Biomimetic Cu/Nitroxyl Catalyst Systems for Selective Alcohol Oxidation
Abstract
:1. Introduction
2. Copper in Biomimetic Oxidation Catalysis
3. Nitroxyl Radicals in Biomimetic Oxidation Catalysis
4. Cu/TEMPO· Catalyst Systems
5. Mechanistic Aspects of Cu/TEMPO·-Catalysed Alcohol Oxidation
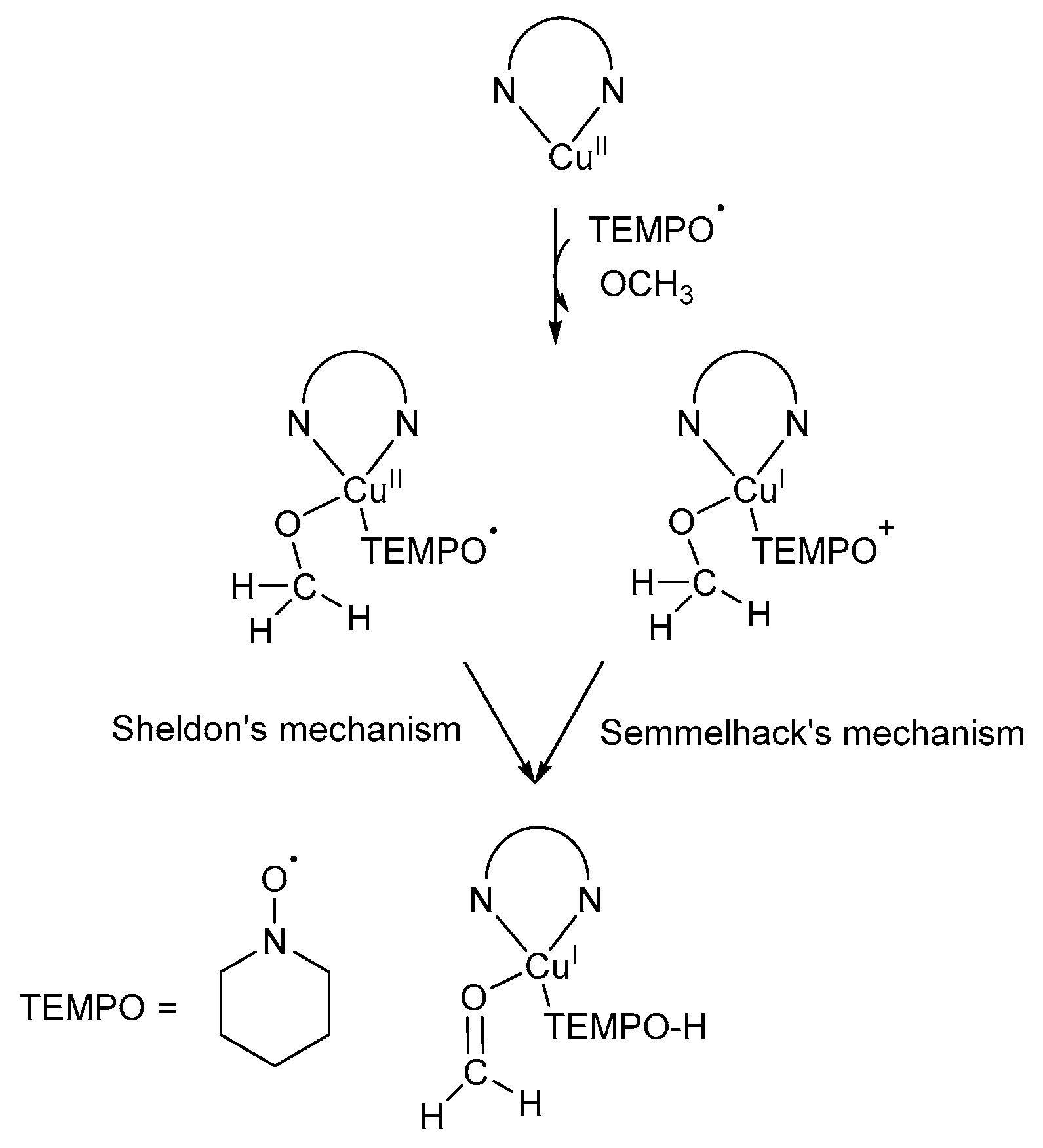
5.1. Option 1: Oxoammonium Cation (TEMPO+) Mechanistic Pathway
5.2. Option 2: Monomeric CuII-TEMPO-H Mechanistic Pathway
5.3. Option 3: A Dimeric CuII-Species-Based Mechanistic Pathway
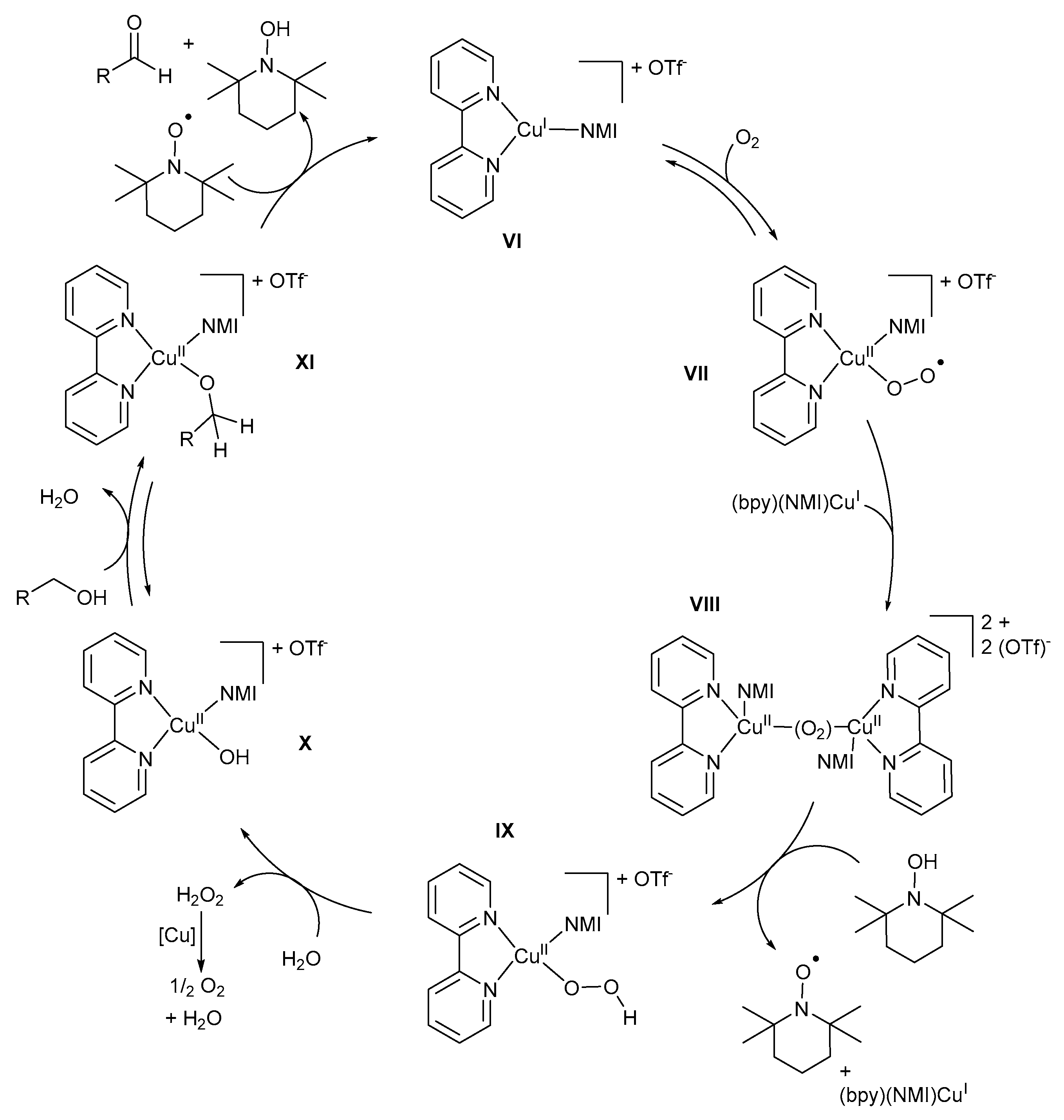
5.4. Option 4: Mononuclear (bpy)(NMI)CuII-O2-TEMPO-Based Mechanistic Pathway
6. Alternative Co-Catalysts in Cu/Nitroxyl-Catalysed Alcohol Oxidation
7. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Q.; Dornan, L.M.; Rogan, L.; Hughes, N.L.; Muldoon, M.J. Aerobic oxidation catalysis with stable radicals. Chem. Commun. 2014, 50, 4524–4543. [Google Scholar] [Green Version]
- Hoover, J.M.; Stahl, S.S. Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. [Google Scholar] [CrossRef] [PubMed]
- Gamez, P.; Arends, I.W.C.E.; Reedijk, J.; Sheldon, R.A. Copper(ii)-catalysed aerobic oxidation of primary alcohols to aldehydes. Chem. Commun. 2003, 2414–2415. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Ten Brink, G.-J.; Dijksman, A. Green, Catalytic Oxidations of Alcohols. Acc. Chem. Res. 2002, 35, 774–781. [Google Scholar] [CrossRef]
- Stahl, S.S. Palladium Oxidase Catalysis: Selective Oxidation of Organic Chemicals by Direct Dioxygen-Coupled Turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Wang, M.; Wu, Z. Mechanistic Insight into the Alcohol Oxidation Mediated by an Efficient Green [CuBr2 (2, 2′-bipy)]-TEMPO Catalyst by Density Functional Method. Inorg. Chem. 2010, 49, 9392–9399. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Schmid, C.R.; Cortes, D.A.; Chou, C.S. Oxidation of alcohols to aldehydes with oxygen and cupric ion, mediated by nitrosonium ion. J. Am. Chem. Soc. 1984, 106, 3374–3376. [Google Scholar] [CrossRef]
- Dijksman, A.; Arends, I.W.C.E.; Sheldon, R.A. Cu(ii)-nitroxyl radicals as catalytic galactose oxidase mimics. Org. Biomol. Chem. 2003, 1, 3232–3237. [Google Scholar] [CrossRef]
- Rabeah, J.; Bentrup, U.; Stößer, R.; Brückner, A. Selective Alcohol Oxidation by a Copper TEMPO Catalyst: Mechanistic Insights by Simultaneously Coupled Operando EPR/UV-Vis/ATR-IR Spectroscopy. Angew. Chem. Int. Ed. 2015, 127, 11957–11960. [Google Scholar] [CrossRef]
- Marais, L.; Bures, J.; Jordaan, J.H.L.; Mapolie, S.; Swarts, A.J. A bis(pyridyl)-N-alkylamine/Cu(i) catalyst system for aerobic alcohol oxidation. Org. Biomol. Chem. 2017, 15, 6926–6933. [Google Scholar] [CrossRef]
- Hoover, J.M.; Ryland, B.L.; Stahl, S.S. Mechanism of Copper(I)/TEMPO-Catalyzed Aerobic Alcohol Oxidation. J. Am. Chem. Soc. 2013, 135, 2357–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendlandt, A.E.; Suess, A.M.; Stahl, S.S. Copper-Catalyzed Aerobic Oxidative C—H Functionalizations: Trends and Mechanistic Insights. Angew. Chem. Int. Ed. 2011, 50, 11062–11087. [Google Scholar]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Chem. Rev. 2005, 105, 2329–2364. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2008, 252, 134–154. [Google Scholar] [CrossRef]
- Schultz, M.J.; Sigman, M.S. Recent advances in homogeneous transition metal-catalyzed aerobic alcohol oxidations. Tetrahedron 2006, 62, 8227–8241. [Google Scholar] [CrossRef]
- Gamez, P.; Aubel, P.G.; Driessen, W.L.; Reedijk, J. Homogeneous bio-inspired copper-catalyzed oxidation reactions. Chem. Soc. Rev. 2001, 30, 376–385. [Google Scholar] [CrossRef]
- Whittaker, J.W. Free radical catalysis by galactose oxidase. Chem. Rev. 2003, 103, 2347–2364. [Google Scholar] [CrossRef]
- Hatcher, L.Q.; Karlin, K.D. Ligand influences in copper-dioxygen complex-formation and substrate oxidations. Adv. Inorg. Chem. 2006, 58, 131–184. [Google Scholar]
- Michel, F.; Thomas, F.; Hamman, S.; Philouze, C.; Saint-Aman, E.; Pierre, J.-L. Galactose Oxidase Models: Creation and Modification of Proton Transfer Coupled to Copper(II) Coordination Processes in Pro-Phenoxyl Ligands. Eur. J. Inorg. Chem. 2006, 2006, 3684–3696. [Google Scholar] [CrossRef]
- Dijksman, A.; Marino-Gonzalez, A.; Mairata i Payeras, A.; Arends, I.W.; Sheldon, R.A. Efficient and selective aerobic oxidation of alcohols into aldehydes and ketones using ruthenium/TEMPO as the catalytic system. J. Am. Chem. Soc. 2001, 123, 6826–6833. [Google Scholar] [CrossRef]
- Whittaker, M.M.; Whittaker, J.W. Ligand interactions with galactose oxidase: Mechanistic insights. Biophys. J. 1993, 64, 762–772. [Google Scholar] [CrossRef]
- Whittaker, M.M.; Ekberg, C.A.; Peterson, J.; Sendova, M.S.; Day, E.P.; Whittaker, J.W. Spectroscopic and magnetochemical studies on the active site copper complex in galactose oxidase. J. Mol. Catal. B Enzym. 2000, 8, 3–15. [Google Scholar] [CrossRef]
- Bailey, W.F.; Bobbit, J.M.; Wiberg, K.B. Mechanism of the Oxidation of Alcohols by Oxoammonium Cations. J. Org. Chem. 2007, 72, 4504–4509. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar]
- Skibida, I.P.; Sakharov, A.M. Molecular oxygen as environmental acceptable, selective and the most strong oxidant in liquid-phase oxidation. Catal. Today 1996, 27, 187–193. [Google Scholar] [CrossRef]
- Ragagnin, G.; Betzemeier, B.; Quici, S.; Knochel, P. Copper-catalysed aerobic oxidation of alcohols using fluorous biphasic catalysis. Tetrahedron 2002, 58, 3985–3991. [Google Scholar] [CrossRef]
- Kumpulainen, E.T.; Koskinen, A.M. Catalytic activity dependency on catalyst components in aerobic copper-TEMPO oxidation. Chem. Eur. J. 2009, 15, 10901–10911. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Industrial oxidations with organocatalyst TEMPO and its derivatives. Org. Process Res. Dev. 2009, 14, 245–251. [Google Scholar] [CrossRef]
- Rozantsev, E.G.; Sholle, V.D. Synthesis and Reactions of Stable Nitroxyl Radicals I. Synthesis. Synthesis 1971, 190–202. [Google Scholar] [CrossRef]
- Griller, D.; Ingold, K.U. Persistent carbon-centered radicals. Acc. Chem. Res. 1976, 9, 13–19. [Google Scholar] [CrossRef]
- Safaei, E.; Hajikhanmirzaei, L.; Karimi, B.; Wojtczak, A.; Cotič, P.; Lee, Y.-I. TEMPO-mediated aerobic oxidation of alcohols using copper (II) complex of bis (phenol) di-amine ligand as biomimetic model for Galactose oxidase enzyme. Polyhedron 2016, 106, 153–162. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E. Catalytic oxidation mediated by metal ions and nitroxyl radicals. J. Mol. Catal. A Chem. 2006, 251, 200–214. [Google Scholar] [CrossRef]
- De Nooy, A.E.; Besemer, A.C.; van Bekkum, H. On the use of stable organic nitroxyl radicals for the oxidation of primary and secondary alcohols. Synthesis 1996, 1996, 1153–1176. [Google Scholar] [CrossRef]
- Gamez, P.; Arends, I.W.C.E.; Sheldon, R.A.; Reedijk, J. Room Temperature Aerobic Copper-Catalysed Selective Oxidation of Primary Alcohols to Aldehydes. Adv. Synth. Catal. 2004, 346, 805–811. [Google Scholar] [CrossRef]
- Karimi, B.; Badreh, E. SBA-15-functionalized TEMPO confined ionic liquid: an efficient catalyst system for transition-metal-free aerobic oxidation of alcohols with improved selectivity. Org. Biomol. Chem. 2011, 9, 4194–4198. [Google Scholar] [CrossRef]
- Cecchetto, A.; Fontana, F.; Minisci, F.; Recupero, F. Efficient Mn–Cu and Mn–Co–TEMPO-catalysed oxidation of alcohols into aldehydes and ketones by oxygen under mild conditions. Tetrahedron Lett. 2001, 42, 6651–6653. [Google Scholar] [CrossRef]
- Karimi, B.; Biglari, A.; Clark, J.H.; Budarin, V. Green, Transition-Metal-Free Aerobic Oxidation of Alcohols Using a Highly Durable Supported Organocatalyst. Angew. Chem. Int. Ed. 2007, 46, 7210–7213. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, X.; Dong, C.; Hu, X. Transition-metal-free: A highly efficient catalytic aerobic alcohol oxidation process. J. Am. Chem. Soc. 2004, 126, 4112–4113. [Google Scholar] [CrossRef] [PubMed]
- Brackman, W.; Gaasbeek, C. Studies in homogeneous catalysis.: Radicals of the R2NO-radical type as catalysts for the oxidation of methanol by cupric complexes and as promoters for various oxidations catalysed by copper. Recl. Trav. Chim. Pays-Bas 1966, 85, 221–241. [Google Scholar] [CrossRef]
- Mannam, S.; Alamsetti, S.K.; Sekar, G. Aerobic, Chemoselective Oxidation of Alcohols to Carbonyl Compounds Catalyzed by a DABCO-Copper Complex under Mild Conditions. Adv. Synth. Catal. 2007, 349, 2253–2258. [Google Scholar] [CrossRef]
- Geißlmeir, D.; Jary, W.G.; Falk, H. The TEMPO/copper catalyzed oxidation of primary alcohols to aldehydes using oxygen as stoichiometric oxidant. Monatsh. Chem. 2005, 136, 1591–1599. [Google Scholar] [CrossRef]
- Ansari Imtiaz, A.; Gree, R. TEMPO-Catalyzed Aerobic Oxidation of Alcohols to Aldehydes and Ketones in Ionic Liquid [bmim][PF6]. Org. Lett. 2002, 4, 1507–1509. [Google Scholar] [CrossRef]
- Betzemeier, B.; Cavazzini, M.; Quici, S.; Knochel, P. Copper-catalyzed aerobic oxidation of alcohols under fluorous biphasic conditions. Tetrahedron Lett. 2000, 41, 4343–4346. [Google Scholar] [CrossRef]
- Mirica, L.M.; Ottenwaelder, X.; Stack, T.D.P. Structure and spectroscopy of copper−dioxygen complexes. Chem. Rev. 2004, 104, 1013–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bie, Z.; Shang, S.; Lv, Y.; Li, G.; Niu, J.; Gao, S. Bioinspired aerobic oxidation of alcohols with a bifunctional ligand based on bipyridine and TEMPO. RSC Adv. 2016, 6, 35008–35013. [Google Scholar] [CrossRef]
- Hoover, J.M.; Ryland, B.L.; Stahl, S.S. Copper/TEMPO-Catalyzed Aerobic Alcohol Oxidation: Mechanistic Assessment of Different Catalyst Systems. ACS Catal. 2013, 3, 2599–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, N.; Ragauskas, A.J. TEMPO-catalyzed oxidation of benzylic alcohols to aldehydes with the H2O 2/HBr/ionic liquid [bmim] PF 6 system. Tetrahedron Lett. 2005, 46, 3323–3326. [Google Scholar] [CrossRef]
- Miller, R.A.; Hoerrner, R.S. Iodine as a chemoselective reoxidant of TEMPO: Application to the oxidation of alcohols to aldehydes and ketones. Org. Lett. 2003, 5, 285–287. [Google Scholar] [CrossRef]
- Mestres, R.; Palenzuela, J. High atomic yield bromine-less benzylic bromination. Green Chem. 2002, 4, 314–316. [Google Scholar] [CrossRef]
- Velusamy, S.; Punniyamurthy, T. Copper (II)-Catalyzed Oxidation of Alcohols to Carbonyl Compounds with Hydrogen Peroxide. Eur. J. Org. Chem. 2003, 2003, 3913–3915. [Google Scholar] [CrossRef]
- Velusamy, S.; Srinivasan, A.; Punniyamurthy, T. Copper(II) catalyzed selective oxidation of primary alcohols to aldehydes with atmospheric oxygen. Tetrahedron Lett. 2006, 47, 923–926. [Google Scholar] [CrossRef]
- Figiel, P.J.; Sibaouih, A.; Ahmad, J.U.; Nieger, M.; Räisänen, M.T.; Leskelä, M.; Repo, T. Aerobic Oxidation of Benzylic Alcohols in Water by 2, 2, 6, 6-Tetramethylpiperidine-1-oxyl (TEMPO)/Copper (II) 2-N-Arylpyrrolecarbaldimino Complexes. Adv. Synth. Catal. 2009, 351, 2625–2632. [Google Scholar] [CrossRef]
- Wagner-Jauregg, T.; Hackley, B.E., Jr.; Lies, T.; Owens, O.; Proper, R. Model Reactions of Phosphorus-containing Enzyme Inactivators. IV. 1a The Catalytic Activity of Certain Metal Salts and Chelates in the Hydrolysis of Diisopropyl Fluorophosphate1b. J. Am. Chem. Soc. 1955, 77, 922–929. [Google Scholar] [CrossRef]
- Figiel, P.J.; Leskelä, M.; Repo, T. TEMPO-Copper(II) Diimine-Catalysed Oxidation of Benzylic Alcohols in Aqueous Media. Adv. Synth. Catal. 2007, 349, 1173–1179. [Google Scholar] [CrossRef]
- Duan, R.; Cheng, L.; Zhang, Q.; Ma, L.; Ma, H.; Yang, J. Mechanistic insight into the aerobic oxidation of benzyl alcohol catalyzed by the Cu II–TEMPO catalyst in alkaline water solution. RSC Adv. 2015, 5, 83976–83984. [Google Scholar] [CrossRef]
- Ryland, B.L.; Stahl, S.S. Practical Aerobic Oxidations of Alcohols and Amines with Homogeneous Copper/TEMPO and Related Catalyst Systems. Angew. Chem. Int. Ed. 2014, 53, 8824–8838. [Google Scholar] [CrossRef]
- Steves, J.E.; Stahl, S.S. Copper(I)/ABNO-Catalyzed Aerobic Alcohol Oxidation: Alleviating Steric and Electronic Constraints of Cu/TEMPO Catalyst Systems. J. Am. Chem. Soc. 2013, 135, 15742–15745. [Google Scholar] [CrossRef]
- Ryland, B.L.; McCann, S.D.; Brunold, T.C.; Stahl, S.S. Mechanism of Alcohol Oxidation Mediated by Copper(II) and Nitroxyl Radicals. J. Am. Chem. Soc. 2014, 136, 12166–12173. [Google Scholar] [CrossRef] [Green Version]
- van Albada, G.A.; Mutikainen, I.; Roubeau, O.; Turpeinen, U.; Reedijk, J. Ferromagnetic trinuclear carbonato-bridged and tetranuclear hydroxo-bridged Cu (II) compounds with 4, 4′-dimethyl-2, 2′-bipyridine as ligand. X-ray structure, spectroscopy and magnetism. Inorg. Chim. Acta 2002, 331, 208–215. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Lin, J.-L. Crystal structures of [Cu2(bpy)2(H2O)(OH)2(SO4)]·4H2O and [Cu(bpy)(H2O)2]SO4 with bpy = 2,2′-bipyridine. Z. Anorg. Allg. Chem. 2003, 629, 1622–1626. [Google Scholar] [CrossRef]
- Faucherre, J.; Petitfaux, C. Copper(II) chelates of pyridine-2-aldoxime. Bull. Soc. Chim. Fr. 1965, 347–359. [Google Scholar]
- Perlepes, S.P.; Huffman, J.C.; Christou, G. Preparation and characterization of dinuclear copper(II) complexes containing the [Cu2(μ-OAc)2]2+ core. Polyhedron 1992, 11, 1471–1479. [Google Scholar] [CrossRef]
- van Albada, G.A.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. Structure, spectroscopy and magnetism of a strong ferromagnetically coupled dinuclear hydroxo-bridged Cu (II) compound with 4, 4′-dimethyl-2, 2′-bipyridine as a ligand. The first X-ray structure of a dinuclear Cu (II) compound with dmbipy. Inorg. Chim. Acta 2001, 324, 273–277. [Google Scholar] [CrossRef]
- Mei, Q.; Liu, H.; Yang, Y.; Liu, H.; Li, S.; Zhang, P.; Han, B. Base-Free Aerobic Oxidation of Alcohols over Copper-Based Complex under Ambient Condition. ACS Sustain. Chem. Eng. 2018, 6, 2362–2369. [Google Scholar]
- Liu, Z.; Shen, Z.; Zhang, N.; Zhong, W.; Liu, X. Aerobic Oxidation of Alcohols Catalysed by Cu (I)/NMI/TEMPO System and Its Mechanistic Insights. Catal. Lett. 2018, 148, 2709–2718. [Google Scholar] [CrossRef]
- Anelli, P.L.; Biffi, C.; Montanari, F.; Quici, S. Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 1987, 52, 2559–2562. [Google Scholar] [CrossRef]
- Rychnovsky, S.D.; Vaidyanathan, R. TEMPO-catalyzed oxidations of alcohols using m-CPBA: The role of halide ions. J. Org. Chem. 1999, 64, 310–312. [Google Scholar] [CrossRef]
- Shibuya, M.; Tomizawa, M.; Iwabuchi, Y. Oxidative rearrangement of tertiary allylic alcohols employing oxoammonium salts. J. Org. Chem. 2008, 73, 4750–4752. [Google Scholar] [CrossRef]
- Bobbit, J.M. Oxoammonium Salts. 6.6 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium Perchlorate: A Stable and Convenient Reagent for the Oxidation of Alcohols. Silica Gel Catalysis. J. Org. Chem. 1998, 63, 9367–9374. [Google Scholar] [CrossRef]
- Semmelhack, M.; Chou, C.S.; Cortes, D.A. Nitroxyl-mediated electrooxidation of alcohols to aldehydes and ketones. J. Am. Chem. Soc. 1983, 105, 4492–4494. [Google Scholar] [CrossRef]
- Michel, C.; Belanzoni, P.; Gamez, P.; Reedijk, J.; Baerends, E.J. Activation of the C− H bond by electrophilic attack: theoretical study of the reaction mechanism of the aerobic oxidation of alcohols to aldehydes by the Cu (bipy) 2+/2, 2, 6, 6-tetramethylpiperidinyl-1-oxy cocatalyst system. Inorg. Chem. 2009, 48, 11909–11920. [Google Scholar] [CrossRef]
- Semmelhack, M.; Schmid, C.R.; Cortés, D.A. Mechanism of the oxidation of alcohols by 2, 2, 6, 6-tetramethylpiperidine nitrosonium cation. Tetrahedron Lett. 1986, 27, 1119–1122. [Google Scholar] [CrossRef]
- Iron, M.A.; Szpilman, A.M. Mechanism of the Copper/TEMPO-Catalyzed Aerobic Oxidation of Alcohols. Chem. Eur. J. 2017, 23, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stack, T. Galactose oxidase model complexes: Catalytic reactivities. J. Am. Chem. Soc. 1996, 118, 13097–13098. [Google Scholar] [CrossRef]
- Caneschi, A.; Grand, A.; Laugier, J.; Rey, P.; Subra, R. Three-center binding of a nitroxyl free radical to copper (II) bromide. J. Am. Chem. Soc. 1988, 110, 2307–2309. [Google Scholar] [CrossRef]
- Laugier, J.; Latour, J.M.; Caneschi, A.; Rey, P. Structural and redox properties of the Tempo adducts of copper (II) halides. Inorg. Chem. 1991, 30, 4474–4477. [Google Scholar] [CrossRef]
- Ben-Daniel, R.; Alsters, P.; Neumann, R. Selective Aerobic Oxidation of Alcohols with a Combination of a Polyoxometalate and Nitroxyl Radical as Catalysts. J. Org. Chem. 2001, 66, 8650–8653. [Google Scholar] [CrossRef]
- Lewis, E.A.; Tolman, W.B. Reactivity of dioxygen-copper systems. Chem. Rev. 2004, 104, 1047–1076. [Google Scholar] [CrossRef]
- Kim, C.; Chen, K.; Kim, J.; Que, L. Stereospecific Alkane Hydroxylation with H2O2 Catalyzed by an Iron (II)− Tris (2-pyridylmethyl) amine Complex. J. Am. Chem. Soc. 1997, 119, 5964–5965. [Google Scholar] [CrossRef]
- Bordwell, F.G.; Liu, W.-Z. Equilibrium Acidities and Homolytic Bond Dissociation Energies of N− H and/or O− H Bonds in N-Phenylhydroxylamine and Its Derivatives. J. Am. Chem. Soc. 1996, 118, 8777–8781. [Google Scholar] [CrossRef]
- Olmstead, W.N.; Margolin, Z.; Bordwell, F.G. Acidities of water and simple alcohols in dimethyl sulfoxide solution. J. Org. Chem. 1980, 45, 3295–3299. [Google Scholar] [CrossRef]
- Walroth, R.C.; Miles, K.C.; Lukens, J.T.; MacMillan, S.N.; Stahl, S.S.; Lancaster, K.M. Electronic Structural Analysis of Copper (II)–TEMPO/ABNO Complexes Provides Evidence for Copper (I)–Oxoammonium Character. J. Am. Chem. Soc. 2017, 139, 13507–13517. [Google Scholar] [CrossRef]
- Holland, P.L.; Rodgers, K.R.; Tolman, W.B. Is the Bis(µ-oxo) dicopper Core Capable of Hydroxylating an Arene? Angew. Chem. Int. Ed. 1999, 28, 1139–1142. [Google Scholar] [CrossRef]
- Kitajima, N.; Moro-oka, Y. Copper-dioxygen complexes. Inorganic and bioinorganic perspectives. Chem. Rev. 1994, 94, 737–757. [Google Scholar] [CrossRef]
- Adomeit, S.; Rabeah, J.; Surkus, A.E.; Bentrup, U.; Brückner, A. Effects of Imidazole-Type Ligands in CuI/TEMPO-Mediated Aerobic Alcohol Oxidation. Inorg. Chem. 2017, 56, 684–691. [Google Scholar] [CrossRef]
- Tebben, L.; Studer, A. Nitroxides: applications in synthesis and in polymer chemistry. Angew. Chem. Int. Ed. 2011, 50, 5034–5068. [Google Scholar]
- Bowman, D.; Gillan, T.; Ingold, K. Kinetic applications of electron paramagnetic resonance spectroscopy. III. Self-reactions of dialkyl nitroxide radicals. J. Am. Chem. Soc. 1971, 93, 6555–6561. [Google Scholar] [CrossRef]
- Malatesta, V.; Ingold, K. Protonated nitroxide radicals. J. Am. Chem. Soc. 1973, 95, 6404–6407. [Google Scholar] [CrossRef]
- Keana, J.F. Newer aspects of the synthesis and chemistry of nitroxide spin labels. Chem. Rev. 1978, 78, 37–64. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Kessel, C.R.; Brien, D.J. Bredt’s rule kinetically stabilized nitrogen-centered radical cations and radicals in the 9-azabicyclo [3.3.1] nonyl system. J. Am. Chem. Soc. 1980, 102, 702–711. [Google Scholar] [CrossRef]
- Sasano, Y.; Nagasawa, S.; Yamazaki, M.; Shibuya, M.; Park, J.; Iwabuchi, Y. Highly chemoselective aerobic oxidation of amino alcohols into amino carbonyl compounds. Angew. Chem. Int. Ed. 2014, 53, 3236–3240. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Konz, Z.M.; Graaf, M.D.; Koolman, H.F.; Stahl, S.S. Electrochemical Oxidation of Alcohols and Aldehydes to Carboxylic Acids Catalyzed by 4-Acetamido-TEMPO: An Alternative to “Anelli” and “Pinnick” Oxidations. ACS Catal. 2018, 8, 6738–6744. [Google Scholar] [CrossRef]
- Shibuya, M.; Tomizawa, M.; Sasano, Y.; Iwabuchi, Y. An expeditious entry to 9-Azabicyclo [3.3.1] nonane N-Oxyl (ABNO): Another highly active organocatalyst for oxidation of alcohols. J. Org. Chem. 2009, 74, 4619–4622. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Sasano, Y.; Nagasawa, S.; Shibuya, M.; Iwabuchi, Y. 9-Azanoradamantane N-oxyl (Nor-AZADO): A highly active organocatalyst for alcohol oxidation. Chem. Pharm. Bull. 2011, 59, 1570–1573. [Google Scholar] [CrossRef]
- Xu, B.; Lumb, J.P.; Arndtsen, B.A. A TEMPO-Free Copper-Catalyzed Aerobic Oxidation of Alcohols. Angew. Chem. Int. Ed. 2015, 54, 4208–4211. [Google Scholar] [CrossRef]
- McCann, S.D.; Lumb, J.-P.; Arndtsen, B.A.; Stahl, S.S. Second-Order Biomimicry: In Situ Oxidative Self-Processing Converts Copper(I)/Diamine Precursor into a Highly Active Aerobic Oxidation Catalyst. ACS Cent. Sci. 2017, 3, 314–321. [Google Scholar] [CrossRef]
- Chaloner, L.; Khomutovskaya, A.; Thomas, F.; Ottenwaelder, X. Supramolecular control of monooxygenase reactivity in a copper (II) cryptate. Dalton Trans. 2016, 45, 11109–11119. [Google Scholar] [CrossRef]
- Zhang, C.X.; Liang, H.-C.; Kim, E.-I.; Gan, Q.-F.; Tyeklár, Z.; Lam, K.-C.; Rheingold, A.L.; Kaderli, S.; Zuberbühler, A.D.; Karlin, K.D. Dioxygen mediated oxo-transfer to an amine and oxidative N-dealkylation chemistry with a dinuclear copper complex. Chem. Commun. 2001, 631–632. [Google Scholar] [CrossRef]
- Belanzoni, P.; Michel, C.; Baerends, E.J. Cu (bipy) 2+/TEMPO-catalyzed oxidation of alcohols: Radical or nonradical mechanism? Inorg. Chem. 2011, 50, 11896–11904. [Google Scholar] [CrossRef]
- Lu, Z.; Costa, J.S.; Roubeau, O.; Mutikainen, I.; Turpeinen, U.; Teat, S.J.; Gamez, P.; Reedijk, J. A copper complex bearing a TEMPO moiety as catalyst for the aerobic oxidation of primary alcohols. Dalton Trans. 2008, 3567–3573. [Google Scholar] [CrossRef]
- Lu, Z.; Ladrak, T.; Roubeau, O.; Van Der Toorn, J.; Teat, S.J.; Massera, C.; Gamez, P.; Reedijk, J. Selective, catalytic aerobic oxidation of alcohols using CuBr2 and bifunctional triazine-based ligands containing both a bipyridine and a TEMPO group. Dalton Trans. 2009, 3559–3570. [Google Scholar] [CrossRef]
- Liu, X.; Xia, Q.; Zhang, Y.; Chen, C.; Chen, W. Cu-NHC-TEMPO catalyzed aerobic oxidation of primary alcohols to aldehydes. J. Org. Chem. 2013, 78, 8531–8536. [Google Scholar] [CrossRef]
- Prathap, K.J.; Maayan, G. Metallopeptoids as efficient biomimetic catalysts. Chem. Commun. 2015, 51, 11096–11099. [Google Scholar] [CrossRef]
- Kwon, O.; Esguerra, K.V.N.; Glazerman, M.; Petitjean, L.; Xu, Y.; Ottenwaelder, X.; Lumb, J.-P. Development of 3, 5-Di-tert-butylphenol as a Model Substrate for Biomimetic Aerobic Copper Catalysis. Synlett 2017, 28, 1548–1553. [Google Scholar]
- Hamann, J.N.; Tuczek, F. New catalytic model systems of tyrosinase: fine tuning of the reactivity with pyrazole-based N-donor ligands. Chem. Commun. 2014, 50, 2298–2300. [Google Scholar] [CrossRef] [Green Version]
- Esguerra, K.V.N.; Fall, Y.; Petitjean, L.; Lumb, J.-P. Controlling the Catalytic Aerobic Oxidation of Phenols. J. Am. Chem. Soc. 2014, 136, 7662–7668. [Google Scholar] [CrossRef]


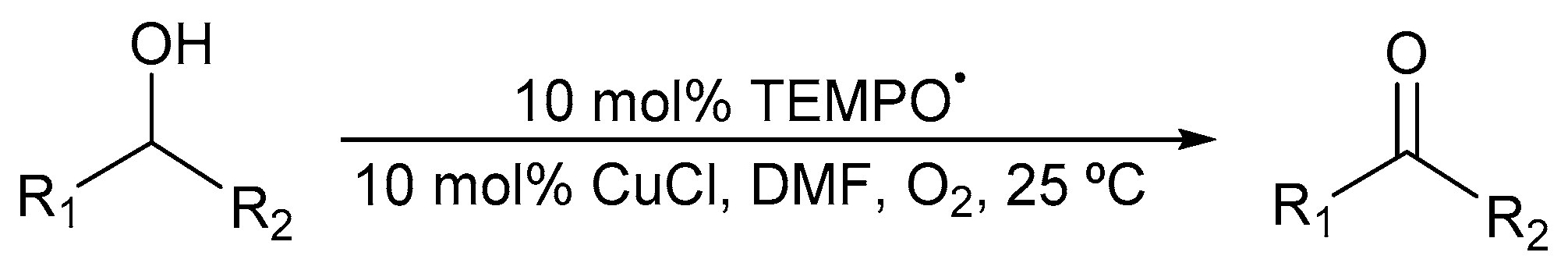





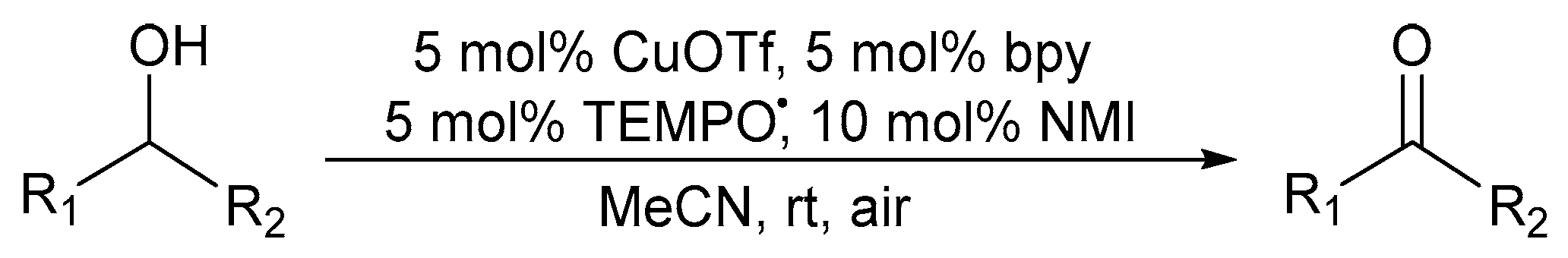














| 1. Stable (persistent) Radicals: Inhibitors | |
| 1.1. Conjugated |  |
| 1.2. Non-conjugated | 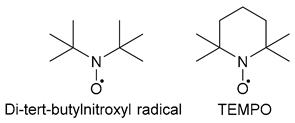 |
| 2. Reactive (non–persistent) Radicals: Catalysts | 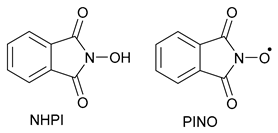 |
| Catalyst System | [Cu] | Ligand | Base | Solvent | Benzylic | Allylic | Aliphatic | Secondary |
|---|---|---|---|---|---|---|---|---|
| Semmelhack [7] | CuCl | - | - | DMF | X | X | ||
| Knockel [44] | Cu | Fluoroalkyl substituted bpy | PhCl/perfluoro-octane | X | X | X | X | |
| Minisci [37] | Cu/Mn | - | - | AcOH | X | X | X | X |
| Ansari and Gree [43] | CuCl | - | - | [bmim]PF6 | X | X | X | |
| Sheldon [8] | CuBr2 | Bpy | KOtBu | MeCN/H2O (2:1) | X | X | X | |
| Geißlmeir [42] | Fine copper powder | Bpy | NaOH | MeCN/H2O (2:1) | X | X | X | |
| Mannam [41] | CuCl | DABCO | - | Toluene | X | X | ||
| Koskinen [28] | Cu(OTf)2 | Bpy | DBU/ NMI | MeCN | X | X | X | |
| Stahl [2] | CuOTf | Bpy | NMI | MeCN | X | X | X | |
| Wang [46] | CuI | Bpy-TEMPO | NMI | MeCN | X | X | X | X |
| Swarts [10] | [Cu(MeCN)4]OTf | L3 | NMI | MeCN | X | X | X |
| Pathway | Cu-Precursor | TEMPO+ | TEMPO· | TEMPO-H | GOase Mimic |
|---|---|---|---|---|---|
| Oxoammonium [7] | CuCl | X | X | ||
| CuII-TEMPO-H [8] | CuBr2 | X | X | ||
| CuII-binuclear [11,28] | Cu(OTf)2/CuOTf | X | |||
| CuII-monomer [9] | CuOTf | X | X |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marais, L.; Swarts, A.J. Biomimetic Cu/Nitroxyl Catalyst Systems for Selective Alcohol Oxidation. Catalysts 2019, 9, 395. https://doi.org/10.3390/catal9050395
Marais L, Swarts AJ. Biomimetic Cu/Nitroxyl Catalyst Systems for Selective Alcohol Oxidation. Catalysts. 2019; 9(5):395. https://doi.org/10.3390/catal9050395
Chicago/Turabian StyleMarais, Lindie, and Andrew John Swarts. 2019. "Biomimetic Cu/Nitroxyl Catalyst Systems for Selective Alcohol Oxidation" Catalysts 9, no. 5: 395. https://doi.org/10.3390/catal9050395
APA StyleMarais, L., & Swarts, A. J. (2019). Biomimetic Cu/Nitroxyl Catalyst Systems for Selective Alcohol Oxidation. Catalysts, 9(5), 395. https://doi.org/10.3390/catal9050395





