A Review of Low Temperature NH3-SCR for Removal of NOx
Abstract
:1. Introduction
2. Binary Transition Metal-Based Catalysts
3. Ternary and Multi-Transition Metal-Based Catalysts
4. Supported Single Transition Metal-Based Catalysts
5. Supported Binary and Multi Transition Metal-Based Catalysts
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wan, Y.; Zhao, W.; Tang, Y.; Li, L.; Wang, H.; Cui, Y.; Gu, J.; Li, Y.; Shi, J. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B 2014, 148–149, 114–122. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic materials for DeNOx selective catalytic reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct decomposition of NOx over TiO2 supported transition metal oxides at low temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D. DRIFT study of CuO−CeO2−TiO2 mixed oxides for NOx reduction with NH3 at low temperatures. ACS Appl. Mater. Interfaces 2014, 6, 8134–8145. [Google Scholar] [CrossRef]
- Chen, M.; Yang, J.; Liu, Y.; Li, W.; Fan, J.; Ran, X.; Teng, W.; Sun, Y.; Zhang, W.-X.; Li, G.; et al. TiO2 interpenetrating networks decorated with SnO2 nanocrystals: Enhanced activity of selective catalytic reduction of NO with NH3. J. Mater. Chem. A 2015, 3, 1405–1409. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Tsang, S.C. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Boningari, T.; Koirala, R.; Smirniotis, P.G. Low-temperature selective catalytic reduction of NO with NH3 over V/ZrO2 prepared by flame-assisted spray pyrolysis: Structural and catalytic properties. Appl. Catal. B 2012, 127, 255–264. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. DRIFT study on cerium-tungsten/titiania catalyst for selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, D.; Zhang, L.; Huang, L.; Li, H.; Gao, R.; Shi, L.; Zhang, J. Comparative study of 3D ordered macroporous Ce0.75Zr0.2M0.05O2−δ (M = Fe, Cu, Mn, Co) for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2014, 4, 93–101. [Google Scholar] [CrossRef]
- Wang, X.; Wen, W.; Su, Y.; Wang, R. Influence of transition metals (M = Co, Fe and Mn) on ordered mesoporous CuM/CeO2 catalysts and applications in selective catalytic reduction of NOx with H2. RSC Adv. 2015, 5, 63135–63141. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhao, Q.; Ke, J.; Xiao, H.; Lv, X.; Liu, S.; Tadé, M.; Wang, S. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity correlation. Appl. Catal. B 2017, 200, 297–308. [Google Scholar] [CrossRef]
- Schneider, H.; Maciejewski, M.; Köhler, K.; Wokaun, A.; Baiker, A. Chromia supported on titania, VI Properties of different chromium oxide phases in the catalytic reduction of NO by NH3 studied by in situ diffuse reflectance FTIR spectroscopy. J. Catal. 1995, 157, 312–320. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.; Li, X. Low-temperature selective catalytic reduction of NOx with NH3 over Fe‒Mn mixed-oxide catalysts containing Fe3Mn3O8 phase. Ind. Eng. Chem. Res. 2012, 51, 202–212. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-temperature selective catalytic reduction (SCR) of NO with NH3 by using Mn, Cr, and Cu oxides supported on hombikat TiO2. Angew. Chem. Int. Ed. 2001, 40, 2479–2482. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Feng, Z.; Zheng, L.; Xie, Y.; Hu, T. Selective catalytic reduction of NO with NH3 over iron titanate catalyst: Catalytic performance and characterization. Appl. Catal. B 2010, 96, 408–420. [Google Scholar] [CrossRef]
- Yao, X.; Kong, T.; Chen, L.; Ding, S.; Yang, F.; Dong, L. Enhanced low-temperature NH3-SCR performance of MnOx/CeO2 catalysts by optimal solvent effect. Appl. Surf. Sci. 2017, 420, 407–415. [Google Scholar] [CrossRef]
- Sreekanth, P.M.; Peña, D.A.; Smirniotis, P.G. Titania supported bimetallic transition metal oxides for low-temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 2006, 45, 6444–6449. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Effect of nickel as dopant in Mn/TiO2 catalysts for the low-temperature selective reduction of NO with NH3. Catal. Lett. 2011, 141, 1399–1404. [Google Scholar] [CrossRef]
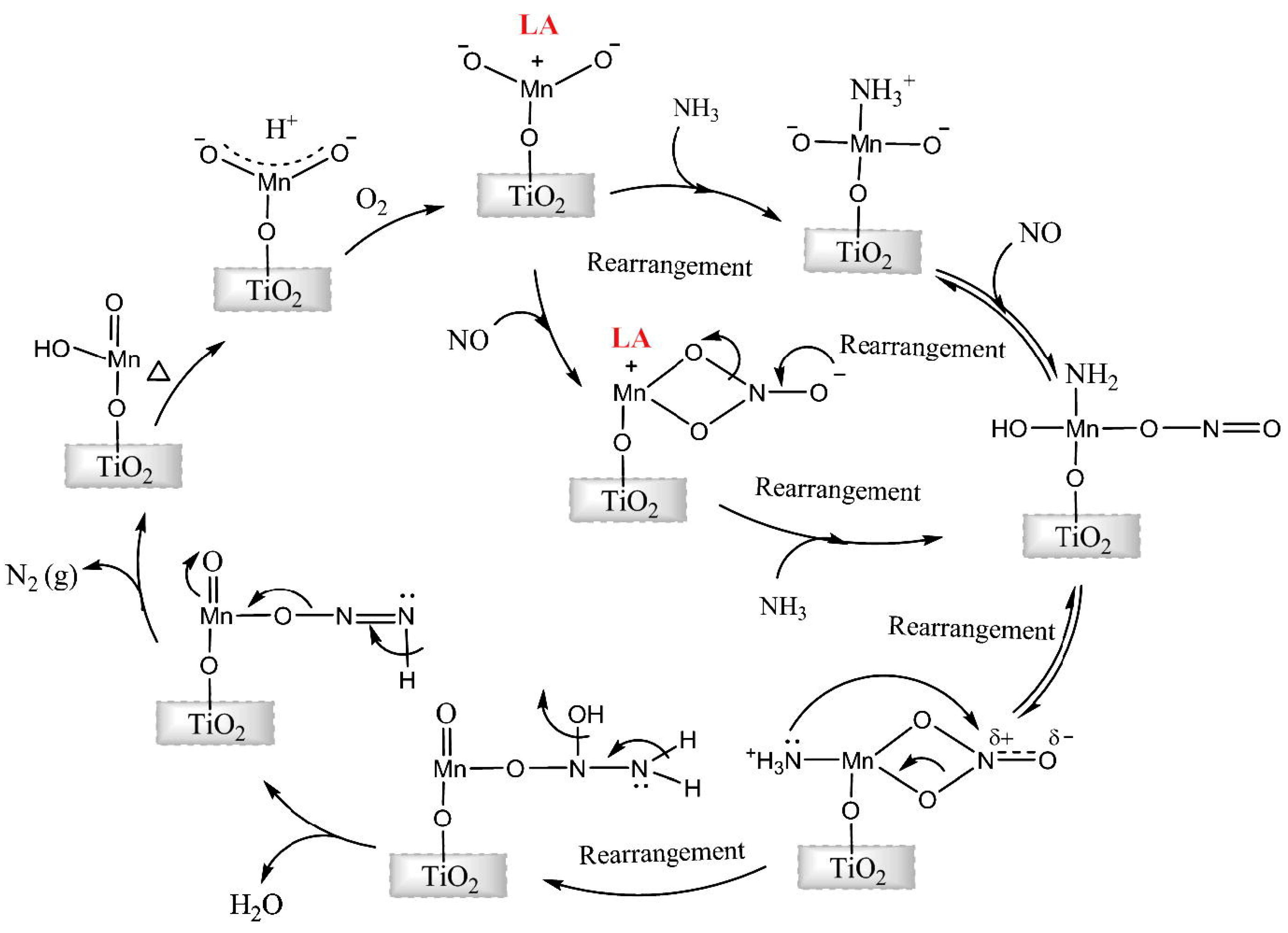
- Ettireddy, P.R.; Ettireddy, N.; Boningari, T.; Pardemann, R.; Smirniotis, P.G. Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies. J. Catal. 2012, 292, 53–63. [Google Scholar] [CrossRef]
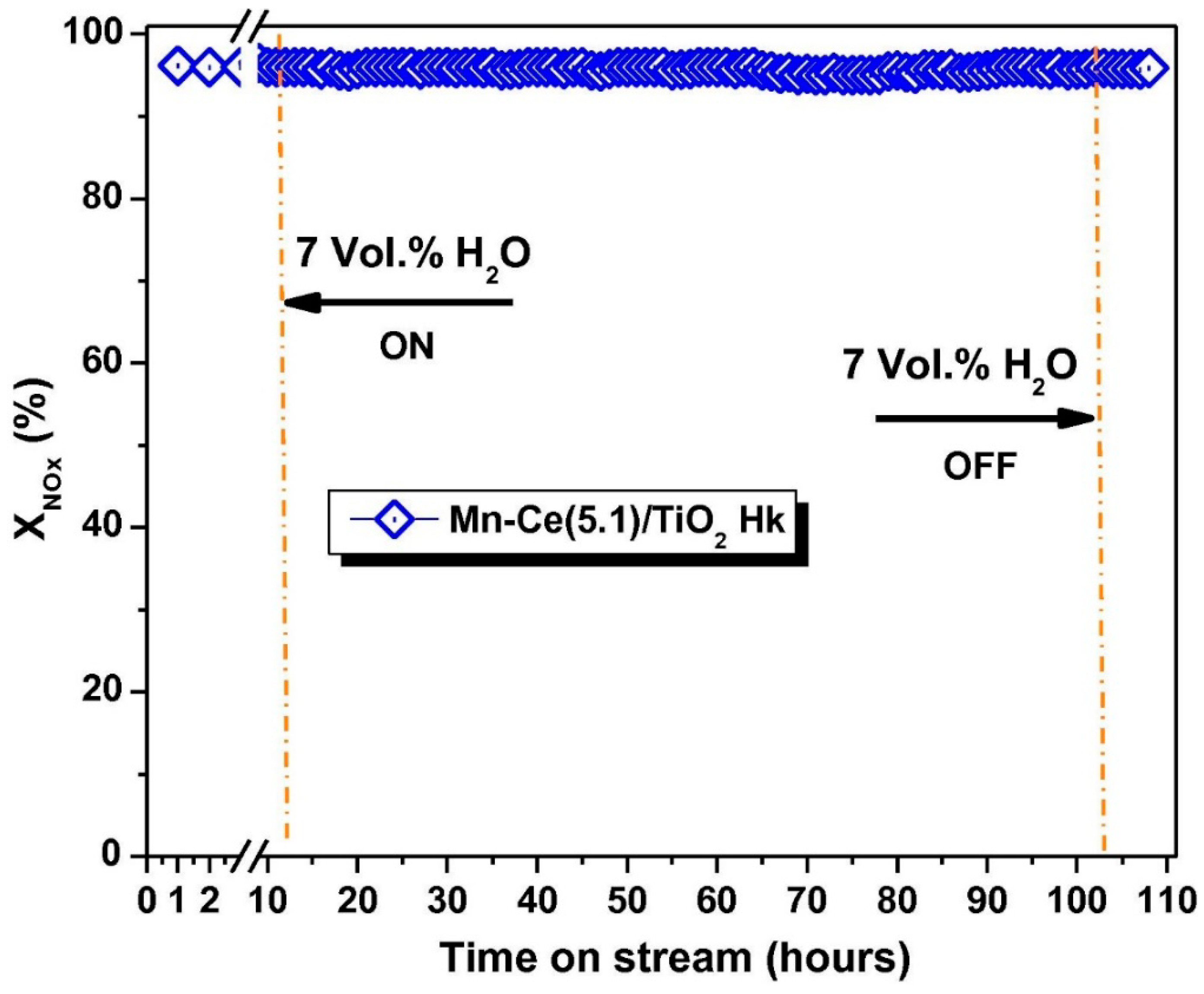
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
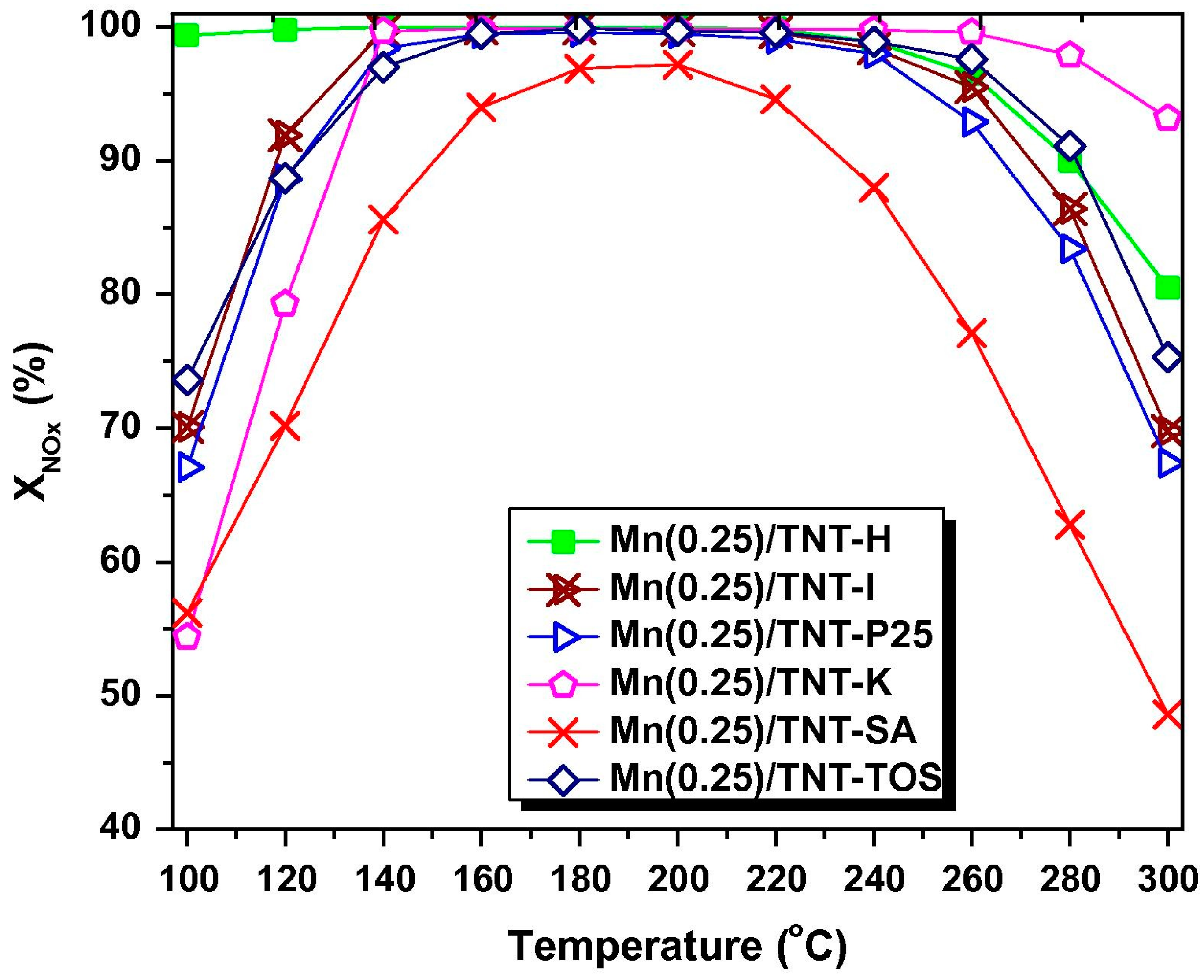
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature selective catalytic reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, D.; Shi, L.; Xu, J.; Zhang, L.; Huang, L.; Li, H.; Zhang, J. Porous Ni–Mn oxide nanosheets in situ formed on nickel foam as 3D hierarchical monolith de-NOx catalysts. Nanoscale 2014, 6, 7346–7353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, B.; Liu, B.; Sun, S. A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: Reaction mechanism and catalyst deactivation. RSC Adv. 2017, 7, 26226–26242. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.; Huang, L.; Zhang, J.; Gao, R.; Zhang, D. Rational design of high-performance DeNOx catalysts based on MnxCo3−xO4 nanocages derived from metal−organic frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Hou, X.; Chu, B.; Nan, B.; Qin, Q.; Fan, M.; Sun, C.; Li, B.; Dong, L.; et al. Investigation of two-phase intergrowth and coexistence in Mn−Ce−Ti−O catalysts for the selective catalytic reduction of NO with NH3: Structure−activity relationship and reaction mechanism. Ind. Eng. Chem. Res. 2019, 58, 849–862. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Wang, J.; Gu, T. Improvement of activity, selectivity and H2O&SO2-tolerance of micromesoporous CrMn2O4 spinel catalyst for low-temperature NH3-SCR of NOx. Appl. Surf. Sci. 2019, 466, 411–424. [Google Scholar]
- Li, X.J.; Du, Y.; Guo, X.M.; Wang, R.N.; Hou, B.H.; Wu, X. Synthesis of a novel NiMnTi mixed metal oxides from LDH precursor and its catalytic application for selective catalytic reduction of NOx with NH3. Catal. Lett. 2019, 149, 456–464. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, H.; Luo, X.; Shi, K.; Zhao, H.; Wang, W.; Chen, Q.; Fan, H.; Wu, T. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/γ-Al2O3 catalysts. Appl. Catal. B 2019, 245, 743–752. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Chen, Z.; Wang, R. Bauxite-supported transition metal oxides: Promising low-temperature and SO2-tolerant catalysts for selective catalytic reduction of NOx. Sci. Rep. 2015, 5, 9766. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Guo, R.-T.; Shi, X.; Pan, W.-G.; Liu, J.; Sun, X.; Liu, S.-W.; Liu, X.-Y.; Qin, H. The enhanced performance of Sb-modified Cu/TiO2 catalyst for selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2019, 475, 334–341. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. Mechanistic aspects of deNOx processing over TiO2 supported Co−Mn oxide catalysts: Structure−activity relationships and in situ DRIFTs analysis. ACS Catal. 2015, 5, 6069–6077. [Google Scholar] [CrossRef]
- Meng, B.; Zhao, Z.; Chen, Y.; Wang, X.; Li, Y.; Qiu, J. Low-temperature synthesis of Mn-based mixed metal oxides with novel fluffy structures as efficient catalysts for selective reduction of nitrogen oxides by ammonia. Chem. Commun. 2014, 50, 12396–12399. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Zhu, D.; Yu, H.; Shi, Q. NH3-SCR performance improvement of mesoporous Sn modified Cr-MnOx catalysts at low temperatures. Catal. Today 2015, 258, 103–111. [Google Scholar] [CrossRef]
- Shi, J.-W.; Gao, C.; Liu, C.; Fan, Z.; Gao, G.; Niu, C. Porous MnOx for low-temperature NH3-SCR of NOx: The intrinsic relationship between surface physicochemical property and catalytic activity. J. Nanopart. Res. 2017, 19, 194–204. [Google Scholar] [CrossRef]
- Husnain, N.; Wang, E.; Li, K.; Anwar, M.T.; Mehmood, A.; Gul, M.; Li, D.; Mao, J. Iron oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3. Rev. Chem. Eng. 2019, 35, 239–264. [Google Scholar] [CrossRef]
- Liu, F.; Shan, W.; Lian, Z.; Xie, L.; Yang, W.; He, H. Novel MnWOx catalyst with remarkable performance for low temperature NH3-SCR of NOx. Catal. Sci. Technol. 2013, 3, 2699–2707. [Google Scholar] [CrossRef]
- Fan, G.; Shi, J.-W.; Gao, C.; Gao, G.; Wang, B.; Niu, C. Rationally designed porous MnOx−FeOx nanoneedles for low-temperature selective catalytic reduction of NOx by NH3. ACS Appl. Mater. Interfaces 2017, 9, 16117–16127. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Shi, J.-W.; Fan, G.; Gao, C.; Niu, C. MnM2O4 microspheres (M = Co, Cu, Ni) for selective catalytic reduction of NO with NH3: Comparative study on catalytic activity and reaction mechanism via in-situ diffuse reflectance infrared Fourier transform spectroscopy. Chem. Eng. J. 2017, 325, 91–100. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on selective catalytic reduction of NOx by NH3 over Mn–based catalysts at low temperatures: Catalysts, mechanisms, kinetics and DFT calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.-W.; Fan, Z.; Gao, G.; Niu, C. Sulfur and water resistance of Mn-based catalysts for low-temperature selective catalytic reduction of NOx: A review. Catalysts 2018, 8, 11. [Google Scholar] [CrossRef]
- Hu, X.; Huang, L.; Zhang, J.; Li, H.; Zha, K.; Shi, L.; Zhang, D. Facile and template-free fabrication of mesoporous 3D nanosphere-like MnxCo3‒xO4 as highly effective catalysts for low temperature SCR of NOx with NH3. J. Mater. Chem. A 2018, 6, 2952–2963. [Google Scholar] [CrossRef]
- Meng, D.; Xu, Q.; Jiao, Y.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G.; Zhan, W. Spinel structured CoaMnbOx mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2018, 221, 652–663. [Google Scholar] [CrossRef]
- Yan, Q.; Nie, Y.; Yang, R.; Cui, Y.; O’Hare, D.; Wang, Q. Highly dispersed CuyAlOx mixed oxides as superior low-temperature alkali metal and SO2 resistant NH3-SCR catalysts. Appl. Catal. A 2017, 538, 37–50. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly effective catalyst of Sm-MnOx for the NH3-SCR of NOx at low temperature: Promotional role of Sm and its catalytic performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
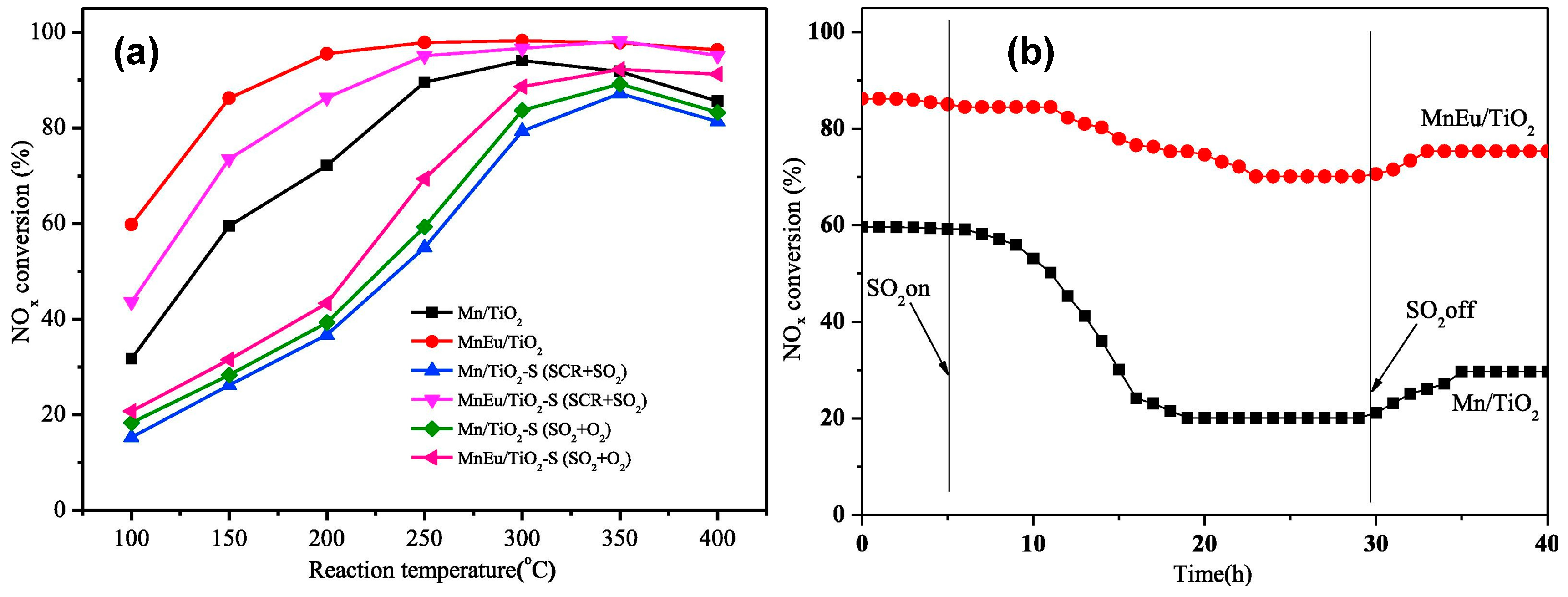
- Sun, P.; Guo, R.-T.; Liu, S.-M.; Wang, S.-X.; Pan, W.-G.; Li, M.-Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Xin, Y.; Li, H.; Zhang, N.; Li, Q.; Zhang, Z.; Cao, X.; Hu, P.; Zheng, L.; Anderson, J.A. Molecular-level insight into selective catalytic reduction of NOx with NH3 to N2 over a highly efficient bifunctional Va-MnOx catalyst at low temperature. ACS Catal. 2018, 8, 4937–4949. [Google Scholar] [CrossRef]
- Han, Y.; Mu, J.; Li, X.; Gao, J.; Fan, S.; Tan, F.; Zhao, Q. Triple-shelled NiMn2O4 hollow spheres as an efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. Chem. Commun. 2018, 54, 9797–9800. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Wang, J.; Shi, Y.; Meng, X. Novel Co– or Ni–Mn binary oxide catalysts with hydroxyl groups for NH3–SCR of NOx at low temperature. Appl. Surf. Sci. 2018, 443, 103–113. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Zhao, Q.; Mu, J.; Chen, J. Fe–Mn mixed oxide catalysts synthesized by one-step urea-precipitation method for the selective catalytic reduction of NOx with NH3 at low temperatures. Catal. Lett. 2018, 148, 227–234. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B 2015, 166–167, 37–44. [Google Scholar] [CrossRef]
- Fan, G.; Shi, J.-W.; Gao, C.; Gao, G.; Wang, B.; Wang, Y.; He, C.; Niu, C. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: The promoting effect of Gd on the catalytic performance and sulfur resistance. Chem. Eng. J. 2018, 348, 820–830. [Google Scholar] [CrossRef]
- Li, C.; Tang, X.; Yi, H.; Wang, L.; Cui, X.; Chu, C.; Li, J.; Zhang, R.; Yu, Q. Rational design of template-free MnOx-CeO2 hollow nanotube as de-NOx catalyst at low temperature. Appl. Surf. Sci. 2018, 428, 924–932. [Google Scholar] [CrossRef]
- Liu, F.; Yu, Y.; He, H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: Structure–activity relationship and reaction mechanism aspects. Chem. Commun. 2014, 50, 8445–8463. [Google Scholar] [CrossRef]
- Zhan, S.; Qiu, M.; Yang, S.; Zhu, D.; Yu, H.; Li, Y. Facile preparation of MnO2 doped Fe2O3 hollow nanofibers for low temperature SCR of NO with NH3. J. Mater. Chem. A 2014, 2, 20486–20493. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Li, Y.; Zhan, S.; Guan, Q.; Tian, Y. Low-temperature selective catalytic reduction of NO with NH3 over Mn2O3-doped Fe2O3 hexagonal microsheets. ACS Appl. Mater. Interfaces 2016, 8, 5224–5233. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Zha, K.; Li, H.; Shi, L.; Zhang, D. Scale-activity relationship of MnOx-FeOy nanocage catalysts derived from prussian blue analogues for low-temperature NO reduction: Experimental and DFT studies. ACS Appl. Mater. Interfaces 2017, 9, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Li, X.; Sun, W.; Fan, S.; Wang, X.; Wang, L.; Qin, M.; Gan, G.; Yin, Z.; Zhang, D. Inductive effect boosting catalytic performance of advanced Fe1−xVxOδ catalysts in low-temperature NH3 selective catalytic reduction: Insight into the structure, interaction, and mechanisms. ACS Catal. 2018, 8, 6760–6774. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Hou, Y.; Guo, Y.; Liu, Y.; Cui, Y.; Huang, Z. Role of CTAB in the improved H2O resistance for selective catalytic reduction of NO with NH3 over iron titanium catalyst. Chem. Eng. J. 2018, 347, 313–321. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D. Low-temperature selective catalytic reduction of NO with NH3 over ordered mesoporous MnxCo3−xO4 catalyst. Catal. Commun. 2015, 62, 107–111. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D.; Wang, S. Facile preparation of ordered mesoporous MnCo2O4 for low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2015, 7, 2568–2577. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. In Situ DRIFTs investigation of the low-temperature reaction mechanism over Mn-doped Co3O4 for the selective catalytic reduction of NOx with NH3. J. Phys. Chem. C 2015, 119, 22924–22933. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, N.; Wang, Z.; Sun, W.; Sun, K. Porous bimetallic Mn2Co1Ox catalysts prepared by a one-step combustion method for the low temperature selective catalytic reduction of NOx with NH3. Catal. Commun. 2015, 72, 111–115. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Cu–Mn mixed oxides for low temperature NO reduction with NH3. Catal. Today 2006, 111, 236–241. [Google Scholar] [CrossRef]
- Guo, R.-T.; Zhen, W.-L.; Pan, W.-G.; Zhou, Y.; Hong, J.-N.; Xu, H.-J.; Jin, Q.; Ding, C.-G.; Guo, S.-Y. Effect of Cu doping on the SCR activity of CeO2 catalyst prepared by citric acid method. J. Ind. Eng. Chem. 2014, 20, 1577–1580. [Google Scholar] [CrossRef]
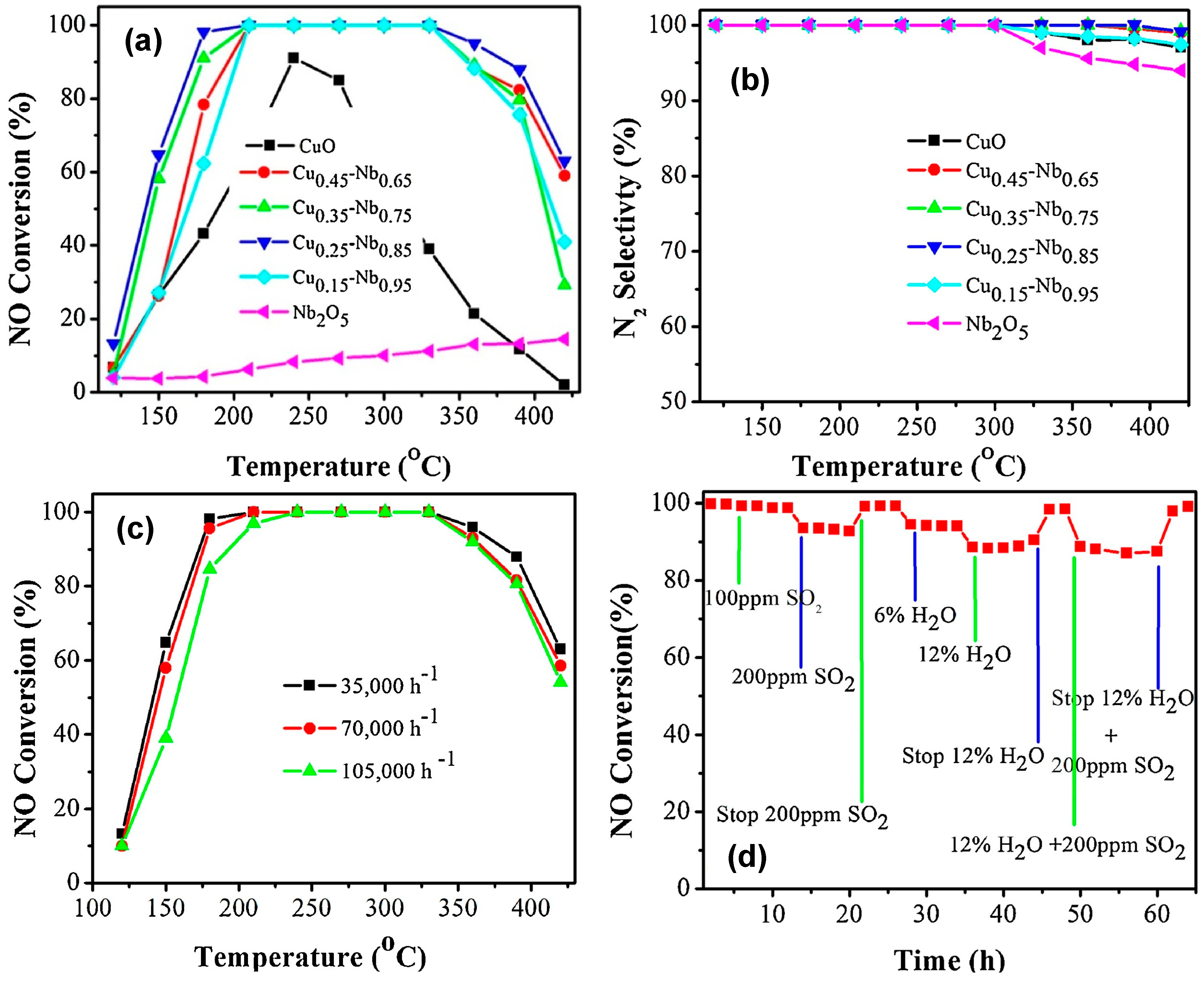
- Ali, S.; Chen, L.; Li, Z.; Zhang, T.; Li, R.; Bakhtiar, S.U.H.; Leng, X.; Yuan, F.; Niu, X.; Zhu, Y. Cux-Nb1.1-x (x = 0.45, 0.35, 0.25, 0.15) bimetal oxides catalysts for the low temperature selective catalytic reduction of NO with NH3. Appl. Catal. B 2018, 236, 25–35. [Google Scholar] [CrossRef]
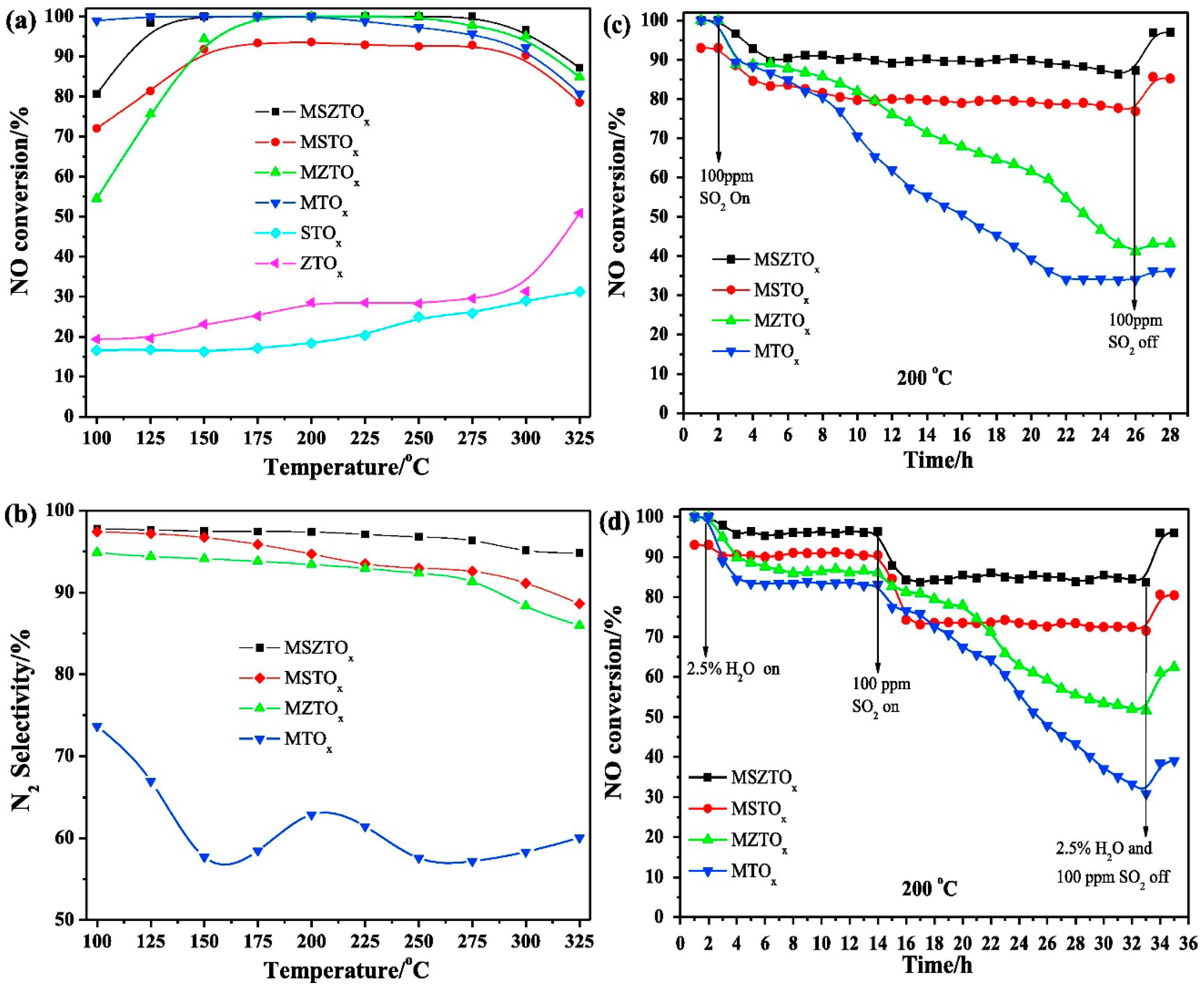
- Yan, Q.; Chen, S.; Zhang, C.; Wang, Q.; Louis, B. Synthesis and catalytic performance of Cu1Mn0.5Ti0.5Ox mixed oxide as low-temperature NH3-SCR catalyst with enhanced SO2 resistance. Appl. Catal. B 2018, 238, 236–247. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, R.; Zhao, Q.; Ke, J.; Xiao, H.; Wang, L.; Liu, S.; Tadé, M.; Wang, S. Facile synthesis of tube-shaped Mn-Ni-Ti solid solution and preferable Langmuir-Hinshelwood mechanism for selective catalytic reduction of NOx by NH3. Appl. Catal. A 2018, 549, 289–301. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Wang, X.; Dan, J.; Zhang, J.; Cao, P.; Dai, B. Microspherical MnO2-CeO2-Al2O3 mixed oxide for monolithic honeycomb catalyst and application in selective catalytic reduction of NOx with NH3 at 50–150 °C. Chem. Eng. J. 2018, 346, 182–192. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, B.; Song, W.; Liu, J.; Chen, Y.; Zhao, Z.; Wei, Y. Effect of Nb promoter on the structure and performance of iron titanate catalysts for the selective catalytic reduction of NO with NH3. Ind. Eng. Chem. Res. 2018, 57, 7802–7810. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Qiu, L.; Gao, Y.; O’Hare, D.; Wang, Q. The synthesis of CuyMnzAl1−zOx mixed oxide as a low-temperature NH3-SCR catalyst with enhanced catalytic performance. Dalton Trans. 2018, 47, 2992–3004. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Shan, W.; Yang, S.; Liu, F. W-modified Mn−Ti mixed oxide catalyst for the selective catalytic reduction of NO with NH3. Ind. Eng. Chem. Res. 2018, 57, 9112–9119. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low Temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Xiong, Z.-B.; Hu, Q.; Liu, D.-Y.; Wu, C.; Zhou, F.; Wang, Y.-Z.; Jin, J.; Lu, C.-M. Influence of partial substitution of iron oxide by titanium oxide on the structure and activity of iron–cerium mixed oxide catalyst for selective catalytic reduction of NOx with NH3. Fuel 2016, 165, 432–439. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, Q.; Sun, W.; Tade, M.; Liu, S. Improved activity of W-modified MnOx–TiO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2016, 288, 216–222. [Google Scholar] [CrossRef]
- Zamudio, M.A.; Russo, N.; Fino, D. Low temperature NH3 selective catalytic reduction of NOx over substituted MnCr2O4 spinel-oxide catalysts. Ind. Eng. Chem. Res. 2011, 50, 6668–6672. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chang, H.; Peng, Y.; Li, J. Novel W-modified SnMnCeOx catalyst for the selective catalytic reduction of NOx with NH3. Catal. Commun. 2017, 100, 117–120. [Google Scholar] [CrossRef]
- Ali, S.; Chen, L.; Yuan, F.; Li, R.; Zhang, T.; Bakhtiar, S.U.H.; Leng, X.; Niu, X.; Zhu, Y. Synergistic effect between copper and cerium on the performance of Cux-Ce0.5-x-Zr0.5 (x = 0.1–0.5) oxides catalysts for selective catalytic reduction of NO with ammonia. Appl. Catal. B 2017, 210, 223–234. [Google Scholar] [CrossRef]
- Wei, Y.; Fan, H.; Wang, R. Transition metals (Co, Zr, Ti) modified iron-samarium oxide as efficient catalysts for selective catalytic reduction of NOx at low-temperature. Appl. Surf. Sci. 2018, 459, 63–73. [Google Scholar] [CrossRef]
- Gao, X.; Du, X.-S.; Cui, L.-W.; Fu, Y.-C.; Luo, Z.-Y.; Cen, K.-F. A Ce–Cu–Ti oxide catalyst for the selective catalytic reduction of NO with NH3. Catal. Commun. 2010, 12, 255–258. [Google Scholar] [CrossRef]
- Xiong, Z.B.; Wu, C.; Hu, Q.; Wang, Y.Z.; Jin, J.; Lu, C.M.; Guo, D.X. Promotional effect of microwave hydrothermal treatment on the low-temperature NH3-SCR activity over iron-based catalyst. Chem. Eng. J. 2016, 286, 459–466. [Google Scholar] [CrossRef]
- Cheng, K.; Song, W.; Cheng, Y.; Zheng, H.; Wang, L.; Liu, J.; Zhao, Z.; Wei, Y. Enhancing the low temperature NH3-SCR activity of FeTiOx catalysts via Cu doping: A combination of experimental and theoretical study. RSC Adv. 2018, 8, 19301–19309. [Google Scholar] [CrossRef]
- Fang, N.; Guo, J.; Shu, S.; Luo, H.; Chu, Y.; Li, J. Enhancement of low-temperature activity and sulfur resistance of Fe0.3Mn0.5Zr0.2 catalyst for NO removal by NH3-SCR. Chem. Eng. J. 2017, 325, 114–123. [Google Scholar] [CrossRef]
- Guo, R.-T.; Sun, X.; Liu, J.; Pan, W.-G.; Li, M.-Y.; Liu, S.-M.; Sun, P.; Liu, S.-W. Enhancement of the NH3-SCR catalytic activity of MnTiOx catalyst by the introduction of Sb. Appl. Catal. A 2018, 558, 1–8. [Google Scholar] [CrossRef]
- Shi, J.-W.; Gao, G.; Fan, Z.; Gao, C.; Wang, B.; Wang, Y.; Li, Z.; He, C.; Niu, C. NiyCo1-yMn2Ox microspheres for the selective catalytic reduction of NOx with NH3: The synergetic effects between Ni and Co for improving low-temperature catalytic performance. Appl. Catal. A 2018, 560, 1–11. [Google Scholar] [CrossRef]
- Wu, X.; Feng, Y.; Du, Y.; Liu, X.; Zou, C.; Li, Z. Enhancing DeNOx performance of CoMnAl mixed metal oxides in low-temperature NH3-SCR by optimizing layered double hydroxides (LDHs) precursor template. Appl. Surf. Sci. 2019, 467–468, 802–810. [Google Scholar] [CrossRef]
- Leng, X.; Zhang, Z.; Li, Y.; Zhang, T.; Ma, S.; Yuan, F.; Niu, X.; Zhu, Y. Excellent low temperature NH3-SCR activity over MnaCe0.3TiOx (a = 0.1–0.3) oxides: Influence of Mn addition. Fuel Process. Technol. 2018, 181, 33–43. [Google Scholar] [CrossRef]
- Ali, S.; Li, Y.; Zhang, T.; Bakhtiar, S.U.H.; Leng, X.; Li, Z.; Yuan, F.; Niu, X.; Zhu, Y. Promotional effects of Nb on selective catalytic reduction of NO with NH3 over Fex-Nb0.5-x-Ce0.5 (x = 0.45, 0.4, 0.35) oxides catalysts. Mol. Catal. 2018, 461, 97–107. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Chen, W.; Chen, D.; Yu, S.; Liu, A.; Dong, L.; Feng, S. Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem. Eng. J. 2018, 347, 27–40. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Zhang, C.; O’Hare, D.; Wang, Q. Synthesis of Cu0.5Mg1.5Mn0.5Al0.5Ox mixed oxide from layered double hydroxide precursor as highly efficient catalyst for low-temperature selective catalytic reduction of NOx with NH3. J. Colloid Interface Sci. 2018, 526, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, F.; Li, Z.; Niu, X.; Zhu, Y. Synergistic effect between the redox property and acidity on enhancing the low temperature NH3-SCR activity for NOx removal over the Co0.2CexMn0.8-xTi10 (x = 0–0.40) oxides catalysts. Chem. Eng. J. 2018, 354, 393–406. [Google Scholar] [CrossRef]
- Peña, D.A.; Uphade, B.S.; Reddy, E.P.; Smirniotis, P.G. Identification of surface species on titania-supported manganese, chromium, and copper oxide low-temperature SCR catalysts. J. Phys. Chem. B 2004, 108, 9927–9936. [Google Scholar] [CrossRef]
- Zhuang, K.; Qiu, J.; Tang, F.; Xu, B.; Fan, Y. The structure and catalytic activity of anatase and rutile titania supported manganese oxide catalysts for selective catalytic reduction of NO by NH3. Phys. Chem. Chem. Phys. 2011, 13, 4463–4469. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Ettireddy, P.R.; Kotrba, A.; Smirniotis, P.G. Influence of SiO2 on M/TiO2 (M = Cu, Mn, and Ce) formulations for low-temperature selective catalytic reduction of NOx with NH3: Surface properties and key components in relation to the activity of NOx reduction. Ind. Eng. Chem. Res. 2015, 54, 2261–2273. [Google Scholar] [CrossRef]
- Sheng, Z.; Ma, D.; Yu, D.; Xiao, X.; Huang, B.; Yang, L.; Wang, S. Synthesis of novel MnOx@TiO2 core-shell nanorod catalyst for low-temperature NH3-selective catalytic reduction of NOx with enhanced SO2 tolerance. Chin. J. Catal. 2018, 39, 821–830. [Google Scholar] [CrossRef]
- Cimino, S.; Totarella, G.; Tortorelli, M.; Lisi, L. Combined poisoning effect of K+ and its counter-ion (Cl− or NO3−) on MnOx/TiO2 catalyst during the low temperature NH3-SCR of NO. Chem. Eng. J. 2017, 330, 92–101. [Google Scholar] [CrossRef]
- Fang, D.; Xie, J.; Hu, H.; Yang, H.; He, F.; Fu, Z. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem. Eng. J. 2015, 271, 23–30. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H.; Liu, L.; Jiang, H. Revisit the effect of manganese oxidation state on activity in low-temperature NO-SCR. Mol. Catal. 2018, 446, 49–57. [Google Scholar] [CrossRef]
- Yang, S.; Qi, F.; Xiong, S.; Dang, H.; Liao, Y.; Wong, P.K.; Li, J. MnOx supported on Fe–Ti spinel: A novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl. Catal. B 2016, 181, 570–580. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Peña, D.A.; Uphade, B.S.; Smirniotis, P.G. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3, I. Evaluation and characterization of first row transition metals. J. Catal. 2004, 221, 421–431. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Sreekanth, P.M.; Peña, D.A.; Jenkins, R.G. Manganese oxide catalysts supported on TiO2, Al2O3, and SiO2: A comparison for low-temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 2006, 45, 6436–6443. [Google Scholar] [CrossRef]
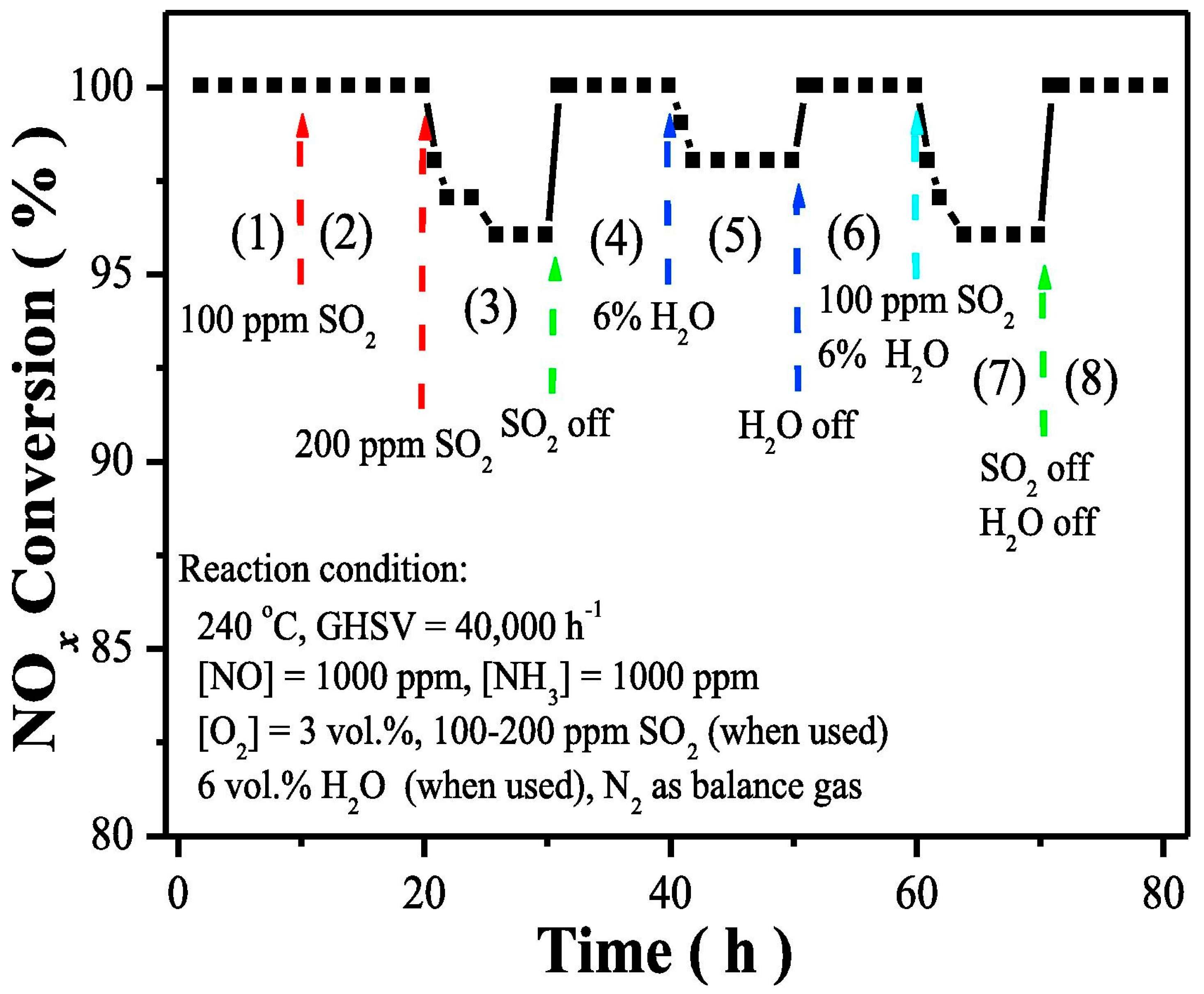
- Ettireddy, P.R.; Ettireddy, N.; Mamedov, S.; Boolchand, P.; Smirniotis, P.G. Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl. Catal. B 2007, 76, 123–134. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Jia, B.; Guo, J.; Luo, H.; Shu, S.; Fang, N.; Li, J. Study of NO removal and resistance to SO2 and H2O of MnOx/TiO2, MnOx/ZrO2 and MnOx/ZrO2–TiO2. Appl. Catal. A 2018, 553, 82–90. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Liu, B.; Shen, H.; Li, L. High N2 selectivity in selective catalytic reduction of NO with NH3 over Mn/Ti–Zr catalysts. RSC Adv. 2018, 8, 12733–12741. [Google Scholar] [CrossRef]
- Han, J.; Zhang, D.; Maitarad, P.; Shi, L.; Cai, S.; Li, H.; Huang, L.; Zhang, J. Fe2O3 nanoparticles anchored in situ on carbon nanotubes via an ethanol-thermal strategy for the selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2015, 5, 438–446. [Google Scholar] [CrossRef]
- Pourkhalil, M.; Moghaddam, A.Z.; Rashidi, A.; Towfighi, J.; Mortazavi, Y. Preparation of highly active manganese oxides supported on functionalized MWNTs for low temperature NOx reduction with NH3. Appl. Surf. Sci. 2013, 279, 250–259. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, D.; Shi, L.; Gao, R.; Li, H.; Ye, L.; Zhang, J. Highly dispersed CeO2 on carbon nanotubes for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2013, 3, 803–811. [Google Scholar] [CrossRef]
- Qu, Z.; Miao, L.; Wang, H.; Fu, Q. Highly dispersed Fe2O3 on carbon nanotubes for low-temperature selective catalytic reduction of NO with NH3. Chem. Commun. 2015, 51, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Li, H.; Wang, L.; Guan, Y.; Jiang, S. The properties and mechanism of CuO modified carbon nanotube for NOx removal. Catal. Lett. 2014, 144, 216–221. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx–CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn–Fe/TiO2 catalysts synthesized by deposition precipitation‒promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B 2015, 165, 628–635. [Google Scholar] [CrossRef]
- You, X.; Sheng, Z.; Yu, D.; Yang, L.; Xiao, X.; Wang, S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 423, 845–854. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Liu, X.; Qi, K.; Xie, J.; Li, F.; Yu, C. Low temperature NH3-SCR of NO over an unexpected Mn-based catalyst: Promotional effect of Mg doping. Appl. Surf. Sci. 2018, 427, 45–55. [Google Scholar] [CrossRef]
- Lu, X.; Song, C.; Jia, S.; Tong, Z.; Tang, X.; Teng, Y. Low-temperature selective catalytic reduction of NOX with NH3 over cerium and manganese oxides supported on TiO2–graphene. Chem. Eng. J. 2015, 260, 776–784. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Zou, W.; Yu, S.; Gui, K.; Dong, L. Fe-Mn/Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chin. J. Catal. 2016, 37, 1314–1323. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Z.; Chen, M.; Zhang, Z.; Shangguan, W. Promotion effect of tungsten and iron co-addition on the catalytic performance of MnOx/TiO2 for NH3-SCR of NOx. Fuel 2017, 210, 783–789. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Liang, H.; Chen, X.; Tang, J.; Wang, X. N-doped graphene and TiO2 supported manganese and cerium oxides on low-temperature selective catalytic reduction of NOx with NH3. J. Adv. Ceram. 2018, 7, 197–206. [Google Scholar] [CrossRef]
- Fan, Y.; Ling, W.; Huang, B.; Dong, L.; Yu, C.; Xi, H. The synergistic effects of cerium presence in the framework and the surface resistance to SO2 and H2O in NH3-SCR. J. Ind. Eng. Chem. 2017, 56, 108–119. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, W.; Cui, S.; Street, J.; Luo, Y. Sulfur resistance of Ce-Mn/TiO2 catalysts for low-temperature NH3–SCR. R. Soc. Open Sci. 2018, 5, 171846. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Lee, C.-Y.; Zhang, Y.-R.; Bai, H. Aerosol-assisted deposition of Mn-Fe oxide catalyst on TiO2 for superior selective catalytic reduction of NO with NH3 at low temperatures. Catal. Commun. 2018, 111, 36–41. [Google Scholar] [CrossRef]
- Lee, T.; Bai, H. Metal Sulfate poisoning effects over MnFe/TiO2 for selective catalytic reduction of NO by NH3 at low temperature. Ind. Eng. Chem. Res. 2018, 57, 4848–4858. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Sun, W.; Fan, S.; Wang, X.; Wang, L.; Qin, M.; Gan, G.; Yin, Z.; Zhang, D. Enhancement of low-temperature catalytic activity over a highly dispersed Fe−Mn/Ti catalyst for selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 10159–10169. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R.-T.; Li, M.-Y.; Sun, P.; Liu, S.-M.; Pan, W.-G.; Liu, S.-W.; Sun, X. Enhancement of the SO2 resistance of Mn/TiO2 SCR catalyst by Eu modification: A mechanism study. Fuel 2018, 223, 385–393. [Google Scholar] [CrossRef]
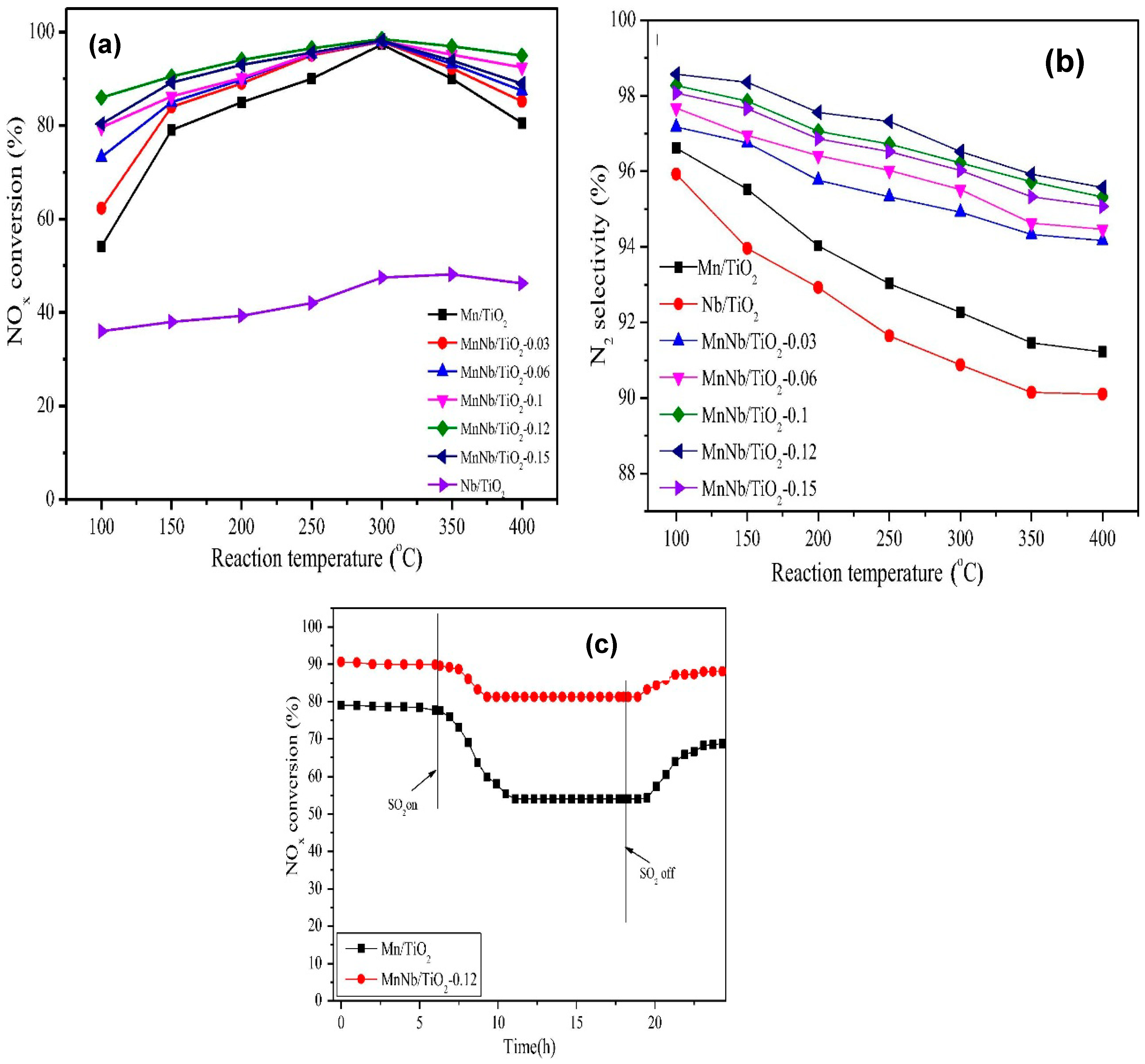
- Sun, P.; Huang, S.-X.; Guo, R.-T.; Li, M.-Y.; Liu, S.-M.; Pan, W.-G.; Fu, Z.-G.; Liu, S.-W.; Sun, X.; Liu, J. The enhanced SCR performance and SO2 resistance of Mn/TiO2 catalyst by the modification with Nb: A mechanistic study. Appl. Surf. Sci. 2018, 447, 479–488. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.-T.; Liu, J.; Fu, Z.-G.; Liu, S.-W.; Pan, W.-G.; Shi, X.; Qin, H.; Wang, Z.-Y.; Liu, X.-Y. The enhanced SCR performance of Mn/TiO2 catalyst by Mo modification: Identification of the promotion mechanism. Int. J. Hydrogen Energy 2018, 43, 16038–16048. [Google Scholar] [CrossRef]
- Fan, J.; Lv, M.; Luo, W.; Ran, X.; Deng, Y.; Zhang, W.-X.; Yang, J. Exposed metal oxide active sites on mesoporous titania channels: A promising design for low-temperature selective catalytic reduction of NO with NH3. Chem. Commun. 2018, 54, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, B.; Wang, H.; Ma, J.; Xu, W.Q.; Li, Y.; Han, Y.; Sun, Q. Fe and/or Mn oxides supported on fly ash-derived SBA-15 for low-temperature NH3-SCR. Catal. Commun. 2018, 108, 82–87. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Wang, Z.; Li, Z.; Sun, Q.; Xu, W.Q.; Li, Y. Reaction mechanism of low-temperature selective catalytic reduction of NOx over Fe-Mn oxides supported on fly-ash-derived SBA-15 molecular sieves: Structure−activity relationships and in situ DRIFT analysis. J. Phys. Chem. C 2018, 122, 20210–20231. [Google Scholar] [CrossRef]
- Tang, X.; Li, C.; Yi, H.; Wang, L.; Yu, Q.; Gao, F.; Cui, X.; Chu, C.; Li, J.; Zhang, R. Facile and fast synthesis of novel Mn2CoO4@rGO catalysts for the NH3-SCR of NOx at low temperature. Chem. Eng. J. 2018, 333, 467–476. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Zhao, Y.; Yan, X.; Cao, P. Poisoning effect of SO2 on honeycomb cordierite-based Mn–Ce/Al2O3 catalysts for NO reduction with NH3 at low temperature. Appl. Sci. 2018, 8, 95. [Google Scholar] [CrossRef]
- Meng, H.; Liu, J.; Du, Y.; Hou, B.; Wu, X.; Xie, X. Novel Cu-based oxides catalyst from one-step carbothermal reduction decomposition method for selective catalytic reduction of NO with NH3. Catal. Commun. 2019, 119, 101–105. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Ma, K.; Zou, W.; Cao, Y.; Xiong, Y.; Tang, C.; Dong, L. Ultra-low loading of copper modified TiO2/CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 207, 366–375. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Xiao, R.; Huang, T.; Shen, K. Novel holmium-modified Fe-Mn/TiO2 catalysts with a broad temperature window and high sulfur dioxide tolerance for low-temperature SCR. Catal. Commun. 2017, 88, 64–67. [Google Scholar] [CrossRef]
- Nam, K.B.; Kwon, D.W.; Hong, S.C. DRIFT study on promotion effects of tungsten-modified Mn/Ce/Ti catalysts for the SCR reaction at low-temperature. Appl. Catal. A 2017, 542, 55–62. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Guo, X.; Cao, L.; Xu, T.; Chen, Z.; Wu, X.; Si, Z.; Weng, D. Nb-modified Mn/Ce/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A 2017, 545, 64–71. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Lu, Y.; Hao, W.; Wei, Z.; Liu, J.; Zhang, Y. Denitrification performance of non-pitch coal-based activated coke by the introduction of MnOx–CeOx–M (FeOx, CoOx) at low temperature. Mol. Catal. 2018, 445, 21–28. [Google Scholar] [CrossRef]
- Pan, Y.; Shen, Y.; Jin, Q.; Zhu, S. Promotional effect of Ba additives on MnCeOx/TiO2 catalysts for NH3-SCR of NO at low temperature. J. Mater. Res. 2018, 33, 2414–2422. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Ying, Q.; Yao, W.; Wu, Z. The superior performance of Nb-modified Cu-Ce-Ti mixed oxides for the selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A 2018, 562, 19–27. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Li, X.; Tan, P.; Zhou, A.; Fang, Q.; Chen, G. Ho-modified Mn–Ce/TiO2 for low-temperature SCR of NOx with NH3: Evaluation and characterization. Chin. J. Catal. 2018, 39, 1653–1663. [Google Scholar] [CrossRef]
- Lu, P.; Li, R.; Xing, Y.; Li, Y.; Zhu, T.; Yue, H.; Wu, W. Low temperature selective catalytic reduction of NOX with NH3 by activated coke loaded with FexCoyCezOm: The enhanced activity, mechanism and kinetics. Fuel 2018, 233, 188–199. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Dong, G. Mn-Ce-V-WOx/TiO2 SCR catalysts: Catalytic activity, stability and interaction among catalytic oxides. Catalysts 2018, 8, 76. [Google Scholar] [CrossRef]
















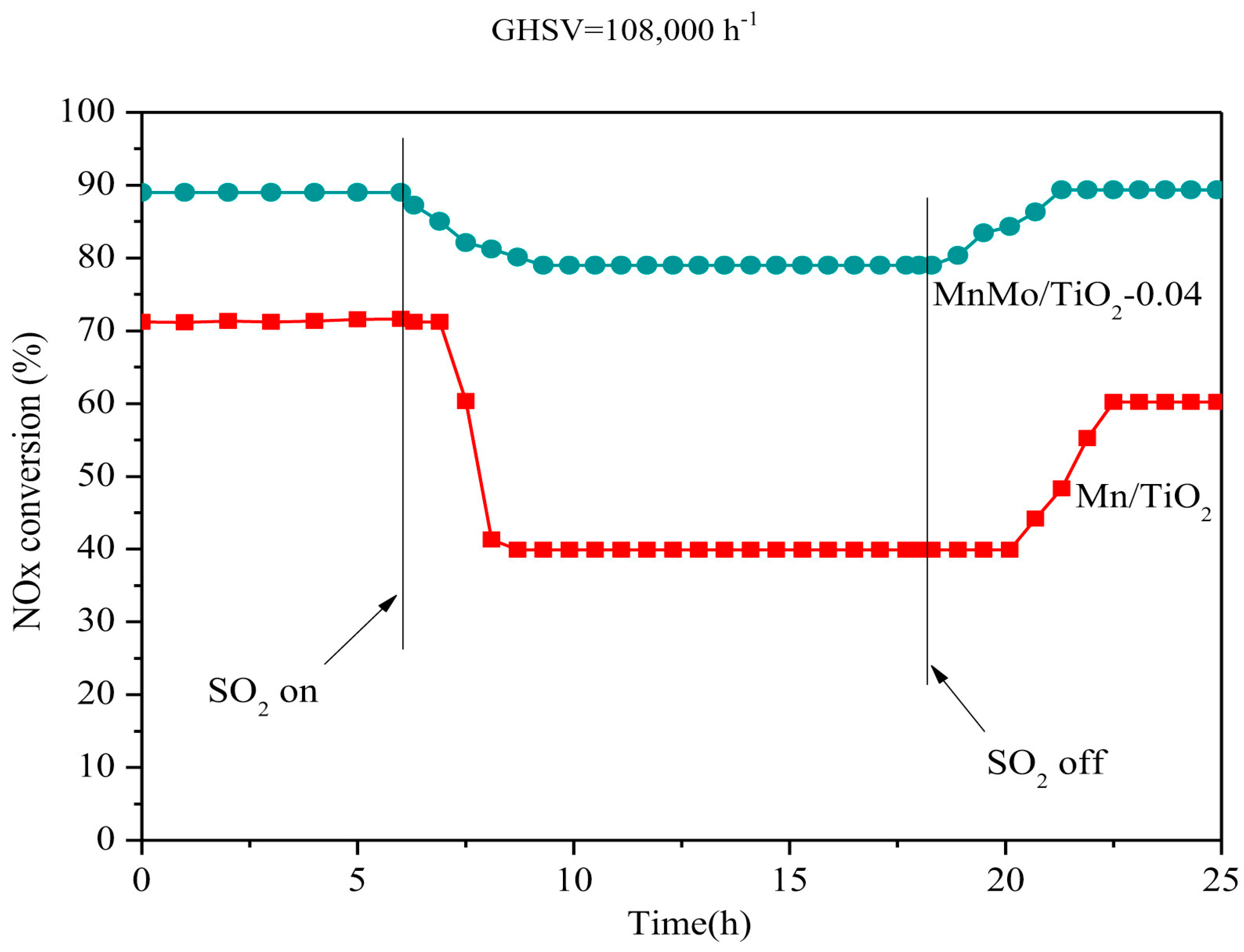

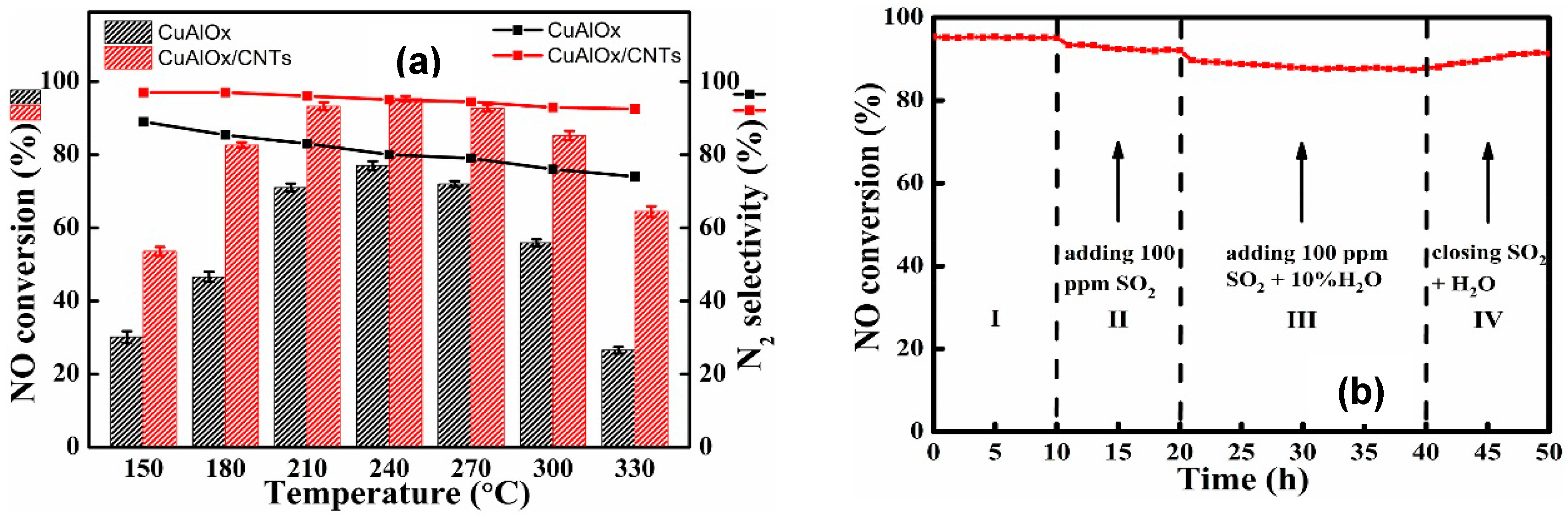
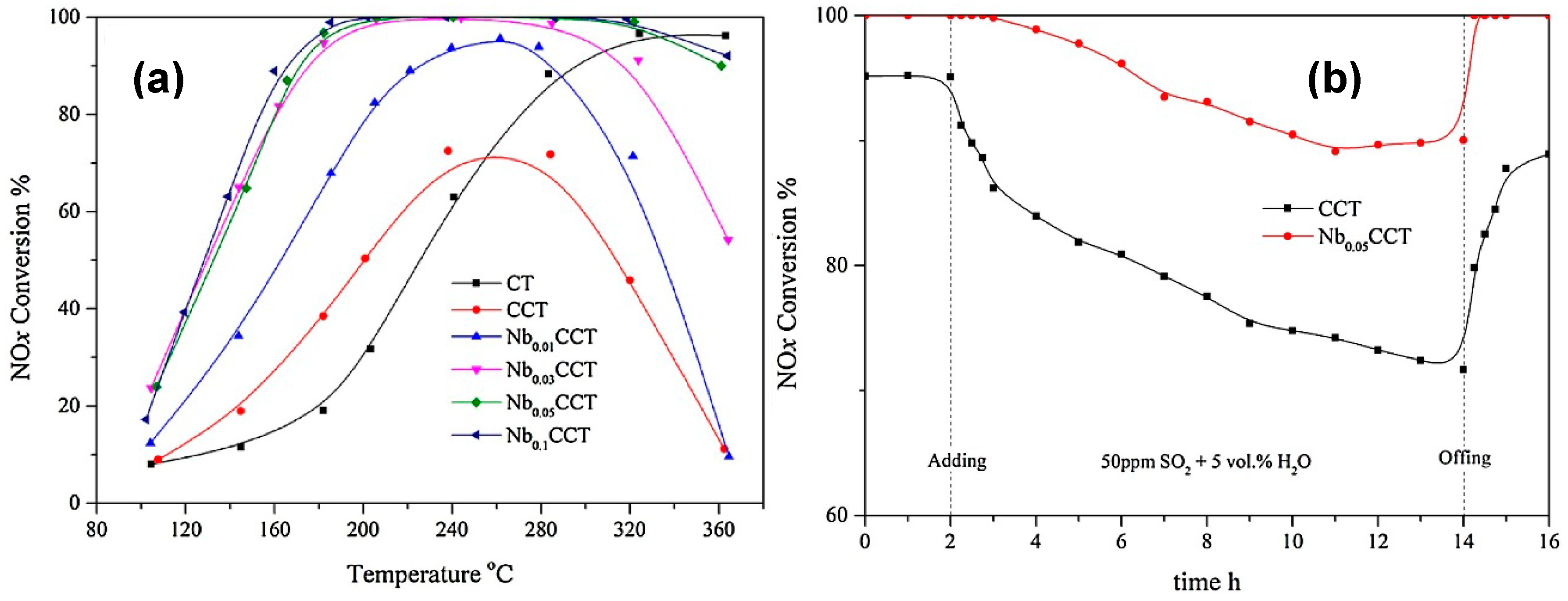

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. https://doi.org/10.3390/catal9040349
Damma D, Ettireddy PR, Reddy BM, Smirniotis PG. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts. 2019; 9(4):349. https://doi.org/10.3390/catal9040349
Chicago/Turabian StyleDamma, Devaiah, Padmanabha R. Ettireddy, Benjaram M. Reddy, and Panagiotis G. Smirniotis. 2019. "A Review of Low Temperature NH3-SCR for Removal of NOx" Catalysts 9, no. 4: 349. https://doi.org/10.3390/catal9040349
APA StyleDamma, D., Ettireddy, P. R., Reddy, B. M., & Smirniotis, P. G. (2019). A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts, 9(4), 349. https://doi.org/10.3390/catal9040349






