Highly Dispersed Ni Nanocatalysts Derived from NiMnAl-Hydrotalcites as High-Performing Catalyst for Low-Temperature Syngas Methanation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology of LDHs and Catalysts
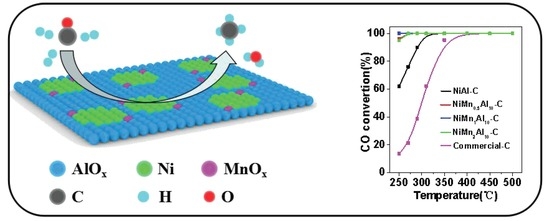
2.2. Catalytic Activity
3. Materials and Methods
3.1. Chemicals
3.2. Catalysts Preparation
3.3. Catalysts Characterization
3.4. Catalytic Performance Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Razzaq, R.; Li, C.; Zhang, S. Coke oven gas: Availability, properties, purification, and utilization in China. Fuel 2013, 113, 287–299. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Z.; Meng, X.; Tao, M. Synthesis, characterization and properties of anti-sintering nickel incorporated MCM-41 methanation catalysts. Fuel 2013, 109, 693–701. [Google Scholar] [CrossRef]
- Höhlein, B.; Menzer, R.; Range, J. High Temperature Methanation in the Long-distance Nuclear energy transport system. Appl. Catal. 1981, 1, 125–139. [Google Scholar] [CrossRef]
- Höhlein, B.; Niessen, J.; Schiebahn, H.J.R.; Vorwerk, M. Methane from synthesis gas and operation of high-temperature methanation. Nucl. Eng. Des. 1984, 78, 241–250. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Wissing, L.; Skjøth-Rasmussen, M.S. High temperature methanation: Catalyst considerations. Catal. Today 2013, 215, 233–238. [Google Scholar] [CrossRef]
- Ma, S.; Tan, Y.; Han, Y. Methanation of syngas over coral reef-like Ni/Al2O3 catalysts. J. Nat. Gas. Chem. 2011, 20, 435–440. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, F.; Li, J.; Chen, K.; Yao, Y.; Li, P.; Zhu, M.; Shi, Y.; Wang, Q.; Guo, X. High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation. Catalysts 2018, 8, 363. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced Investigation of CO Methanation over Ni/Al2O3Catalysts for Synthetic Natural Gas Production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, J.; Zhang, M.; Li, H.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Highly active and stable Ni/γ-Al2O3 catalysts selectively deposited with CeO2 for CO methanation. RSC Adv. 2014, 4, 16094–16103. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, F.; Zhong, Z.; Xu, G.; Su, F. Anti-sintering ZrO2-modified Ni/α-Al2O3 catalyst for CO methanation. RSC Adv. 2016, 6, 20979–20986. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, J.; Gu, F.; Lu, X.; Liu, Y.; Li, H.; Zhong, Z.; Liu, B.; Xu, G.; Su, F. One-pot synthesis of ordered mesoporous Ni–V–Al catalysts for CO methanation. J. Catal. 2015, 326, 127–138. [Google Scholar] [CrossRef]
- Lu, X.; Gu, F.; Liu, Q.; Gao, J.; Jia, L.; Xu, G.; Zhong, Z.; Su, F. Ni-MnOx Catalysts Supported on Al2O3-Modified Si Waste with Outstanding CO Methanation Catalytic Performance. Ind. Eng. Chem. Res. 2015, 54, 12516–12524. [Google Scholar] [CrossRef]
- Tang, H.; Li, S.; Gong, D.; Guan, Y.; Liu, Y. Bimetallic Ni-Fe catalysts derived from layered double hydroxides for CO methanation from syngas. Front. Chem. Sci. Eng. 2017, 11, 613–623. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Nickel Catalysts Supported on Barium Hexaaluminate for Enhanced CO Methanation. Ind. Eng. Chem. Res. 2012, 51, 10345–10353. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Zhang, M.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Template preparation of high-surface-area barium hexaaluminate as nickel catalyst support for improved CO methanation. RSC Adv. 2013, 3, 18156. [Google Scholar] [CrossRef]
- Jia, C.; Gao, J.; Li, J.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Nickel catalysts supported on calcium titanate for enhanced CO methanation. Catal. Sci. Technol. 2013, 3, 490–499. [Google Scholar] [CrossRef]
- Jin, G.; Gu, F.; Liu, Q.; Wang, X.; Jia, L.; Xu, G.; Zhong, Z.; Su, F. Highly stable Ni/SiC catalyst modified by Al2O3 for CO methanation reaction. RSC Adv. 2016, 6, 9631–9639. [Google Scholar] [CrossRef]
- Sun, J.; Feng, Q.; Liu, Q.; Ji, S.; Fang, Y.; Peng, X.; Wang, Z.-J. An Al2O3-Coated SiC-Supported Ni Catalyst with Enhanced Activity and Improved Stability for Production of Synthetic Natural Gas. Ind. Eng. Chem. Res. 2018, 57, 14899–14909. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Jiang, J.-X.; Jin, G.; Li, H.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. SiO2-stabilized Ni/t-ZrO2 catalysts with ordered mesopores: One-pot synthesis and their superior catalytic performance in CO methanation. Catal. Sci. Technol. 2016, 6, 3529–3543. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.; Dong, L.; Su, T.; Li, B.; Luo, X.; Xie, X.; Qin, Z.; Xu, C.; Ji, H. Mn Modified Ni/Bsentonite for CO2 Methanation. Catalysts 2018, 8, 646. [Google Scholar] [CrossRef]
- Feng, F.; Song, G.; Xiao, J.; Shen, L.; Pisupati, S.V. Carbon deposition on Ni-based catalyst with TiO2 as additive during the syngas methanation process in a fluidized bed reactor. Fuel 2019, 235, 85–91. [Google Scholar] [CrossRef]
- Dai, X.; Liang, J.; Ma, D.; Zhang, X.; Zhao, H.; Zhao, B.; Guo, Z.; Kleitz, F.; Qiao, S. Large-pore mesoporous RuNi-doped TiO2–Al2O3 nanocomposites for highly efficient selective CO methanation in hydrogen-rich reformate gases. Appl. Catal. B-Environ. 2015, 165, 752–762. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Perathoner, S.; Abate, S.; Centi, G.; Palkovits, R. Hydrotalcite based Ni-Fe/(Mg, Al)Ox catalysts for CO2 methanation-tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 2018, 8, 1016–1027. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, F.; Gao, J.; Li, H.; Xu, G.; Su, F. Coking-resistant Ni-ZrO2/Al2O3 catalyst for CO methanation. J. Energy. Chem. 2014, 23, 761–770. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, H.; Zhang, H.; Ying, W.; Fang, D. Ni-Ce-Al composite oxide catalysts synthesized by solution combustion method: Enhanced catalytic activity for CO methanation. Fuel 2015, 162, 16–22. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni-Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Bian, L.; Zhang, L.; Zhu, Z.; Li, Z. Methanation of carbon oxides on Ni/Ce/SBA-15 pretreated with dielectric barrier discharge plasma. Mol. Catal. 2018, 446, 131–139. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni-Cr-Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, Y.; Tian, Y.; Gu, F.; Zhong, Z.; Su, F. Ordered Mesoporous Ni-Fe-Al Catalysts for CO Methanation with Enhanced Activity and Resistance to Deactivation. Ind. Eng. Chem. Res. 2017, 56, 9809–9820. [Google Scholar] [CrossRef]
- Xu, L.; Lian, X.; Chen, M.; Cui, Y.; Wang, F.; Li, W.; Huang, B. CO2 methanation over Co-Ni bimetal-doped ordered mesoporous Al2O3 catalysts with enhanced low-temperature activities. Int. J. Hydrog. Energy 2018, 43, 17172–17184. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zhao, G.; Yang, H.; Yuan, M.; An, X.; Zhou, H.; Qiao, Y.; Tian, Y. CO2 methanation over ordered mesoporous NiRu-doped CaO-Al2O3 nanocomposites with enhanced catalytic performance. Int. J. Hydrog. Energy 2018, 43, 239–250. [Google Scholar] [CrossRef]
- Hayashi, K.; Miyao, T.; Tabira, Y.; Higashiyama, K. Low-temperature Selective CO Methanation over Unsupported Nickel Catalyst Covered by Silica Thin Layer. J. Jpn. Petrol. Inst. 2016, 59, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Dong, C.; Ping, D.; Geng, J.; Zhang, J.; Dong, X. Zr-Modified SBA-15 Supported Ni Catalysts with Excellent Catalytic Performance of CO Selective Methanation in H2-Rich Fuels. Catal. Lett. 2018, 148, 2967–2973. [Google Scholar] [CrossRef]
- Meng, F.; Song, Y.; Li, X.; Cheng, Y.; Li, Z. Catalytic methanation performance in a low-temperature slurry-bed reactor over Ni-ZrO2 catalyst: effect of the preparation method. J. Sol-Gel. Sci. Technol. 2016, 80, 759–768. [Google Scholar] [CrossRef]
- Cui, D.; Liu, J.; Yu, J.; Su, F.; Xu, G. Attrition-resistant Ni-Mg/SiO2-Al2O3 catalysts with different silica sources for fluidized bed syngas methanation. Int J Hydrog. Energy 2017, 42, 4987–4997. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Ji, F.; Li, M. Effect of ZrO2 on catalyst structure and catalytic methanation performance over Ni-based catalyst in slurry-bed reactor. Int. J. Hydrog. Energy 2015, 40, 8833–8843. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Ma, H.; Qian, W.; Zhang, H.; Ying, W. Antisintering and High-Activity Ni Catalyst Supported on Mesoporous Silica Incorporated by Ce/Zr for CO Methanation. Ind. Eng. Chem. Res. 2018, 57, 14406–14416. [Google Scholar] [CrossRef]
- Chen, G.; Gao, R.; Zhao, Y.; Li, Z.; Waterhouse, G.I.N.; Shi, R.; Zhao, J.; Zhang, M.; Shang, L.; Sheng, G.; et al. Alumina-Supported CoFe Alloy Catalysts Derived from Layered-Double-Hydroxide Nanosheets for Efficient Photothermal CO2 Hydrogenation to Hydrocarbons. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, J.; Liu, P.; Sun, H.; Zhuang, X.; Chen, M.; Feng, X. Accelerated Hydrogen Evolution Kinetics on NiFe-Layered Double Hydroxide Electrocatalysts by Tailoring Water Dissociation Active Sites. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Li, P.; Zhu, M.; Tian, Z.; Han, Y.; Zhang, Y.; Zhou, T.; Kang, L.; Dan, J.; Guo, X.; Yu, F.; et al. Two-Dimensional Layered Double Hydroxide Derived from Vermiculite Waste Water Supported Highly Dispersed Ni Nanoparticles for CO Methanation. Catalysts 2017, 7, 79. [Google Scholar] [CrossRef]
- Fan, Q.; Li, X.; Yang, Z.; Han, J.; Xu, S.; Zhang, F. Double-Confined Nickel Nanocatalyst Derived from Layered Double Hydroxide Precursor: Atomic Scale Insight into Microstructure Evolution. Chem. Mater. 2016, 28, 6296–6304. [Google Scholar] [CrossRef]
- Chen, H.; He, S.; Cao, X.; Zhang, S.; Xu, M.; Pu, M.; Su, D.; Wei, M.; Evans, D.G.; Duan, X. Ru-Cluster-Modified Ni Surface Defects toward Selective Bond Breaking between C-O and C-C. Chem. Mater. 2016, 28, 4751–4761. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhang, S.; Xu, S.; Zhou, J.; Wang, F.; Wei, M.; Evans, D.G.; Duan, X. Ni-In Intermetallic Nanocrystals as Efficient Catalysts toward Unsaturated Aldehydes Hydrogenation. Chem. Mater. 2013, 25, 3888–3896. [Google Scholar] [CrossRef]
- Li, Z.; Bian, L.; Zhu, Q.; Wang, W. Ni-based catalyst derived from Ni/Mg/Al hydrotalcite–like compounds and its activity in the methanation of carbon monoxide. Kinet. Catal. 2014, 55, 217–223. [Google Scholar] [CrossRef]
- Kim, W.Y.; Lee, Y.H.; Park, H.; Choi, Y.H.; Lee, M.H.; Lee, J.S. Coke tolerance of Ni/Al2O3 nanosheet catalyst for dry reforming of methane. Catal. Sci. Technol. 2016, 6, 2060–2064. [Google Scholar] [CrossRef]
- He, S.; Li, C.; Chen, H.; Su, D.; Zhang, B.; Cao, X.; Wang, B.; Wei, M.; Evans, D.G.; Duan, X. A Surface Defect-Promoted Ni Nanocatalyst with Simultaneously Enhanced Activity and Stability. Chem. Mater. 2013, 25, 1040–1046. [Google Scholar] [CrossRef]
- Mitchell, R.W.; Lloyd, D.C.; van de Water, L.G.A.; Ellis, P.R.; Metcalfe, K.A.; Sibbald, C.; Davies, L.H.; Enache, D.I.; Kelly, G.J.; Boyes, E.D.; et al. Effect of Pretreatment Method on the Nanostructure and Performance of Supported Co Catalysts in Fischer-Tropsch Synthesis. ACS Catal. 2018, 8, 8816–8829. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Evans, D.G.; Duan, X. Stoichiometric synthesis of pure MFe2O4 (M = Mg, Co and Ni) spinel ferrites from tailored layered double hydroxide (hydrotalcite–like) precursors. Chem. Mater. 2004, 16, 1597–1602. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal. Sci. Technol. 2013, 3, 2627–2633. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Z.; Wang, G.; Zhu, H.; Dong, M.; Li, S.; Wu, Z.; Li, Z.; Wu, Z.; Zhang, J.; et al. Catalytic performance of MnOx-NiO composite oxide in lean methane combustion at low temperature. Appl. Catal. B-Enriron. 2013, 129, 172–181. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Li, W.; Li, J.; Chen, J.; Chen, Y. Excellent low temperature performance for total benzene oxidation over mesoporous CoMnAl composited oxides from hydrotalcites. J. Mater. Chem. A 2016, 4, 8113–8122. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Centeno, M.A.; Ivanova, S.; Eloy, P.; Gaigneaux, E.M.; Odriozola, J.A. Cu-modified cryptomelane oxide as active catalyst for CO oxidation reactions. Appl. Catal. B 2012, 123–124, 27–35. [Google Scholar] [CrossRef]











| LDHs | d003/nm | d006/nm | d009/nm | d110/nm | a a = 2d110/nm | b c = (d003 + 2d006 + 3d009)/nm |
|---|---|---|---|---|---|---|
| NiAl-LDH | 0.741 | 0.378 | 0.255 | 0.151 | 0.302 | 2.258 |
| NiMn0.5Al10-LDH | 0.752 | 0.378 | 0.255 | 0.151 | 0.302 | 2.269 |
| NiMn1Al10-LDH | 0.758 | 0.378 | 0.255 | 0.151 | 0.302 | 2.275 |
| NiMn2Al10-LDH | 0.752 | 0.378 | 0.255 | 0.151 | 0.302 | 2.269 |
| Catalysts | a Wt% ± 0.1 | b SBET (m2·g−1) | c Vp (cm3·g−1) | d H2 Uptake (μmoL/g) | e De (%) ± 0.1 | ||
|---|---|---|---|---|---|---|---|
| Ni | Al | Mn | |||||
| NiAl-C | 61.7 | 7.3 | 0 | 76 | 0.2 | 534 | 10.2 |
| NiMn0.5Al10-C | 57.5 | 7.3 | 0.6 | 87 | 0.5 | 559 | 11.4 |
| NiMn1Al10-C | 57.8 | 7.7 | 1.7 | 69 | 0.7 | 631 | 12.8 |
| NiMn2Al10-C | 57.9 | 7.9 | 3.2 | 66 | 0.2 | 550 | 11.2 |
| Catalysts | Mn 2p2/3 Position (eV) | Mn4+/Mn3+ | O 1s Position (eV) | OII/(OII + OI) | |||
|---|---|---|---|---|---|---|---|
| Mn2+ | Mn3+ | Mn4+ | OI | OII | |||
| NiAl-C | - | - | - | - | 530.5 | 531.8 | 0.41 |
| NiMn0.5Al10-C | 637.8 | 642.8 | 647.7 | 0.33 | 530.5 | 531.8 | 0.49 |
| NiMn1Al10-C | 637.9 | 642.9 | 647.6 | 0.59 | 530.5 | 531.8 | 0.56 |
| NiMn2Al10-C | 637.9 | 643.0 | 647.8 | 0.48 | 530.4 | 531.7 | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.; Zhuang, J.; Du, J.; Gu, F.; Xu, G.; Zhong, Z.; Liu, Q.; Su, F. Highly Dispersed Ni Nanocatalysts Derived from NiMnAl-Hydrotalcites as High-Performing Catalyst for Low-Temperature Syngas Methanation. Catalysts 2019, 9, 282. https://doi.org/10.3390/catal9030282
Lu B, Zhuang J, Du J, Gu F, Xu G, Zhong Z, Liu Q, Su F. Highly Dispersed Ni Nanocatalysts Derived from NiMnAl-Hydrotalcites as High-Performing Catalyst for Low-Temperature Syngas Methanation. Catalysts. 2019; 9(3):282. https://doi.org/10.3390/catal9030282
Chicago/Turabian StyleLu, Bin, Jiahao Zhuang, Jinping Du, Fangna Gu, Guangwen Xu, Ziyi Zhong, Qing Liu, and Fabing Su. 2019. "Highly Dispersed Ni Nanocatalysts Derived from NiMnAl-Hydrotalcites as High-Performing Catalyst for Low-Temperature Syngas Methanation" Catalysts 9, no. 3: 282. https://doi.org/10.3390/catal9030282