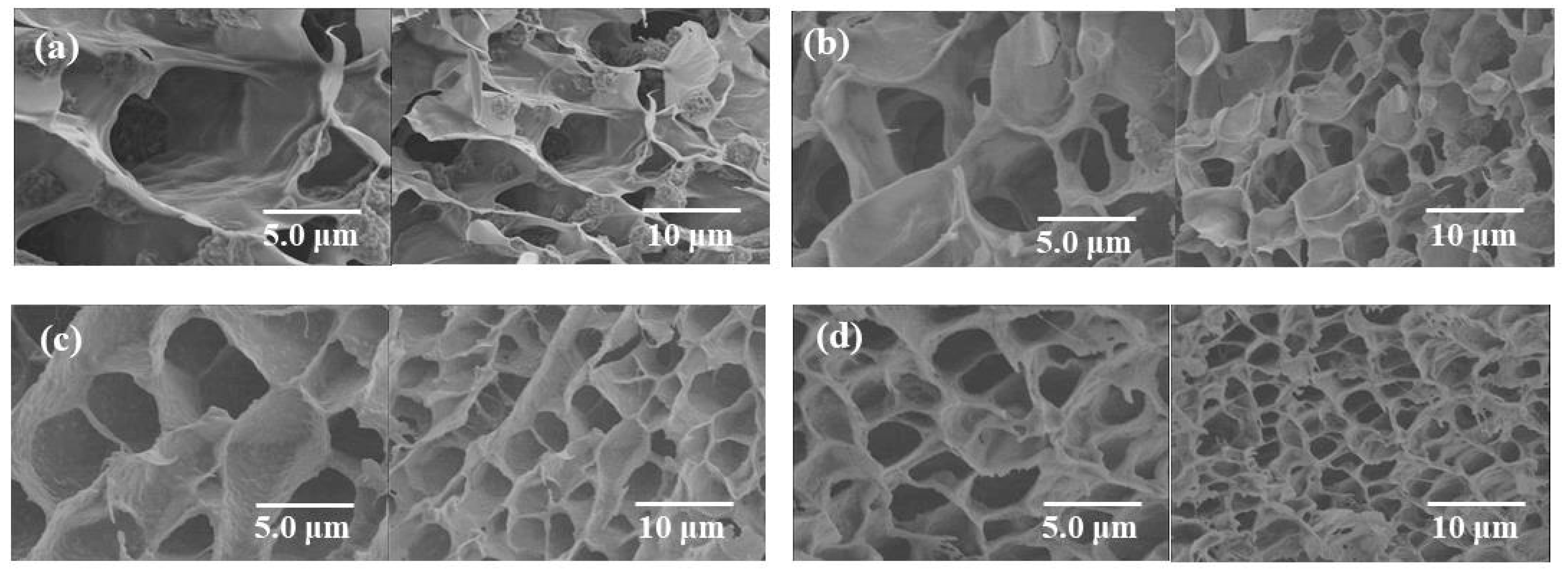
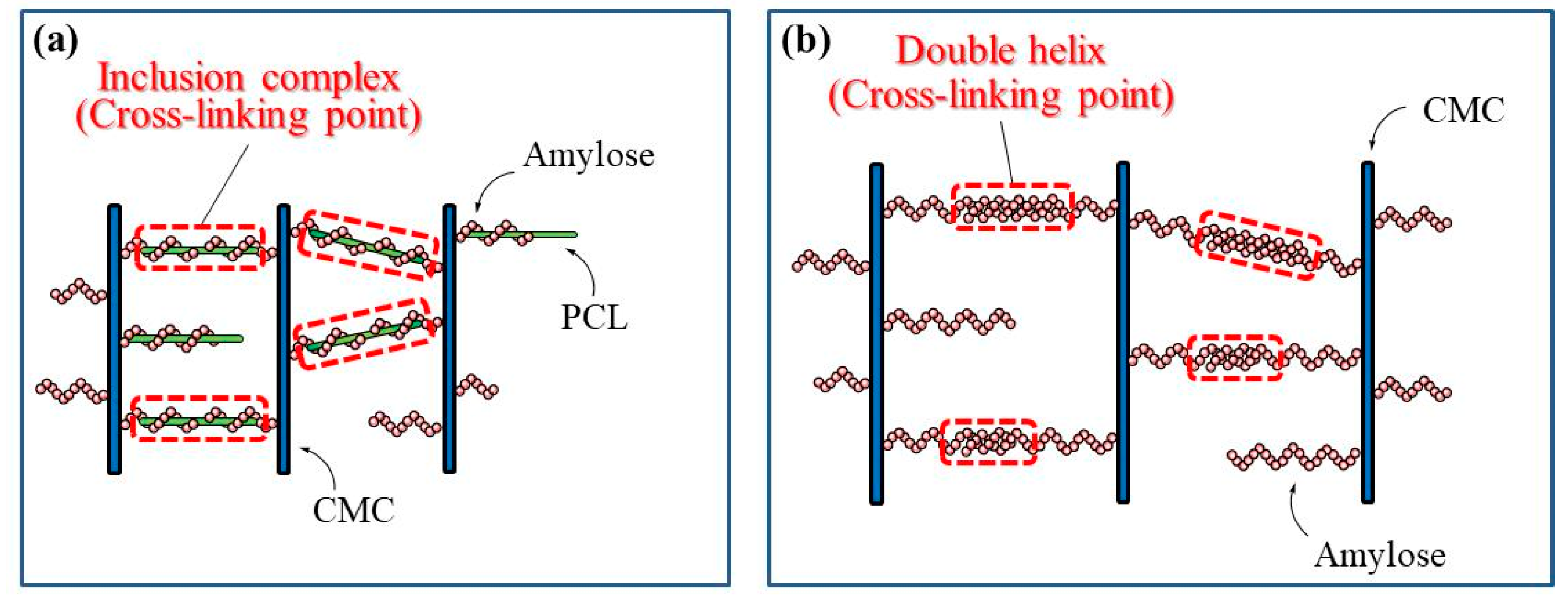
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Phosphorylase-Catalyzed Enzymatic Polymerization of G-1-P Using G7-Grafted PGA in the Presence of PCL
3.3. Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Kobayashi, S. Polymer synthesis by enzymatic catalysis. Curr. Opin. Chem. Biol. 2010, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111, 4308–4345. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Precision synthesis of functional polysaccharide materials by phosphorylase-catalyzed enzymatic reactions. Polymers 2016, 8, 138. [Google Scholar] [CrossRef]
- Kadokawa, J. a-Glucan phosphorylase: A useful catalyst for precision enzymatic synthesis of oligo- and polysaccharides. Curr. Org. Chem. 2017, 21, 1192–1204. [Google Scholar] [CrossRef]
- Ziegast, G.; Pfannemuller, B. Linear and star-shaped hybrid polymers. 4. Phosphorolytic syntheses with di-functional, oligo-functional and multifunctional primers. Carbohydr. Res. 1987, 160, 185–204. [Google Scholar] [CrossRef]
- Ohdan, K.; Fujii, K.; Yanase, M.; Takaha, T.; Kuriki, T. Enzymatic synthesis of amylose. Biocatal. Biotransform. 2006, 24, 77–81. [Google Scholar] [CrossRef]
- Yanase, M.; Takaha, T.; Kuriki, T. a-Glucan phosphorylase and its use in carbohydrate engineering. J. Sci. Food Agric. 2006, 86, 1631–1635. [Google Scholar] [CrossRef]
- Imberty, A.; Chanzy, H.; Perez, S.; Buleon, A.; Tran, V. The double-helical nature of the crystalline part of A-starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef]
- Imberty, A.; Perez, S. A revisit to the three-dimensional structure of B-type starch. Biopolymers 1988, 27, 1205–1221. [Google Scholar] [CrossRef]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kaneko, Y.; Nakaya, A.; Tagaya, H. Formation of an amylose-polyester inclusion complex by means of phosphorylase-catalyzed enzymatic polymerization of a-D-glucose 1-phosphate monomer in the presence of poly(epsilon-caprolactone). Macromolecules 2001, 34, 6536–6538. [Google Scholar] [CrossRef]
- Kadokawa, J.; Nakaya, A.; Kaneko, Y.; Tagaya, H. Preparation of inclusion complexes between amylose and ester-containing polymers by means of vine-twining polymerization. Macromol. Chem. Phys. 2003, 204, 1451–1457. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kadokawa, J. Vine-twining polymerization: A new preparation method for well-defined supramolecules composed of amylose and synthetic polymers. Chem. Rec. 2005, 5, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kadokawa, J. Synthesis of nanostructured bio-related materials by hybridization of synthetic polymers with polysaccharides or saccharide residues. J. Biomater. Sci. Polym. Ed. 2006, 17, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kadokawa, J. Preparation of polymers with well-defined nanostructure in the polymerization field. In Modern Trends in Macromolecular Chemistry; Lee, J.N., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; pp. 199–217. [Google Scholar]
- Kadokawa, J. Preparation and applications of amylose supramolecules by means of phosphorylase-catalyzed enzymatic polymerization. Polymers 2012, 4, 116–133. [Google Scholar] [CrossRef]
- Kadokawa, J. Architecture of amylose supramolecules in form of inclusion complexes by phosphorylase-catalyzed enzymatic polymerization. Biomolecules 2013, 3, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Chemoenzymatic synthesis of functional amylosic materials. Pure Appl. Chem. 2014, 86, 701–709. [Google Scholar] [CrossRef]
- Orio, S.; Yamamoto, K.; Kadokawa, J. Preparation and material application of amylose-polymer inclusion complexes by enzymatic polymerization approach. Polymers 2017, 9, 729. [Google Scholar] [CrossRef]
- Kadokawa, J. Synthesis of amylose-grafted polysaccharide materials by phosphorylase-catalyzed enzymatic polymerization. In Biobased Monomers, Polymers, and Materials; Smith, P.B., Gross, R.A., Eds.; ACS Symposium Series 1105; American Chemical Society: Washington, DC, USA, 2012; pp. 237–255. [Google Scholar]
- Kadokawa, J. Synthesis of new polysaccharide materials by phosphorylase-catalyzed enzymatic α-glycosylations using polymeric glycosyl acceptors. In Green Polymer Chemistry: Biocatalysis and Materials II; Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; ACS Symposium Series 1144; American Chemical Society: Washington, DC, USA, 2013; pp. 141–161. [Google Scholar]
- Nishimura, T.; Akiyoshi, K. Amylose engineering: Phosphorylase-catalyzed polymerization of functional saccharide primers for glycobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, I.; Singhal, R. Poly (glutamic acid)—An emerging biopolymer of commercial interest. Bioresour. Technol. 2011, 102, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-g-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shouji, T.; Yamamoto, K.; Kadokawa, J. Chemoenzyamtic synthesis and self-assembling gelation behavior of amylose-grafted poly(g-glutamic acid). Int. J. Biol. Macromol. 2017, 97, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Shoji, T.; Yamamoto, K. Preparation of supramolecular network materials by means of amylose helical assemblies. Polymer 2018, 140, 73–79. [Google Scholar] [CrossRef]
- Orio, S.; Shoji, T.; Yamamoto, K.; Kadokawa, J.-I. Difference in macroscopic morphologies of amylosic supramolecular networks depending on guest polymers in vine-twining polymerization. Polymers 2018, 10, 1277. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O.; Williams, P.A. Food Polysaccharides and Their Applications, 2nd ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; 733p. [Google Scholar]
- Kadokawa, J.; Arimura, T.; Takemoto, Y.; Yamamoto, K. Self-assembly of amylose-grafted carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, D.; Takemoto, Y.; Yamamoto, K.; Kadokawa, J. Hierarchically self-assembled nanofiber films from amylose-grafted carboxymethyl cellulose. Fibers 2013, 2, 34–44. [Google Scholar] [CrossRef]
- Bhuiyan, S.H.; Rus’d, A.A.; Kitaoka, M.; Hayashi, K. Characterization of a hyperthermostable glycogen phosphorylase from Aquifex aeolicus expressed in Escherichia coli. J. Mol. Catal. B Enzym. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Yanase, M.; Takata, H.; Fujii, K.; Takaha, T.; Kuriki, T. Cumulative effect of amino acid replacements results in enhanced thermostability of potato type L a-glucan phosphorylase. Appl. Environ. Microbiol. 2005, 71, 5433–5439. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Taira, A.; Tomioka, T.; Okada, M. A catalytic approach for cationic living polymerization: Sc(OTf)3-catalyzed ring-opening polymerization of lactones. Macromolecules 2000, 33, 1497–1499. [Google Scholar] [CrossRef]
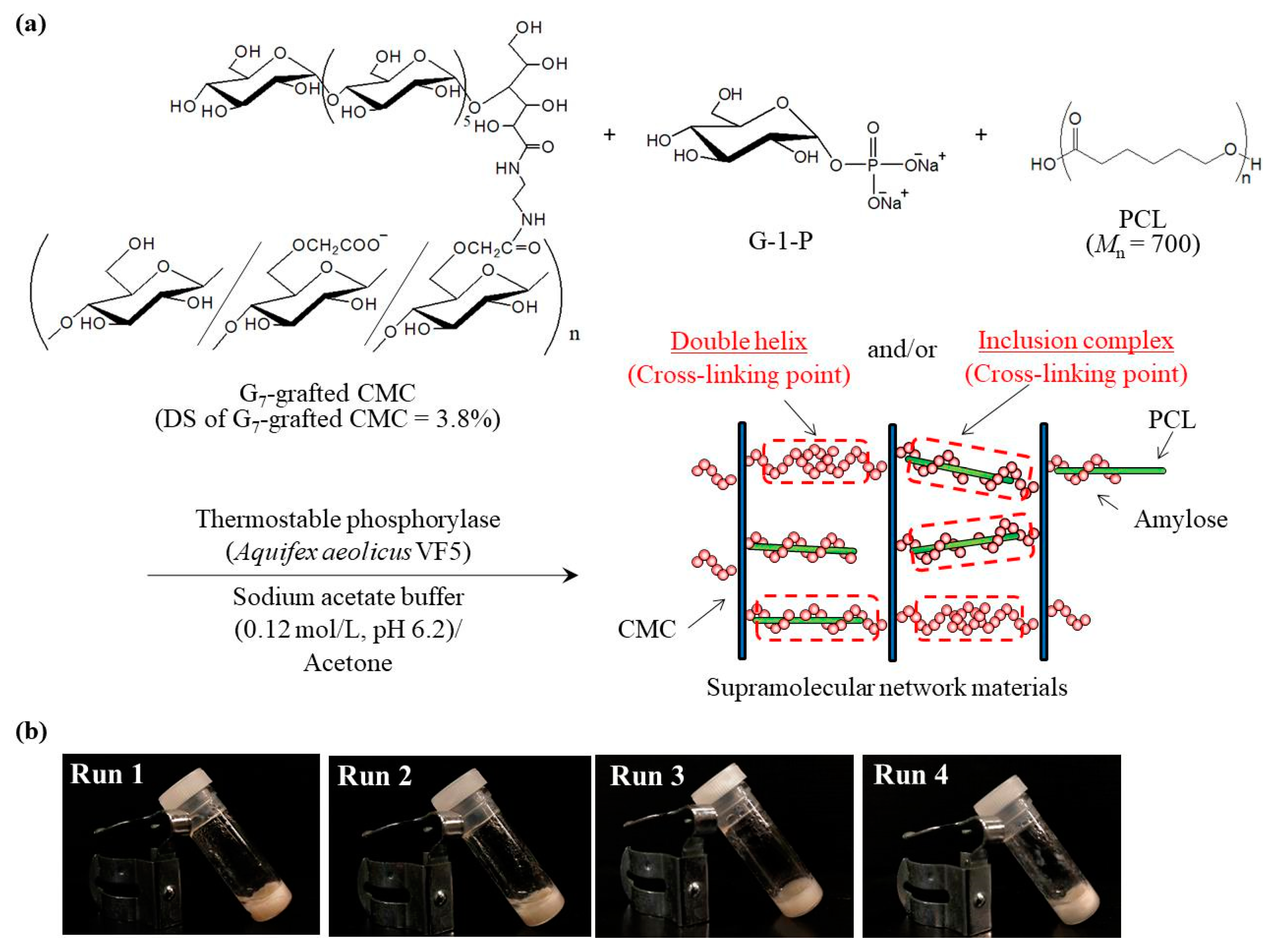



| Run | PCL/G7 | Conversion b (%) | Mn of Amylose b | Amylose Content b (wt%) | Water Content c (wt%) | PCL Content d (wt%) | IC: DH in Amylose e (wt ratio) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 93.3 | 16,000 | 70.1 | 93.2 | 0 | 0:100 |
| 2 | 3 | 86.7 | 15,000 | 66.6 | 86.4 | 3.3 | 43.7:56.3 |
| 3 | 5 | 83.3 | 14,000 | 63.6 | 85.4 | 5.7 | 80.0:20.0 |
| 4 | 10 | 80.0 | 14,000 | 61.7 | 85.2 | 6.8 | 98.4:1.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadokawa, J.-i.; Shoji, T.; Yamamoto, K. Preparation of Amylose-Carboxymethyl Cellulose Conjugated Supramolecular Networks by Phosphorylase-Catalyzed Enzymatic Polymerization. Catalysts 2019, 9, 211. https://doi.org/10.3390/catal9030211
Kadokawa J-i, Shoji T, Yamamoto K. Preparation of Amylose-Carboxymethyl Cellulose Conjugated Supramolecular Networks by Phosphorylase-Catalyzed Enzymatic Polymerization. Catalysts. 2019; 9(3):211. https://doi.org/10.3390/catal9030211
Chicago/Turabian StyleKadokawa, Jun-ichi, Takuya Shoji, and Kazuya Yamamoto. 2019. "Preparation of Amylose-Carboxymethyl Cellulose Conjugated Supramolecular Networks by Phosphorylase-Catalyzed Enzymatic Polymerization" Catalysts 9, no. 3: 211. https://doi.org/10.3390/catal9030211





