Influence of the Titanium Content in the Ti-MCM-41 Catalyst on the Course of the α-Pinene Isomerization Process
Abstract
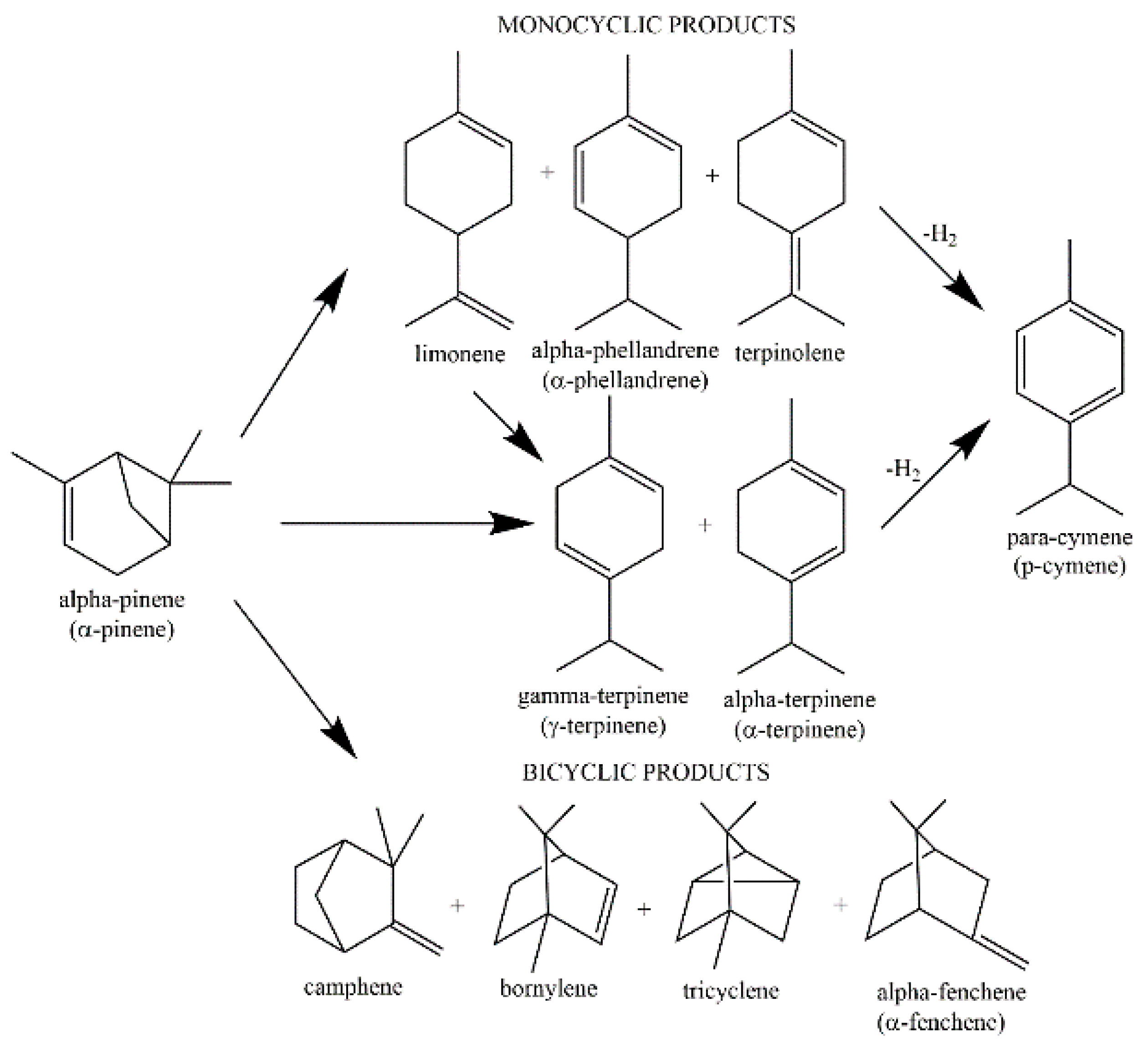
:1. Introduction
2. Results and Discussion
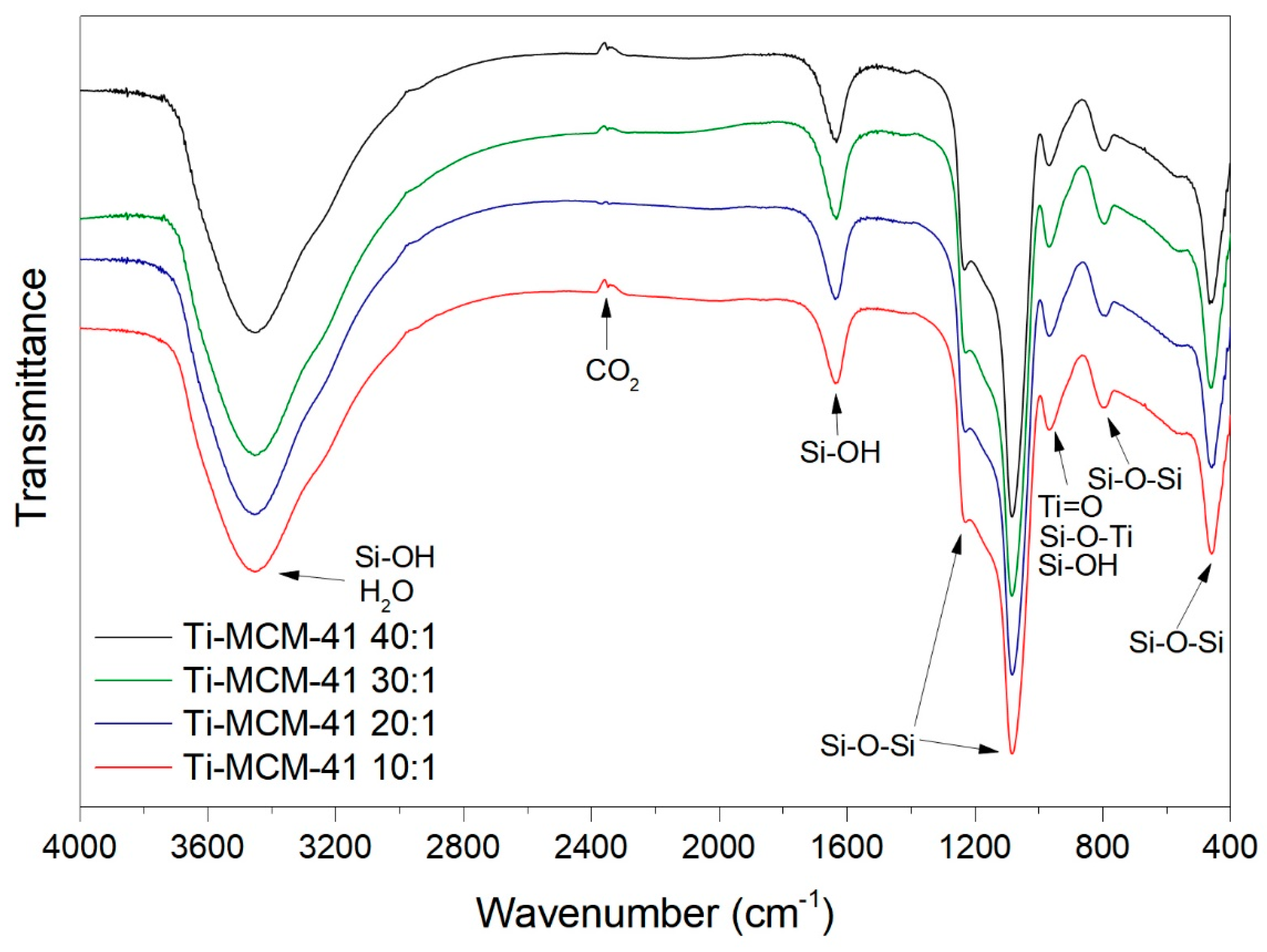
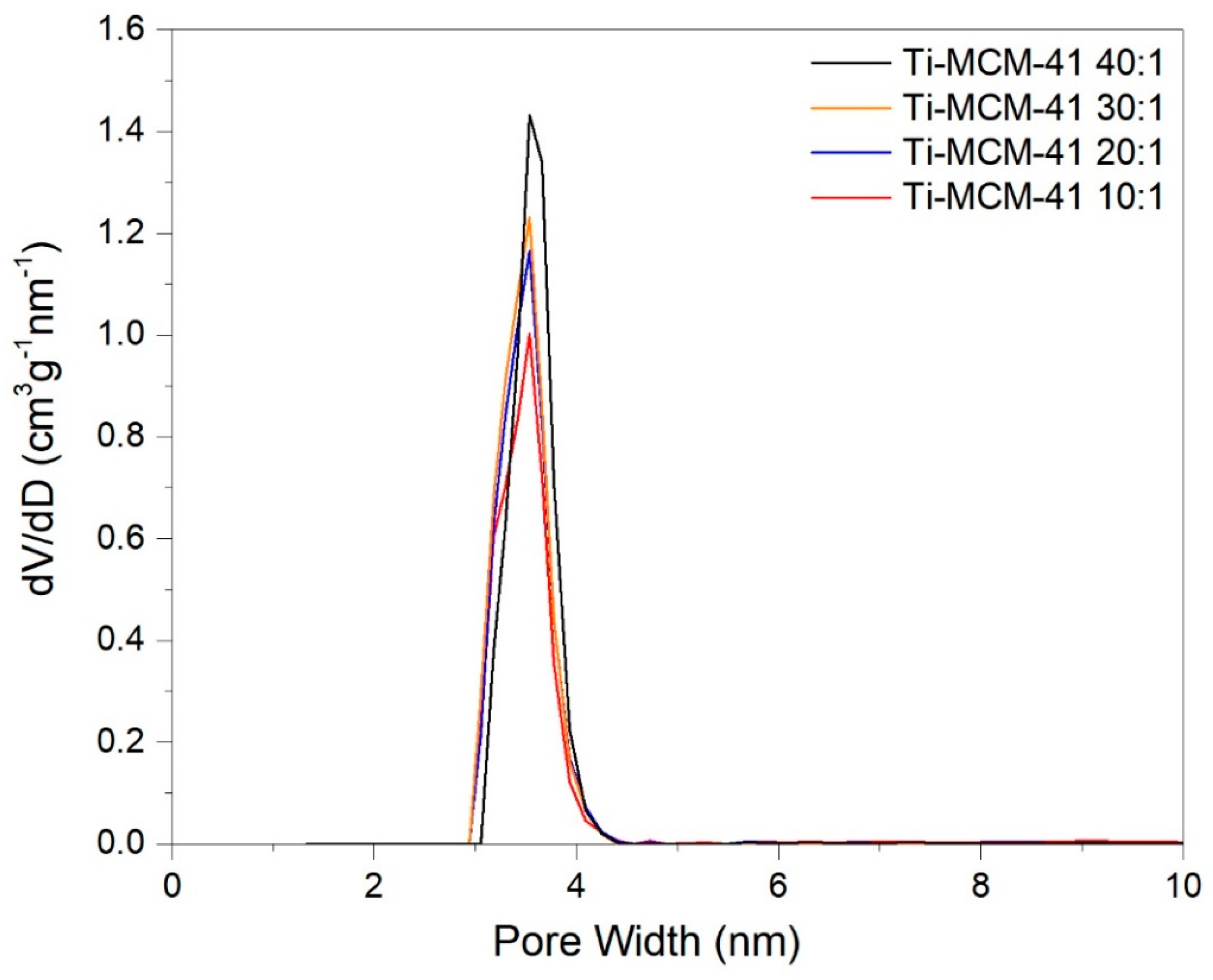
2.1. Characteristics of Catalysts
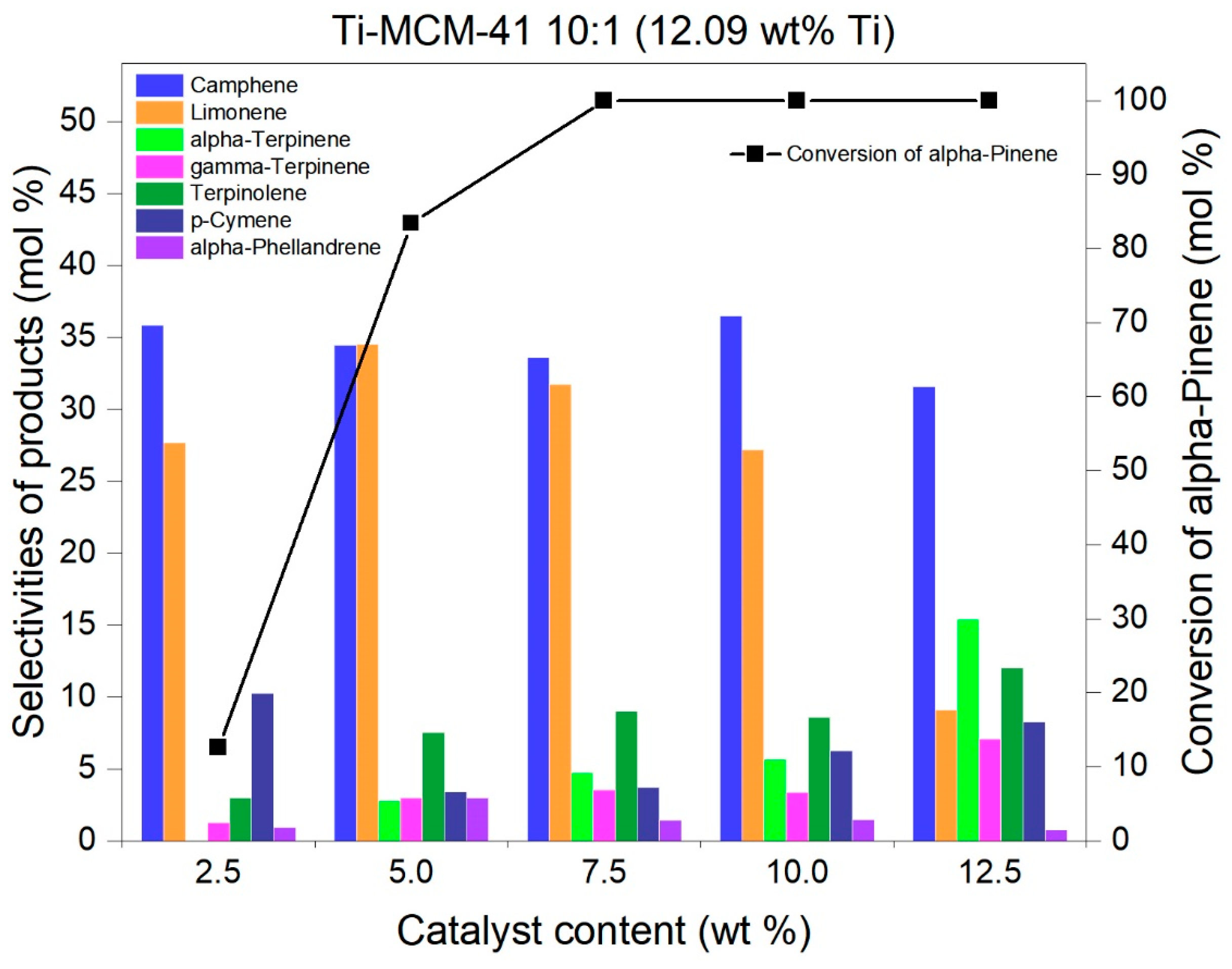
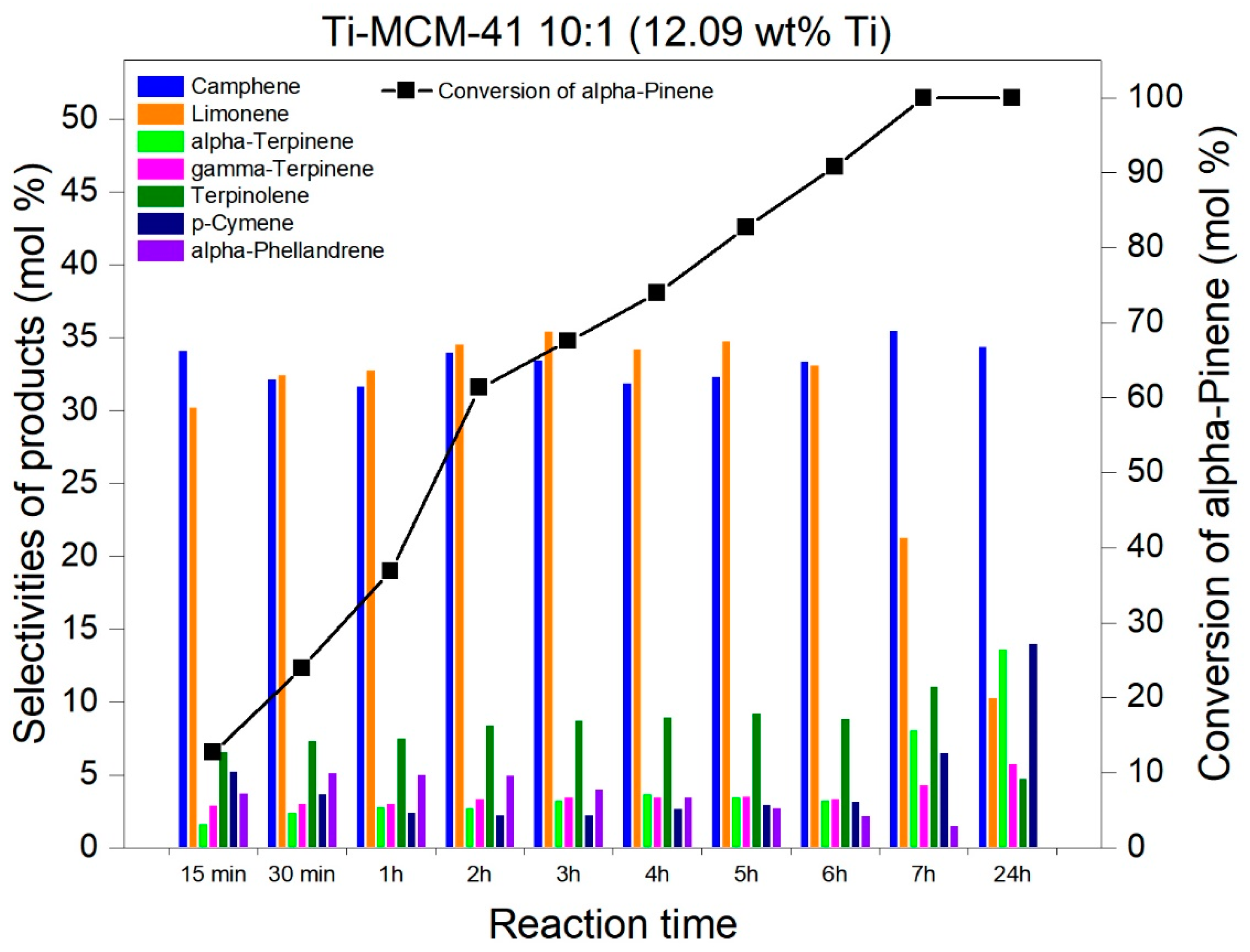
2.2. Studies on the Catalytic Activity of Ti-MCM-41 Materials
3. Materials and Methods
3.1. Synthesis of Ti-MCM-41 Catalysts
3.2. Characteristics of the Ti-MCM-41 Catalysts
3.3. Isomerization of α-Pinene
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Petkovich, N.; Stein, A. Colloidal Crystal Templating Approaches to Materials with Hierarchical Porosity. In Hierarchically Structured Porous Materials; Su, B., Sanchez, C., Yang, X., Zhao, X., Wan, Y., Zhou, W., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 58–61. [Google Scholar]
- Mihai, G.D.; Meynen, V.; Beyers, E.; Mertens, M.; Bilba, N.; Cool, P.; Vansant, E.F. Synthesis, structural characterization and photocatalytic activity of Ti-MCM-41 mesoporous molecular sieves. J. Porous Mater. 2009, 16, 109–118. [Google Scholar] [CrossRef]
- Douroumis, D.; Onyesom, I.; Maniruzzaman, M.; Mitchell, J. Mesoporous silica nanoparticles in nanotechnology. Crit. Rev. Biotechnol. 2013, 33, 229–245. [Google Scholar] [CrossRef]
- Peruzynska, M.; Szelag, S.; Trzeciak, K.; Kurzawski, M.; Cendrowski, K.; Barylak, M. In vitro and in vivo evaluation of sandwich-like mesoporous silica nanoflakes as promising anticancer drug delivery system. Int. J. Pharm. 2016, 506, 458–468. [Google Scholar] [CrossRef]
- Badaničová, M.; Zeleňák, V. Organo-modified mesoporous silica for sorption of carbon dioxide. Monatshefte Chem. 2010, 141, 677–684. [Google Scholar] [CrossRef]
- Brady, R.; Woonton, B.; Gee, M.; O’Connor, A. Hierarchical mesoporous silica materials for separation of functional food ingredients—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 243–248. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.; Li, K. Mesoporous Zeolites: Preparation, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2015; pp. 1–7. [Google Scholar]
- Miądlicki, P.; Wróblewska, A.; Kochmańska, A.; Jędrzejewski, R.; Sreńscek-Nazzal, J. Syntetyczny kanemit jako prekursor mezoporowatych materiałów krzemionkowych. Prz. Geol. 2005, 58, 787. [Google Scholar]
- Melo, R.A.A.; Giotto, M.V.; Rocha, J.; Urquieta-González, E.A. MCM-41 Ordered Mesoporous Molecular Sieves Synthesis and Characterization. Mater. Res. 1991, 2, 173–179. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Contescu, C.I.; Putyera, K. Dekker Encyclopedia of Nanoscience and Nanotechnology; Taylor & Francis Group: New York, NY, USA, 2004; pp. 1797–1811. [Google Scholar]
- Salemi Golezani, A.; Sharifi Fateh, A.; Ahmad Mehrabi, H. Synthesis and characterization of silica mesoporous material produced by hydrothermal continues pH adjusting path way. Prog. Nat. Sci. Mater. Int. 2016, 26, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Barczak, M.; Dąbrowski, A. Mostkowane polisilseskwioksany: Synteza, struktura i właściwości adsorpcyjne. Wiad. Chem. 2008, 62, 977–998. [Google Scholar]
- Wang, S.; Ma, C.; Shi, Y.; Ma, X. Ti incorporation in MCM-41 mesoporous molecular sieves using hydrothermal synthesis. Front. Chem. Sci. Eng. 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Berlini, C.; Guidotti, M.; Moretti, G.; Psaro, R.; Ravasio, N. Catalytic epoxidation of unsaturated alcohols on Ti-MCM-41. Catal. Today 2000, 60, 219–225. [Google Scholar] [CrossRef]
- Silvestre-Alberó, J.; Domine, M.E.; Jordá, J.L.; Navarro, M.T.; Rey, F.; Rodríguez-Reinoso, F.; Corma, A. Spectroscopic, calorimetric, and catalytic evidences of hydrophobicity on Ti-MCM-41 silylated materials for olefin epoxidations. Appl. Catal. A Gen. 2015, 507, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Modified Ti/MCM-41 catalysts for enantioselective epoxidation of styrene. J. Mol. Catal. A Chem. 2016, 420, 282–289. [Google Scholar] [CrossRef]
- Bigi, F.; Piscopo, C.G.; Predieri, G.; Sartori, G.; Scotti, R.; Zanoni, R.; Maggi, R. Molybdenum-MCM-41 silica as heterogeneous catalyst for olefin epoxidation. J. Mol. Catal. A Chem. 2014, 386, 108–113. [Google Scholar] [CrossRef]
- Shu, Y.; Shao, Y.; Wei, X.; Wang, X.; Sun, Q.; Zhang, Q.; Li, L. Synthesis and characterization of Ni-MCM-41 for methyl blue adsorption. Microporous Mesoporous Mater. 2015, 214, 88–94. [Google Scholar] [CrossRef]
- Xin, H.; Ke, T. Preparation and adsorption denitrogenation from model fuel or diesel oil of heteroatoms mesoporous molecular sieve Co-MCM-41. Energy Source Part A. 2016, 38, 2560–2567. [Google Scholar] [CrossRef]
- Collard, X.; Li, L.; Lueangchaichaweng, W.; Bertrand, A.; Aprile, C.; Pescarmona, P.P. Ga-MCM-41 nanoparticles: Synthesis and application of versatile heterogeneous catalysts. Catal. Today. 2014, 235, 184–192. [Google Scholar] [CrossRef]
- Olyaei, A. Rapid and Efficient One-Pot Green Synthesis of 12-Aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-ones Using Zr-MCM-41 Catalyst. Synth. Commun. 2015, 45, 94–104. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Abrokwah, R.Y.; Kuila, D. Synthesis of stable Cu-MCM-41 nanocatalysts for H2 production with high selectivity via steam reforming of methanol. Int. J. Hydrogen Energy 2015, 40, 10439–10452. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Yao, W.; Wu, Z. One-step synthesis of bimetallic Pt-Pd/MCM-41 mesoporous materials with superior catalytic performance for toluene oxidation. Catal. Commun. 2016, 83, 22–26. [Google Scholar] [CrossRef]
- Lan, B.; Huang, R.; Li, L.; Yan, H.; Liao, G.; Wang, X.; Zhang, Q. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chem. Eng. J. 2013, 219, 346–354. [Google Scholar] [CrossRef]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F. Synthesis and characterization of micro-mesoporous MCM-41 using various ionic liquids as co-templates. Microporous Mesoporous Mater. 2015, 217, 219–224. [Google Scholar] [CrossRef]
- Guncheva, M.; Dimitrov, M.; Ossowicz, P.; Janus, E. Tetraalkylammonium acetates and tetraalkylammonium tetrafluoroborates as new templates for room-temperature synthesis of mesoporous silica spheres. J. Porous Mater. 2018, 25, 935–943. [Google Scholar] [CrossRef]
- Agabekov, E.; Sen’kov, G.M.; Sidorenko, A.Y.; Tuyen, N.D.; Tuan, V.A. New α-pinene isomerization catalysts. Catal. Ind. 2011, 3, 319–330. [Google Scholar] [CrossRef]
- Tarbell, I.M. The History of the Standard Oil Company; Cosmico: New York, NY, USA, 2010; p. 274. [Google Scholar]
- Kapp, T.; Kammann, U.; Vobach, M.; Vetter, W. Synthesis of low and high chlorinated toxaphene and comparison of their toxicity by zebrafish (Danio rerio) embryo test. Environ. Toxicol. Chem. 2006, 25, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, D.; Mettee, H. Camphor and its Industrial Synthesis. Chem. Educ. J. 2016, 18, 1–4. [Google Scholar]
- Bin-Tang, L.; Jin-Quan, Y.; Ai-Qun, F.; Ping, Z.; Shu-De, X. Study on selective alkylation of guaiacol with camphene over H-Mordenite. Chin. J. Org. Chem. 1995, 15, 318–322. [Google Scholar]
- Kim, D.H.; Goh, H.J.; Lee, H.W.; Kim, K.S.; Kim, Y.T.; Moon, H.S.; Lee, S.W.; Park, S.Y. The Effect of Terpene Combination on Ureter Calculus Expulsion after Extracorporeal Shock Wave Lithotripsy. Korean J. Urol. 2014, 55, 36–40. [Google Scholar] [CrossRef]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.; Matsuo, A.L.; Travassos, L.R.; Lago, J.H. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, H.Q.; Zhuang, C.F.; Ben, G. Study on Mesoporous PW/SBA-15 for Isomerization of α-Pinene. Appl. Mech. Mater. 2013, 483, 134–137. [Google Scholar] [CrossRef]
- Zou, J.; Chang, N.; Zhang, X.; Wang, L. Isomerization and Dimerization of Pinene using Al-Incorporated MCM-41 Mesoporous Materials. ChemCatChem 2012, 4, 1289–1297. [Google Scholar] [CrossRef]
- Launay, F.; Jarry, B.; Bonardet, J.L. Catalytic activity of mesoporous Ga-SBA-15 materials in α-pinene isomerisation: Similarities and differences with Al-SBA-15 analogues. Appl. Catal. A 2009, 368, 132–138. [Google Scholar] [CrossRef]
- Nie, G.; Zou, J.; Feng, R.; Zhang, X.; Wang, L. HPW/MCM-41 catalyzed isomerization and dimerization of pure pinene and crude turpentine. Catal. Today 2014, 234, 271–277. [Google Scholar] [CrossRef]
- Wróblewska, A.; Miądlicki, P.; Makuch, E. The isomerization of α-pinene over the Ti-SBA-15 catalyst—The influence of catalyst content and temperature. React. Kinet. Mech. Catal. 2016, 119, 641–654. [Google Scholar] [CrossRef]
- Wróblewska, A.; Miądlicki, P.; Sreńscek-Nazzal, J.; Sadłowski, M.; Koren, Z.C.; Michalkiewicz, B. Alpha-pinene isomerization over Ti-SBA-15 catalysts obtained by the direct method: The influence of titanium content, temperature, catalyst amount and reaction time. Microporous Mesoporous Mater. 2018, 258, 72–82. [Google Scholar] [CrossRef]
- Wijayati, N.; Pranowo, H.D.; Jumina, J.; Triyono, T. Synthesis of terpineol from α-pinene catalyzed by TCA/Y-zeolite. Indones. J. Chem. 2011, 11, 234–237. [Google Scholar] [CrossRef]
- Mokrzycki, Ł.; Sulikowski, B.; Olejniczak, Z. Properties of Desilicated ZSM-5, ZSM-12, MCM-22 and ZSM-12/MCM-41 Derivatives in Isomerization of α-Pinene. Catal. Lett. 2009, 127, 296–303. [Google Scholar] [CrossRef]
- Tian, F.; Wu, Y.; Shen, Q.; Li, X.; Chen, Y.; Meng, C. Effect of Si/Al ratio on mesopore formation for zeolite beta via NaOH treatment and the catalytic performance in α-pinene isomerization and benzoylation of naphthalene. Microporous Mesoporous Mater. 2013, 173, 129–138. [Google Scholar] [CrossRef]
- Ecormier, M.A.; Lee, A.F.; Wilson, K. High activity, templated mesoporous SO4/ZrO2/HMS catalysts with controlled acid site density for α-pinene isomerization. Microporous Mesoporous Mater. 2005, 80, 129–138. [Google Scholar] [CrossRef]
- Ünveren, E.; Gündüz, G.; Cakicioğlu-Özkan, F. Isomerization of Alpha-pinene Over Acid Treated Natural Zeolite. Chem. Eng. Commun. 2005, 192, 386–404. [Google Scholar] [CrossRef] [Green Version]
- Gündüz, G.; Murzin, D.Y. Influence of catalyst pretreatment on alpha-pinene isomerization over natural clays. React. Kinet. Mech. Catal. 2002, 75, 231–237. [Google Scholar] [CrossRef]
- Akpolat, O.; Gündüz, G.; Ozkan, F.; Beşün, N. Isomerization of α-pinene over calcined natural zeolites. Appl. Catal. A 2004, 265, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Chudasama, C.; Jasra, R. Isomerisation of α-pinene using modified montmorillonite clays. J. Mol. Catal. A Chem. 2004, 216, 51–59. [Google Scholar] [CrossRef]
- Atalay, B.; Gündüz, G. Isomerizaton of α-pinene over H3PW12O40 catalysts supported on natural zeolite. Chem. Eng. J. 2011, 168, 1311–1318. [Google Scholar] [CrossRef]
- Luan, Z.; He, H.; Zhou, W.; Klinowski, J. Transformation of lamellar silicate into the mesoporous molecular sieve MCM-41. Chem. Soc., Faraday Trans. 1998, 94, 979–983. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Elmorsi, M.A.; Ebeid, E. Corrosion Protection of Aluminum Metal Using MCM-41 Films Supported by Silver Nanoparticles and Distyrylpyrazine Photopolymer. Adv. Sci. Eng. Med. 2015, 7, 423–428. [Google Scholar] [CrossRef]
- Hu, Y.; Martra, G.; Zhang, J.; Higashimoto, S.; Coluccia, S.; Anpo, M. Characterization of the Local Structures of Ti-MCM-41 and Their Photocatalytic Reactivity for the Decomposition of NO into N2 and O2. J. Phys. Chem. B 2006, 110, 1680–1685. [Google Scholar] [CrossRef]
- Balu, A.M.; Hidalgo, J.M.; Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Microwave oxidation of alkenes and alcohols using highly active and stable mesoporous organotitanium silicates. J. Mol. Catal. A Chem. 2008, 293, 17–24. [Google Scholar] [CrossRef]
- Han, Y.; Kim, H.; Park, J.; Lee, S.; Kim, J. Influence of Ti doping level on hydrogen adsorption of mesoporous Ti-SBA-15 materials prepared by direct synthesis. Int. J. Hydrogen Energy. 2012, 37, 14240–14247. [Google Scholar] [CrossRef]
- Azimov, F.; Markova, I.; Stefanova, V.; Sharipov, K. Synthesis and characterization of SBA-15 and Ti-SBA-15 nanopurous materials for DME catalysts. J. Univ. Chem. Technol. Metall. 2012, 47, 333–340. [Google Scholar]
- Angeles-Beltrán, D.; Negrón-Silva, G.; Lomas-Romero, L.; Iglesias-Arteaga, M.A.; Santos-Aires, F.J.C. Titanium-modified MCM-41 Prepared by Ultrasound and by Hydrothermal Treatment, Catalysts for Acetylation Reactions. J. Mex. Chem. Soc. 2008, 52, 175–180. [Google Scholar]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Lee, Y.R. Highly graphitic carbon nanosheets synthesized over tailored mesoporous molecular sieves using acetylene by chemical vapor deposition method. RSC Adv. 2015, 5, 93364–93373. [Google Scholar] [CrossRef]
- Frattini, L.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Kyriakou, G.; Lee, A.F. Support enhanced α-pinene isomerization over HPW/SBA-15. Appl. Catal. B Environ. 2017, 200, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Grün, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel pathways for the preparation of mesoporous MCM-41 materials: Control of porosity and morphology. Microporous Mesoporous Mater. 1999, 27, 207–216. [Google Scholar] [CrossRef]
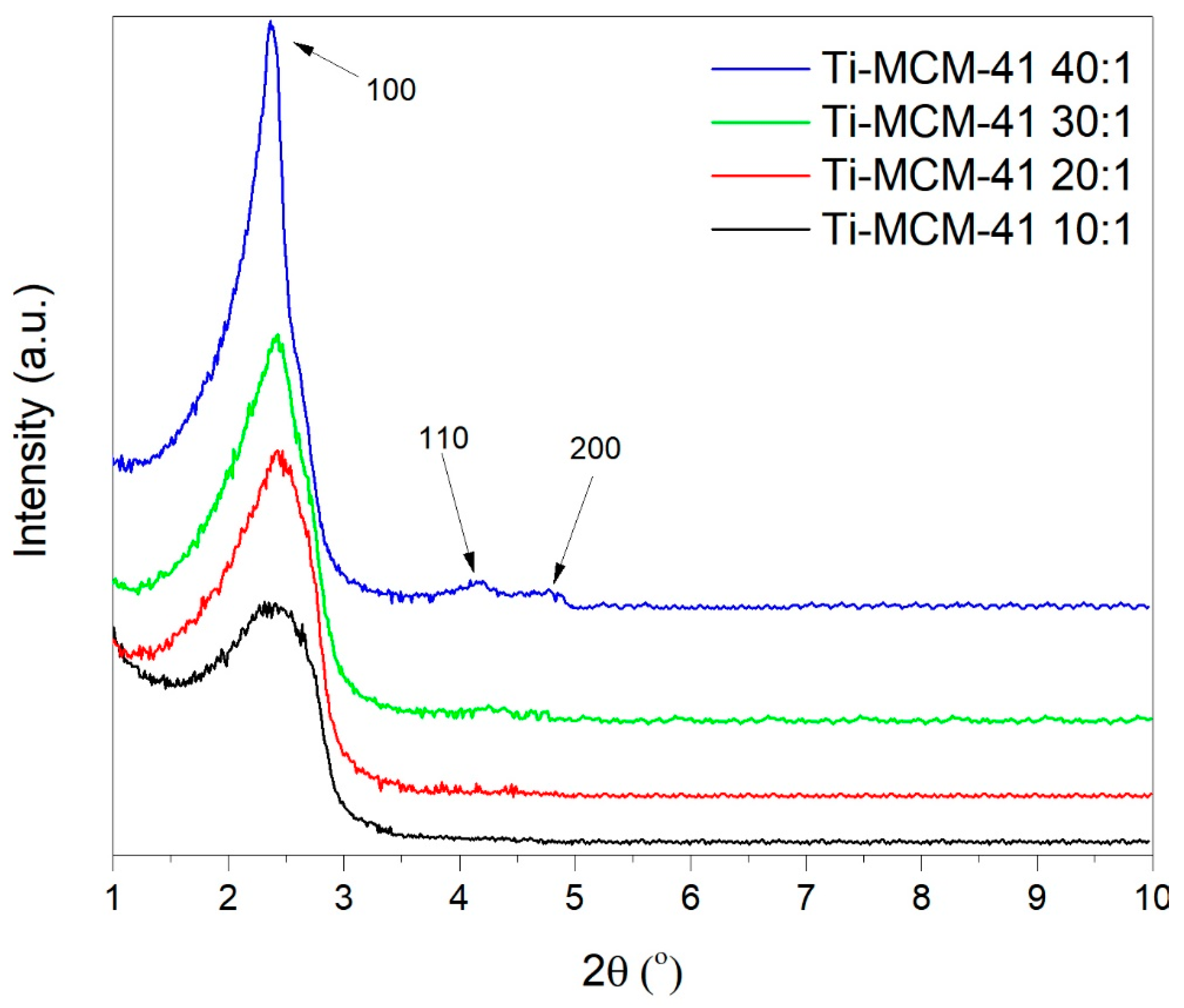
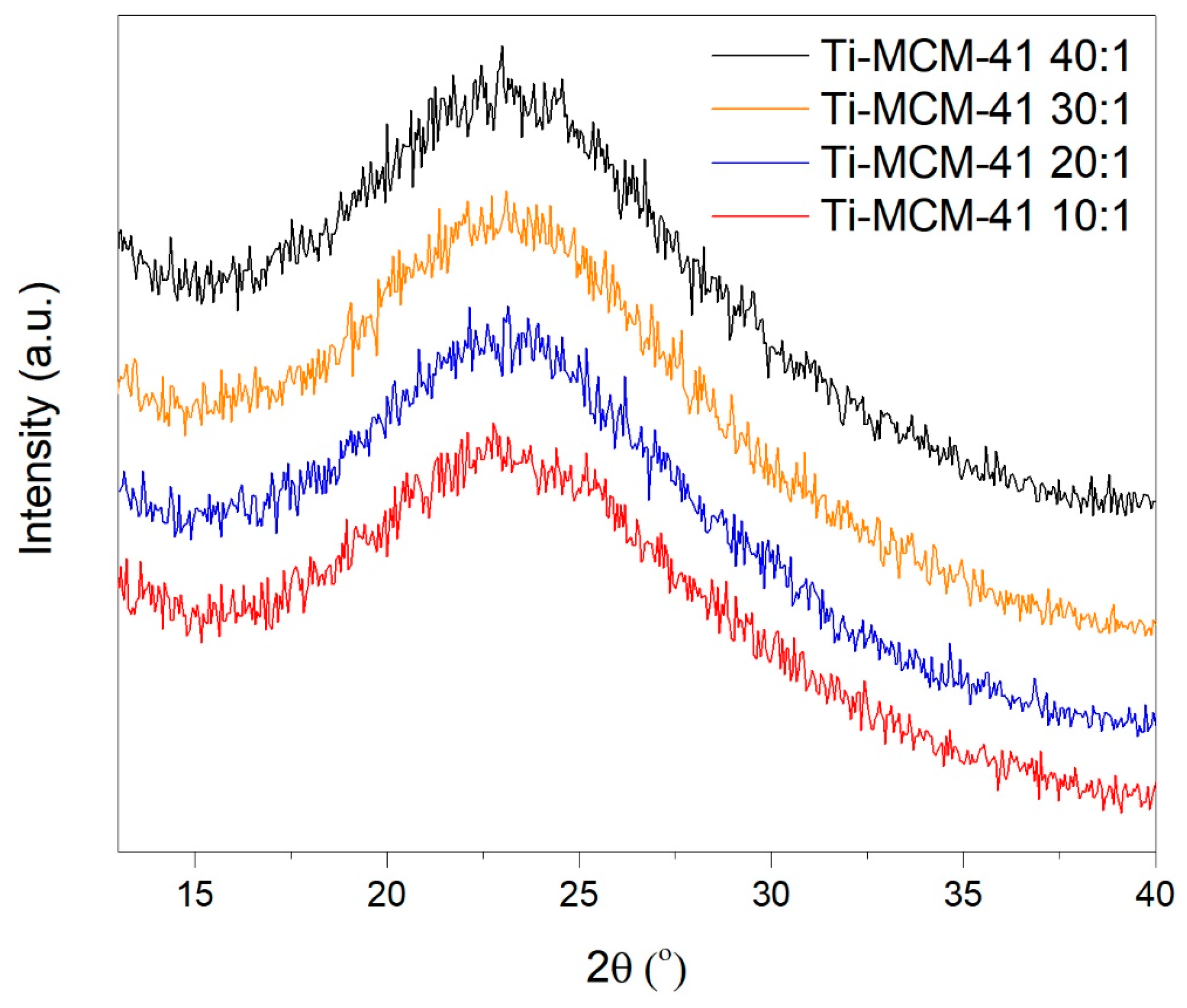
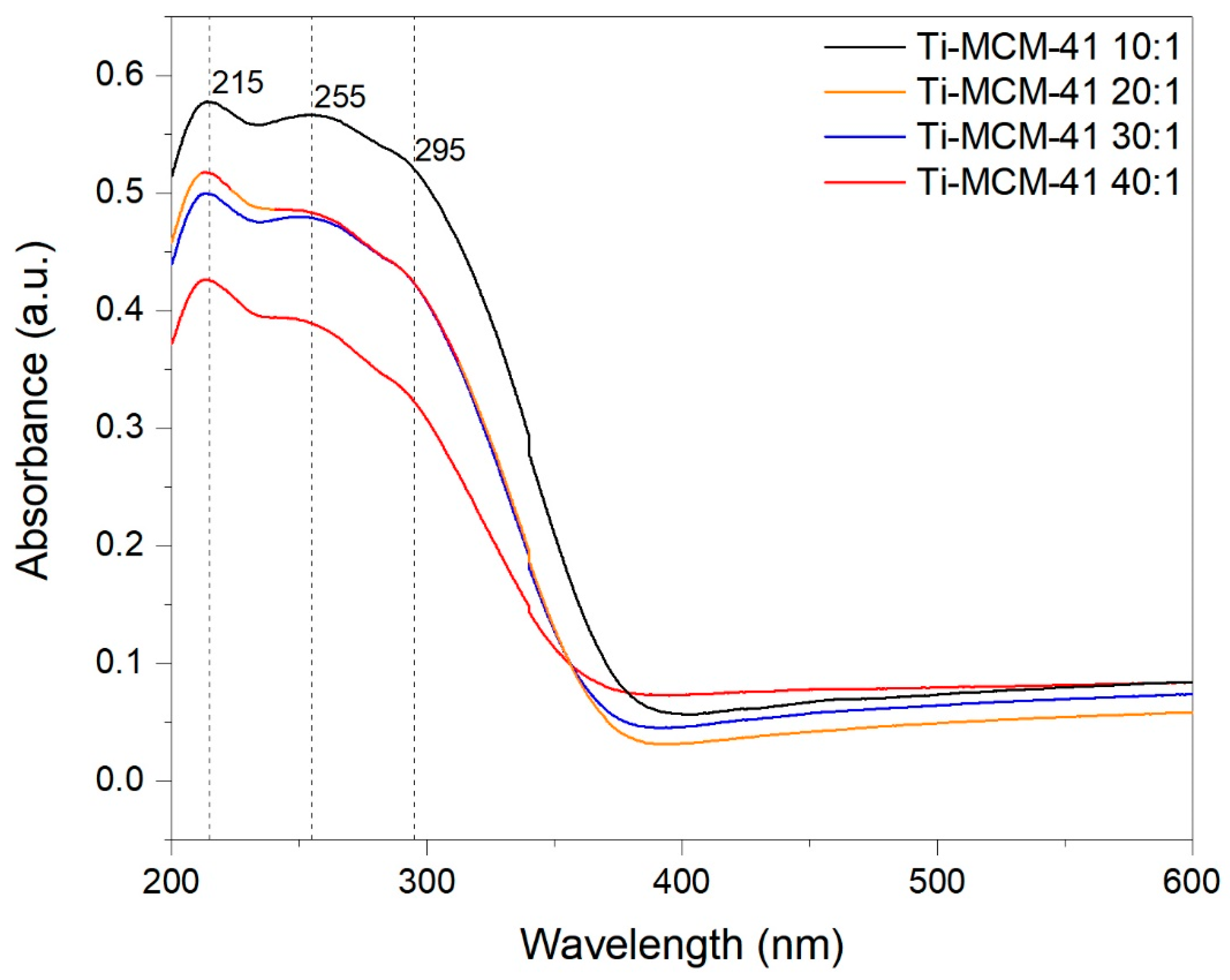




| Material | SBET (m2/g) | Vp (cm3/g) |
|---|---|---|
| Ti-MCM-41 10:1 | 902 | 0.839 |
| Ti-MCM-41 20:1 | 1014 | 0.839 |
| Ti-MCM-41 30:1 | 1071 | 0.826 |
| Ti-MCM-41 40:1 | 1063 | 0.840 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, A.; Miądlicki, P.; Tołpa, J.; Sreńscek-Nazzal, J.; Koren, Z.C.; Michalkiewicz, B. Influence of the Titanium Content in the Ti-MCM-41 Catalyst on the Course of the α-Pinene Isomerization Process. Catalysts 2019, 9, 396. https://doi.org/10.3390/catal9050396
Wróblewska A, Miądlicki P, Tołpa J, Sreńscek-Nazzal J, Koren ZC, Michalkiewicz B. Influence of the Titanium Content in the Ti-MCM-41 Catalyst on the Course of the α-Pinene Isomerization Process. Catalysts. 2019; 9(5):396. https://doi.org/10.3390/catal9050396
Chicago/Turabian StyleWróblewska, Agnieszka, Piotr Miądlicki, Jadwiga Tołpa, Joanna Sreńscek-Nazzal, Zvi C. Koren, and Beata Michalkiewicz. 2019. "Influence of the Titanium Content in the Ti-MCM-41 Catalyst on the Course of the α-Pinene Isomerization Process" Catalysts 9, no. 5: 396. https://doi.org/10.3390/catal9050396






