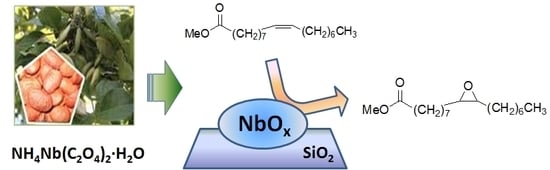
Epoxidation of Karanja (Millettia pinnata) Oil Methyl Esters in the Presence of Hydrogen Peroxide over a Simple Niobium-Containing Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Karanja Oil FAME Mixture
2.2. Preparation of the NbOx-SiO2 Catalyst
2.3. Characterization of the NbOx-SiO2 Catalyst
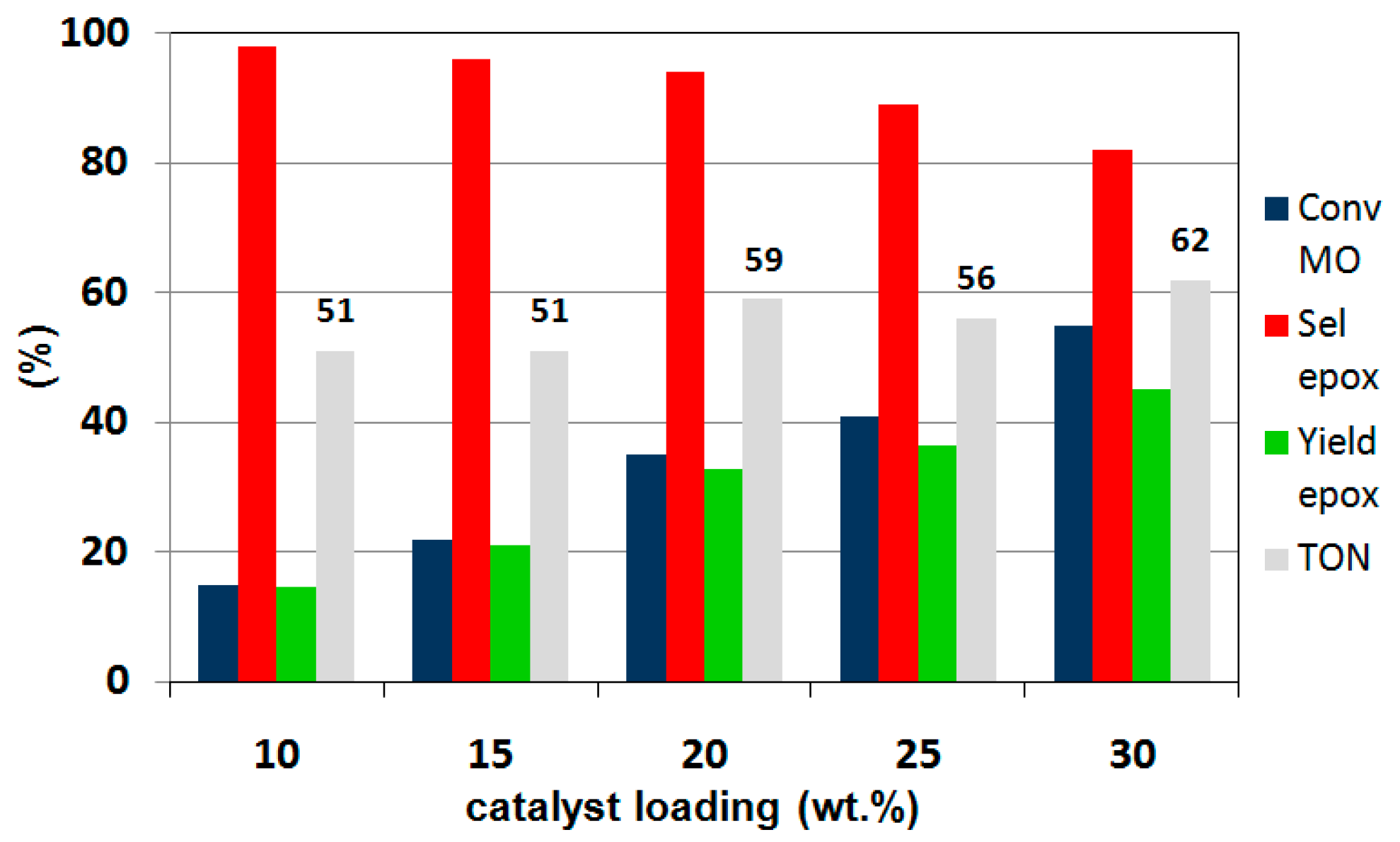
2.4. Catalytic Performance in the Liquid-Phase Epoxidation Methyl Oleate
2.5. Catalytic Performance in the Liquid-Phase Epoxidation Karanja Oil FAME Mixture
3. Materials and Methods
3.1. Materials and Reactants
3.2. Catalyst Preparation
3.3. Preparation of Karanja Oil FAME Mixture
3.4. Characterization of Karanja Oil FAME Mixture
3.5. Catalyst Characterization
3.6. Liquid-Phase Epoxidation Tests of Methyl Oleate and Karanja FAME Mixture
4. Conclusions
- (1)
- a conceptually simple, novel niobium-silica catalyst was prepared starting from cheap and easily available precursors and following a synthesis protocol that does not require the use of controlled atmosphere or organic solvents;
- (2)
- the NbOx-SiO2 catalyst was active in the catalytic epoxidation of unsaturated fatty acid methyl esters in the presence of aqueous hydrogen peroxide, showing a good robustness to repeated recovery and reuse cycles; and
- (3)
- a solid catalyst was successfully used, for the first time here, in the epoxidation of a mixture of FAMEs directly derived from a real sample of karanja (Millettia pinnata) seed oil, without the need of additional co-reactants or promoters.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guidotti, M.; Palumbo, C. Catalytic Epoxidation of Organics from Vegetable Sources. In Encyclopaedia of Inorganic and Bioinorganic Chemistry; Atwood, D.A., Ed.; Wiley: Chichester, UK, 2016; pp. 373–385. [Google Scholar]
- Chowdhury, A.; Anthony, P. A Review on Biodegradable Polymeric Materials Derived from Vegetable Oils for Diverse Applications. Int. J. Sci. Res. 2016, 5, 1786–1791. [Google Scholar]
- Noor Armylisas, A.H.; Siti Hazirah, M.F.; Yeong, S.K.; Hazimah, A.H. Modification of olefinic double bonds of unsaturated fatty acids and other vegetable oil derivatives via epoxidation: A review. Grasas Aceites 2017, 68, e174. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Epoxidation of Castor Oil Fatty Acid Methyl Esters (COFAME) as a Lubricant base Stock Using Heterogeneous Ion-exchange Resin (IR-120) as a Catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef]
- Moser, B.R.; Sharma, B.K.; Doll, K.M.; Erhan, S.Z. Diesters from Oleic Acid: Synthesis, Low Temperature Properties, and Oxidation Stability. J. Am. Oil Chem. Soc. 2007, 84, 675–680. [Google Scholar] [CrossRef]
- Doll, K.M.; Erhan, S.Z. Synthesis of cyclic acetals (ketals) from oleochemicals using a solvent free method. Green Chem. 2008, 10, 712–717. [Google Scholar] [CrossRef]
- Kandula, S.; Stolp, L.; Grass, M.; Woldt, B.; Kodali, D. Synthesis and Functional Evaluation of Soy Fatty Acid Methyl Ester Ketals as Bioplasticizers. J. Am. Oil Chem. Soc. 2014, 91, 1967–1974. [Google Scholar] [CrossRef]
- Zhang, G.; Kodani, S.; Hammock, B.D. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 2014, 53, 108–123. [Google Scholar] [CrossRef]
- Stern, A.; Guidotti, M.; Shaubi, E.; Popov, M.; Linder, C.; Heldman, E.; Grinberg, S. Steric environment around acetylcholine head groups of bolaamphiphilic nanovesicles influences the release rate of encapsulated compounds. Int. J. Nanomed. 2014, 9, 561–574. [Google Scholar]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Lee, H.V. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenković, O.S.; Veljkovic, V.B.; Hung, Y.T. Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Praniesh, R.; Navamani, P.; Swathi, R.; Sharmila, R.; Manoj Kumar, N. Process optimization and kinetic modeling of biodiesel production using non-edible Madhuca indica oil. Fuel 2017, 195, 217–225. [Google Scholar] [CrossRef]
- Patel, R.L.; Sankhavara, C.D. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Bajwa, A.S.; Sathaye, S.; Kulkarni, V.M.; Patwardhan, A.V. Chemoenzymatic epoxidation of Karanja oil: An alternative to chemical epoxidation? Asia-Pac. J. Chem. Eng. 2016, 11, 314–322. [Google Scholar] [CrossRef]
- Goud, V.V.; Pradhan, N.C.; Patwardhan, A.V. Epoxidation of Karanja (Pongamia glabra) oil by H2O2. J. Am. Oil Chem. Soc. 2006, 83, 635–640. [Google Scholar] [CrossRef]
- Goud, V.V.; Patwardhan, A.V.; Dinda, S.; Pradhan, N.C. Epoxidation of karanja (Pongamia glabra) oil catalysed by acidic ion exchange resin. Eur. J. Lipid Sci. Technol. 2007, 109, 575–584. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Ti/SiO2 as a Nanosized Solid Catalyst for the Epoxidation of Fatty Acid Methyl Esters and Triglycerides. Energy Fuels 2012, 26, 2953–2961. [Google Scholar] [CrossRef]
- Kadam, A.; Pawar, M.; Yemul, O.; Thamke, V.; Kodam, K. Biodegradable biobased epoxy resin from karanja oil. Polymer 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Gaikwad, M.S.; Gite, V.V.; Mahulikar, P.P.; Hundiwale, D.G.; Yemul, O.S. Eco-friendly polyurethane coatings from cottonseed and karanja oil. Prog. Org. Coatings 2015, 86, 164–172. [Google Scholar] [CrossRef]
- Sharmin, E.; Ashraf, S.M.; Ahmad, S. Synthesis, characterization, antibacterial and corrosion protective properties of epoxies, epoxy-polyols and epoxy-polyurethane coatings from linseed and Pongamia glabra seed oils. Int. J. Biol. Macromol. 2007, 40, 407–422. [Google Scholar] [CrossRef]
- Bora, M.M.; Deka, R.; Ahmed, N.; Kakati, D.K. Karanja (Millettia pinnata (L.) Panigrahi) seed oil as a renewable raw material for the synthesis of alkyd resin. Ind. Crops Prod. 2014, 61, 106–114. [Google Scholar] [CrossRef]
- Korlipara, V.P.; Mallampalli, S.L.K.; Krishnasamy, S.; Rachapudi, B.N.P. Process for the Preparation of Karanja Oil-Based Epoxy and Acyloxy Compounds as Lubricant Basestocks. Patent WO 2014009972, 16 January 2014. [Google Scholar]
- Mustapha, R.; Razak Rahmat, A.; Abdul Majid, R.; Noor Hidayah Mustapha, S. Vegetable oil-based epoxy resins and their composites with bio-based hardener: A short review. Polym. Plastics Technol. Mater. 2019. [Google Scholar] [CrossRef]
- Fraile, J.M.; Garcia, J.I.; Mayoral, J.A.; Vispe, E. Optimization of cyclohexene epoxidation with dilute hydrogen peroxide and silica-supported titanium catalysts. Appl. Catal. A: Gen. 2003, 245, 363–376. [Google Scholar] [CrossRef]
- Gianotti, E.; Bisio, C.; Marchese, L.; Guidotti, M.; Ravasio, N.; Psaro, R.; Coluccia, S. Ti(IV) catalytic centers grafted on different siliceous materials: A spectroscopic and catalytic study. J. Phys. Chem. C 2007, 111, 5083–5089. [Google Scholar] [CrossRef]
- Gallo, A.; Tiozzo, C.; Psaro, R.; Carniato, F.; Guidotti, M. Niobium metallocenes deposited onto mesoporous silica via dry impregnation as catalysts for selective epoxidation of alkenes. J. Catal. 2013, 298, 77–83. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Ivanchikova, I.D.; Maksimchuk, N.V.; Skobelev, I.Y. H2O2-based selective epoxidations: Nb-silicates versus Ti-silicates. Catal. Today 2018. [Google Scholar] [CrossRef]
- Aronne, A.; Turco, M.; Bagnasco, G.; Ramis, G.; Santacesaria, E.; Di Serio, M.; Marenna, E.; Bevilacqua, M.; Cammarano, C.; Fanelli, E. Gel derived niobium–silicon mixed oxides: Characterization and catalytic activity for cyclooctene epoxidation. Appl. Catal. A Gen. 2008, 347, 179–185. [Google Scholar] [CrossRef]
- Tiozzo, C.; Palumbo, C.; Psaro, R.; Bisio, C.; Carniato, F.; Gervasini, A.; Carniti, P.; Guidotti, M. The stability of niobium-silica catalysts in repeated liquid-phase epoxidation tests: A comparative evaluation of in-framework and grafted mixed oxides. Inorg. Chim. Acta 2015, 431, 190–196. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Gallo, A.; Scott, S.L.; Psaro, R.; Guidotti, M. Niobium-silica catalysts for the selective epoxidation of cyclic alkenes: The generation of the active site by grafting niobocene dichloride. Phys. Chem. Chem. Phys. 2013, 15, 13354–13362. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Nauert, S.L.; Thompson, A.B.; Notestein, J.M. Synthesis−structure–function relationships of silica-supported niobium(V) catalysts for alkene epoxidation with H2O2. ACS Catal. 2016, 6, 6124–6134. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Maksimchuk, N.V.; Skobelev, I.Y.; Kholdeeva, O.A. Mesoporous niobium–silicates prepared by evaporation–induced self–assembly as catalysts for selective oxidations with aqueous H2O2. J. Catal. 2015, 332, 138–148. [Google Scholar] [CrossRef]
- Padula, I.D.; Chagas, P.; Furst, C.G.; Oliveira, L.C.A. Mesoporous Niobium Oxyhydroxide Catalysts for Cyclohexene Epoxidation Reactions. Appl. Sci. 2018, 8, 881. [Google Scholar] [CrossRef]
- Paraguassú Cecchi, C.M.; Cesarín-Sobrinho, D.; Buarque Ferreira, A.B.; Netto-Ferreira, J.C. New Insights on the Oxidation of Unsaturated Fatty Acid Methyl Esters Catalyzed by Niobium(V) Oxide. A Study of the Catalyst Surface Reactivity. Catalysts 2018, 8, 6. [Google Scholar] [CrossRef]
- Nowak, I. Frontiers in mesoporous molecular sieves containing niobium: From model materials to catalysts. Catal. Today 2012, 192, 80–88. [Google Scholar] [CrossRef]
- Yan, W.; Ramanathan, A.; Ghanta, M.; Subramaniam, B. Towards highly selective ethylene epoxidation catalysts using hydrogen peroxide and tungsten- or niobium-incorporated mesoporous silicate (KIT-6). Catal. Sci. Technol. 2014, 4, 4433–4439. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, G.; Yan, H.; Liu, Y.; Chen, X.; Feng, X.; Jin, X.; Yang, C. Liquid-Phase Epoxidation of Light Olefins over W and Nb Nanocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 4423–4452. [Google Scholar] [CrossRef]
- Feliczak, A.; Walczak, K.; Wawrzyńczak, A.; Nowak, I. The use of mesoporous molecular sieves containing niobium for the synthesis of vegetable oil-based products. Catal. Today 2009, 140, 23–29. [Google Scholar] [CrossRef]
- Di Serio, M.; Turco, R.; Pernice, P.; Aronne, A.; Sannino, F.; Santacesaria, E. Valuation of Nb2O5–SiO2 catalysts in soybean oil epoxidation. Catal. Today 2012, 192, 112–116. [Google Scholar] [CrossRef]
- Dworakowska, S.; Tiozzo, C.; Niemczyk-Wrzeszcz, M.; Michorczyk, P.; Ravasio, P.; Psaro, R.; Bogdał, D.; Guidotti, M. Mesoporous molecular sieves containing niobium(V) as catalysts for the epoxidation of fatty acid methyl esters and rapeseed oil. J. Clean. Prod. 2017, 166, 901–909. [Google Scholar] [CrossRef]
- Danov, S.M.; Kazantsev, O.A.; Esipovich, A.L.; Belousov, A.S.; Rogozhin, A.E.; Kanakov, E.A. Recent advances in the field of selective epoxidation of vegetable oils and their derivatives: A review and perspective. Catal. Sci. Technol. 2017, 7, 3659–3675. [Google Scholar] [CrossRef]
- Turco, R.; Vitiello, R.; Tesser, R.; Vergara, A.; Andini, S.; Di Serio, M. Niobium Based Catalysts for Methyl Oleate Epoxidation Reaction. Topics Catal. 2017, 60, 1054–1061. [Google Scholar] [CrossRef]
- Zaccheria, F.; Scotti, N.; Marelli, M.; Psaro, R.; Ravasio, N. Unravelling the properties of supported copper oxide: Can the particle size induce acidic behaviour? Dalton Trans. 2013, 42, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, M.; Ravasio, N.; Psaro, R.; Gianotti, E.; Coluccia, S.; Marchese, L. Epoxidation of unsaturated FAMEs obtained from vegetable source over Ti(IV)-grafted silica catalysts: A comparison between ordered and non-ordered mesoporous materials. J. Mol. Catal. A Chem. 2006, 250, 218–225. [Google Scholar] [CrossRef]
- Kumar Karmee, S.; Chadha, A. Preparation of biodiesel from crude oil of Pongamia pinnata. Biores. Technol. 2005, 96, 1425–1429. [Google Scholar] [CrossRef]
- Pandey, A. Handbook of Plant-Based Biofuels; CRC Press: Boca Raton, FL, USA, 2008; pp. 255–266. [Google Scholar]
- Branca, M.; Micera, G.; Dessì, A.; Sanna, D. Oxovanadium(IV) complex formation by simple sugars in aqueous solution. J. Inorg. Biochem. 1992, 45, 169–177. [Google Scholar] [CrossRef]
- Baran, E.J. Oxovanadium(IV) complexes of carbohydrates: A brief overview. J. Inorg. Biochem. 2009, 103, 547–553. [Google Scholar] [CrossRef]
- Thornburg, N.E.; Notestein, J.M. Rate and Selectivity Control in Thioether and Alkene Oxidation with H2O2 over Phosphonate-Modified Niobium(V)–Silica Catalysts. ChemCatChem 2017, 9, 3714–3724. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E.; Wong, M.S.; Ying, J.Y. Structural and reactivity properties of Nb-MCM-41: Comparison with that of highly dispersed Nb2O5/SiO2 catalysts. J. Catal. 2001, 203, 18–24. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, Z.; Gao, X.; Huang, Y.; Yan, Z.; Zou, J.; Yin, H.; Zou, Q.; Kable, S.H.; Zhao, J.; et al. Structural Evolution in a Hydrothermal Reaction between Nb2O5 and NaOH Solution: From Nb2O5 Grains to Microporous Na2Nb2O6·2/3H2O Fibers and NaNbO3 Cubes. J. Am. Chem. Soc. 2006, 128, 2373–2384. [Google Scholar] [CrossRef]
- Braga, V.S.; Dias, J.A.; Dias, S.C.L.; de Macedo, J.L. Catalyst Materials Based on Nb2O5 Supported on SiO2−Al2O3: Preparation and Structural Characterization. Chem. Mater. 2005, 17, 690–695. [Google Scholar] [CrossRef]
- Wawrzynczak, A.; Nowak, I.; Feliczak-Guzik, A. Toward Exploiting the Behavior of Niobium-Containing Mesoporous Silicates vs. Polyoxometalates in Catalysis. Front. Chem. 2018, 6, 560. [Google Scholar] [CrossRef] [PubMed]
- Bregante, D.T.; Flaherty, D.W. Periodic Trends in Olefin Epoxidation over Group IV and V Framework-Substituted Zeolite Catalysts: A Kinetic and Spectroscopic Study. J. Am. Chem. Soc. 2017, 139, 6888–6898. [Google Scholar] [CrossRef] [PubMed]
- Carniti, P.; Gervasini, A.; Marzo, M. Dispersed NbOx catalytic phases in silica matrixes: Influence of niobium concentration and preparative route. J. Phys. Chem. C. 2008, 112, 14064–14074. [Google Scholar] [CrossRef]
- Guidotti, M.; Lazaro, B. Deactivation of molecular sieves in the synthesis of organic chemicals. In Deactivation and Regeneration of Zeolite Catalysts; Guisnet, M., Ramoa-Ribeiro, F., Eds.; Imperial College Press: London, UK, 2011; pp. 303–334. [Google Scholar]
- Guidotti, M.; Conti, L.; Fusi, A.; Ravasio, N.; Psaro, R. Diastereoselective epoxidation of hydroxy-containing unsaturated terpenes on heterogeneous titanium-catalyst. J. Mol. Catal. A Chem. 2002, 182, 151–156. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Kumar, R.; Bansal, V.; Patel, M.B.; Sarpal, A.S. 1H Nuclear Magnetic Resonance (NMR) Determination of the Iodine Value in Biodiesel Produced from Algal and Vegetable Oils. Energy Fuels 2012, 26, 7005–7008. [Google Scholar] [CrossRef]







| FAME | Content (mol%) |
|---|---|
| C16:0 | 11.7 |
| C18:0 | 6.3 |
| C18:1 | 51.6 |
| C18:2 | 17.7 |
| C20:0 | 0.8 |
| C20:1 | 0.7 |
| C22:0 | 10.1 * |
| C22:1 | 0.8 |
| other minor | 0.3 |
| Sample | SSA a (m2 g−1) | PV b (mL g−1) | PDmax c (nm) |
|---|---|---|---|
| SiO2 | 460 | 0.74 | 5.7 |
| NbOx-SiO2 | 404 | 0.70 | 6.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotti, N.; Ravasio, N.; Evangelisti, C.; Psaro, R.; Penso, M.; Niphadkar, P.S.; Bokade, V.V.; Guidotti, M. Epoxidation of Karanja (Millettia pinnata) Oil Methyl Esters in the Presence of Hydrogen Peroxide over a Simple Niobium-Containing Catalyst. Catalysts 2019, 9, 344. https://doi.org/10.3390/catal9040344
Scotti N, Ravasio N, Evangelisti C, Psaro R, Penso M, Niphadkar PS, Bokade VV, Guidotti M. Epoxidation of Karanja (Millettia pinnata) Oil Methyl Esters in the Presence of Hydrogen Peroxide over a Simple Niobium-Containing Catalyst. Catalysts. 2019; 9(4):344. https://doi.org/10.3390/catal9040344
Chicago/Turabian StyleScotti, Nicola, Nicoletta Ravasio, Claudio Evangelisti, Rinaldo Psaro, Michele Penso, Prashant S. Niphadkar, Vijay V. Bokade, and Matteo Guidotti. 2019. "Epoxidation of Karanja (Millettia pinnata) Oil Methyl Esters in the Presence of Hydrogen Peroxide over a Simple Niobium-Containing Catalyst" Catalysts 9, no. 4: 344. https://doi.org/10.3390/catal9040344
APA StyleScotti, N., Ravasio, N., Evangelisti, C., Psaro, R., Penso, M., Niphadkar, P. S., Bokade, V. V., & Guidotti, M. (2019). Epoxidation of Karanja (Millettia pinnata) Oil Methyl Esters in the Presence of Hydrogen Peroxide over a Simple Niobium-Containing Catalyst. Catalysts, 9(4), 344. https://doi.org/10.3390/catal9040344