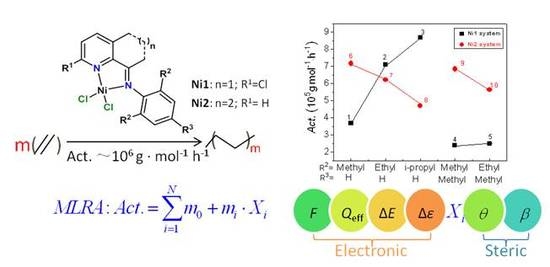
The Catalytic Activities of Carbocyclic Fused Pyridineimine Nickel Complexes Analogues in Ethylene Polymerization by Modeling Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Predicted Activity of Ni1 System
2.2. Predicted Activity of Ni2 System
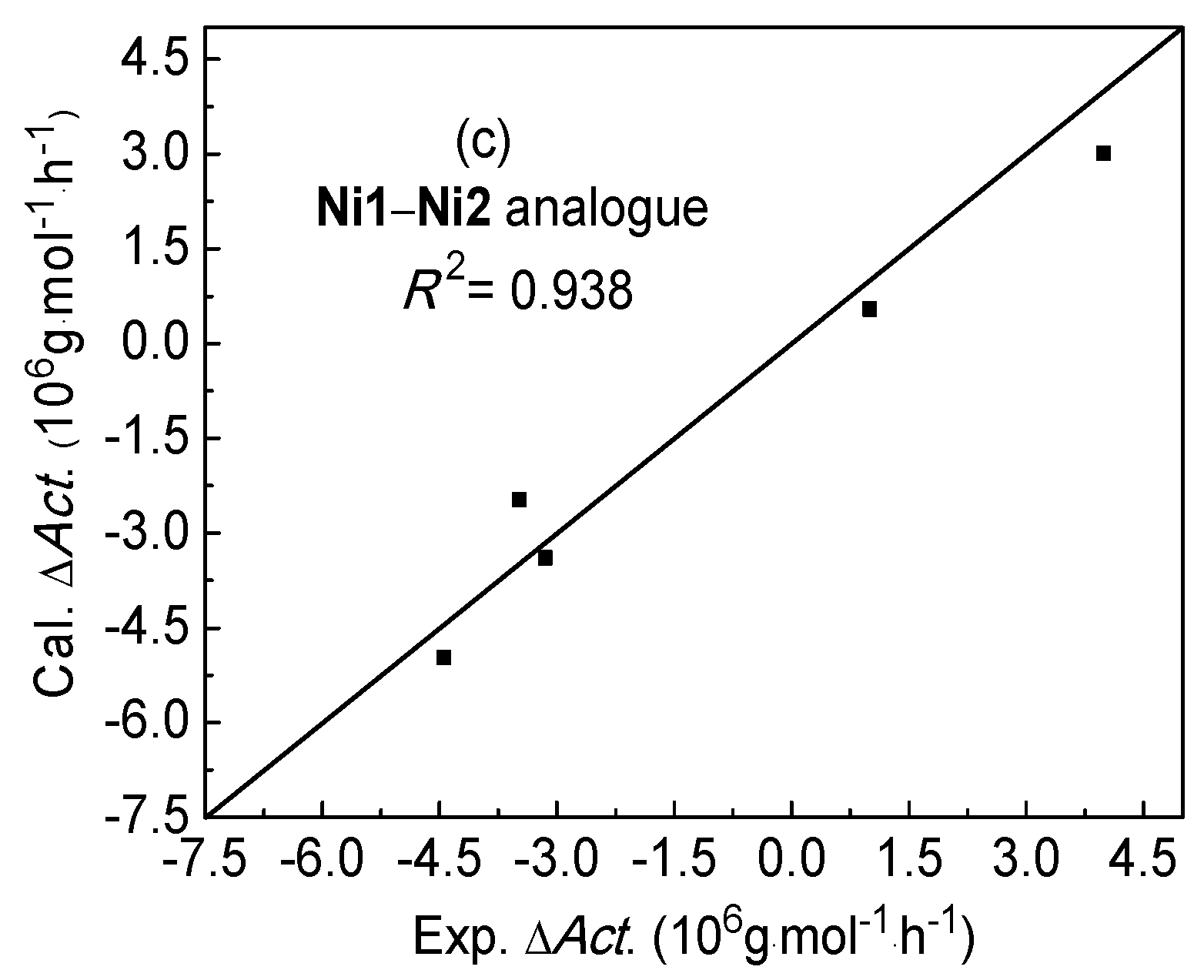
2.3. Difference of Catalytic Activity between Ni1 and Ni2 Systems
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond Metallocenes. Angew. Chem. Int. Ed. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1345. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.E.; Arriagada, D.C.; Labbe, A.T. Mechanistic details of ethylene polymerization reaction using methallyl nickel(II) catalysts. Phys. Chem. Chem. Phys. 2018, 20, 22915. [Google Scholar] [CrossRef]
- Martinez, A.J.I.; Quijada, R.; Toro-Labbe, A. The mechanism of ethylene polymerization reaction catalyzed by group IVB metallocenes. A rational analysis through the use of reaction force. J. Phys. Chem. C 2012, 116, 21318–21325. [Google Scholar] [CrossRef]
- Takeuchi, D. Recent progress in olefin polymerization catalyzed by transition metal complexes: New catalysts and new reactions. Dalton Trans. 2010, 39, 311–328. [Google Scholar] [CrossRef]
- Sun, W.-H. Novel polyethylenes via late transition metal complex pre-catalysts. Adv. Polym. Sci. 2013, 258, 163–178. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.-H.; Redshaw, C. Tailoring iron complexes for ethylene oligomerization and/or polymerization. Dalton Trans. 2013, 42, 8988–8997. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef] [Green Version]
- Piccolrovazzi, N.; Pino, P.; Consiglio, G.; Sironi, A.; Moret, M. Electronic effects in homogeneous indenylzirconium Ziegler-Natta catalysts. Organometallics 1990, 9, 3098–3105. [Google Scholar] [CrossRef]
- Lee, I.-M.; Gauthier, W.J.; Ball, J.M.; Iyengar, B.; Collins, S. Electronic effects in Ziegler-Natta polymerization of propylene and ethylene using soluble metallocene catalysts. Organometallics 1992, 11, 2115–2122. [Google Scholar] [CrossRef]
- Mohring, P.C.; Coville, N.J. Quantification of the influence of steric and electronic parameters on the ethylene polymerisation activity of (CpR)2ZrC12/ethylaluminoxane Ziegler-Natta catalysts. J. Mol. Catal. 1992, 77, 41–50. [Google Scholar] [CrossRef]
- Mohring, P.C.; Coville, N.J. Homogeneous group 4 metallocene Ziegler-Natta catalysts: The influence of cyclopentadienyl-ring substituents. J. Organomet. Chem. 1994, 479, 1–29. [Google Scholar] [CrossRef]
- Mohring, P.C.; Vlachakis, N.; Grimmer, N.E.; Coville, N.J. The influence of steric and electronic effects of substituents on the cyclopentadienyl ring on the behaviour of (CpR)2TiCl2 and (CpR)CpTiCl2/Et3Al2Cl3 catalysts in polymerization of ethane. Organomet. Chem. 1994, 483, 159–166. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Sha, R.; Fu, R.-D.; Sun, W.-H. Correlating net charges and the activity of bis(imino)pyridylcobalt complexes in ethylene polymerization. Inorg. Chim. Acta 2014, 423, 450–453. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.; Sun, W.-H. Assessing catalytic activities through modeling net charges of iron complex precatalysts. Macromol. Chem. Phys. 2014, 215, 1810–1817. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.; Sun, W.-H. Correlating cobalt net charges with catalytic activities of the 2-(Benzimidazolyl)-6-(1-aryliminoethyl) pyridylcobalt complexes toward ethylene polymerization. Macromol. React. Eng. 2015, 9, 473–479. [Google Scholar] [CrossRef]
- Yang, W.; Yi, J.; Sun, W.-H. Revisiting benzylidenequinolinylnickel catalysts through the electronic effects on catalytic activity by DFT studies. Macromol. Chem. Phys. 2015, 216, 1125–1133. [Google Scholar] [CrossRef]
- Yi, J.; Yang, W.; Sun, W.-H. Quantitative investigation of the electronic and steric influences on ethylene Oligo/Polymerization by 2-Azacyclyl-6-aryliminopyridylmetal (Fe, Co and Cr) complexes. Macromol. Chem. Phys. 2016, 217, 757–764. [Google Scholar] [CrossRef]
- Casey, C.P.; Whiteker, G.T. The natural bite angle of chelating diphosphines. Isr. J. Chem. 1990, 30, 299–304. [Google Scholar] [CrossRef]
- Van-Leeuwen, P.W.N.M.; Kamer, P.C.J.; Reek, J.N.H.; Dierkes, P. Ligand bite angle effects in metal-catalyzed C-C bond formation. Chem. Rev. 2000, 100, 2741–2769. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, Z.; Sun, W.-H. Modeling study on the catalytic activities of 2-imino-1,10-phenanthrolinylmetal (Fe, Co, and Ni) precatalysts in ethylene oligomerization. RSC Adv. 2016, 6, 79335–79342. [Google Scholar] [CrossRef]
- Suo, H.; Solan, G.A.; Ma, Y.; Sun, W.-H. Development in compartmentalized bimetallic transition metal ethylene polymerization catalysts. Coord. Chem. Rev. 2018, 372, 101–116. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: N imine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymerstructure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Yu, J.; Hu, X.; Zeng, Y.; Zhang, L.; Ni, C.; Hao, X.; Sun, W.-H. Synthesis, characterisation and ethylene oligomerization behaviour of N-(2-substituted-5,6,7-trihydroquinolin-8-ylidene) arylaminonickel dichlorides. New J. Chem. 2011, 35, 178–183. [Google Scholar] [CrossRef]
- Huang, F.; Sun, Z.; Du, S.; Yue, E.; Ba, J.; Hu, X.; Liang, T.; Galland, G.B.; Sun, W.-H. Ring-tension adjusted ethylene polymerization by aryliminocycloheptapyridyl nickel complexes. Dalton Trans. 2015, 44, 14281–14292. [Google Scholar] [CrossRef]
- Ahmed, S.; Yang, W.; Ma, Z.; Sun, W.-H. Catalytic activities of bis(pentamethylene)pyridyl(Fe/Co) complex analogues in ethylene polymerization by modeling method. J. Phys. Chem. A 2018, 122, 9637–9644. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Le, T.; Epa, V.C.; Burden, F.R.; Winkler, D.A. Quantitative structure—Property relationship modeling of diverse materials properties. Chem. Rev. 2012, 112, 2889–2919. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the Dmol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Becke, A.D. A multicenter numerical integration scheme for polyatomic molecules. J. Chem. Phys. 1988, 88, 2547–2553. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B Condens. Matter Mater. Phys. 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Bergner, A.; Dolg, M.; Kuechle, W.; Stoll, H.; Preub, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Li, Z.; Bian, K.; Zhou, M. Excel for Windows 95 Encyclopaedia; McFedried, P., Ed.; Electronics Industry Press: Beijing, China, 1997. [Google Scholar]
- Sachdev, H.P.S.; Satyanarayan, L.; Kumar, S.; Puri, R.K. Classification of nutritional status as ‘Z score’ or per cent of reference median—Does it alter mortality prediction in malnourished children? Int. J. Epiderm. 1992, 21, 916–921. [Google Scholar] [CrossRef]
- Ayatollahi, S.M.T. Age standardization of weight-for-height in children using a unified z-score method. Ann. Hum. Biol. 1995, 22, 151–162. [Google Scholar] [CrossRef]
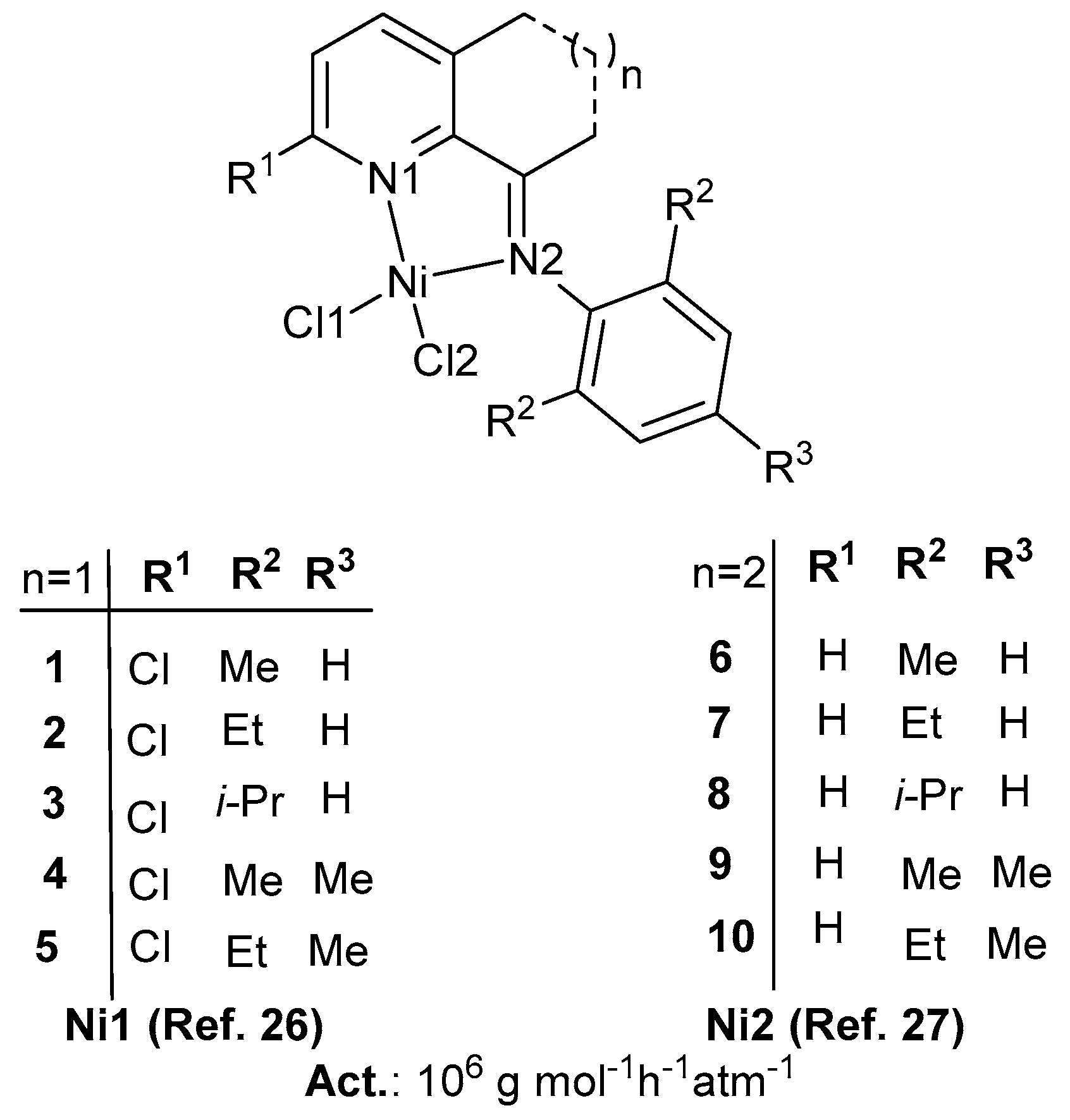
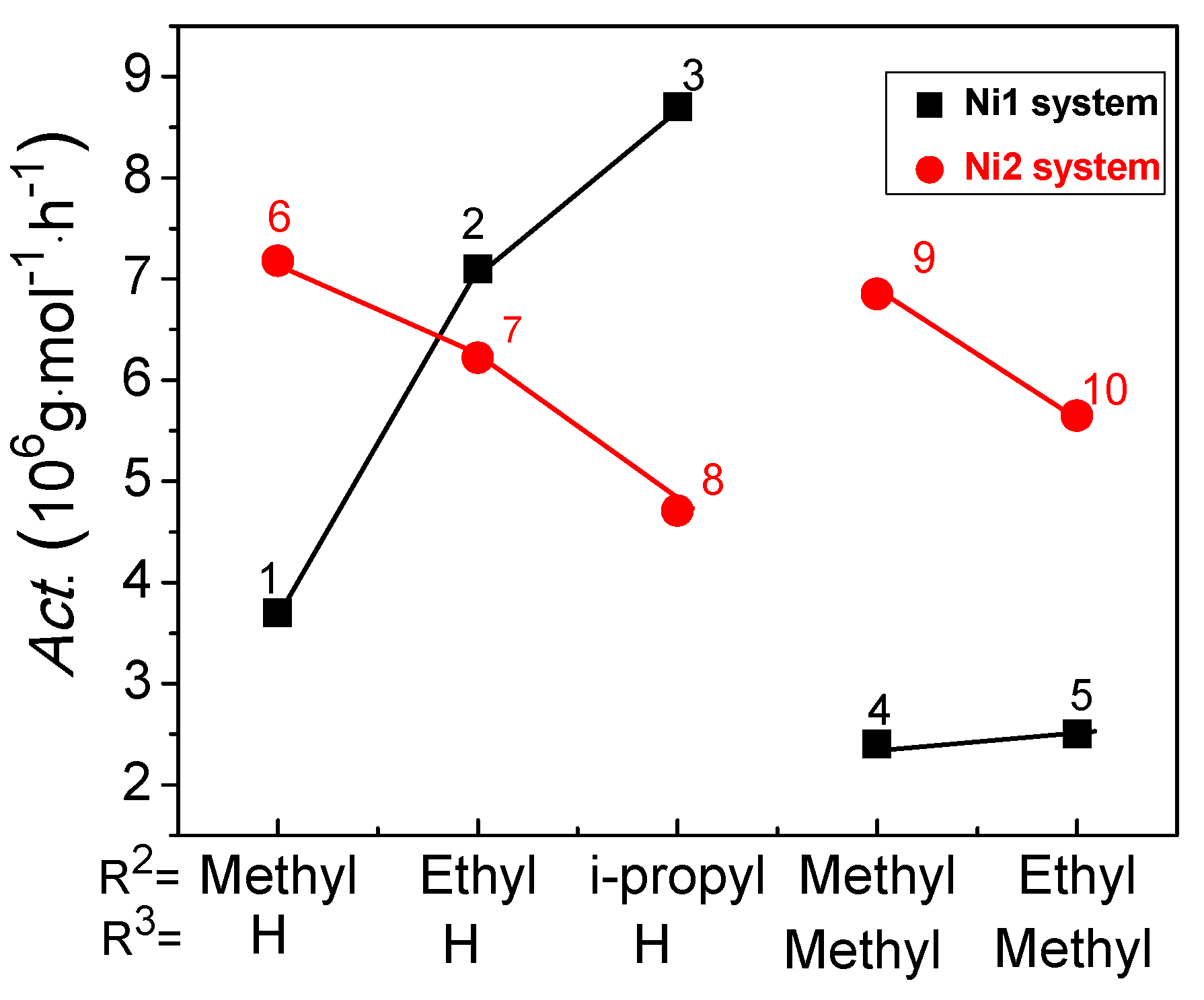

| Complex | Co-catalyst | P a | Ratio b | T c | T d | Precatalyst | Activity e |
|---|---|---|---|---|---|---|---|
| 1 | EASC | 10 | 400 | 20 | 30 | 5 μmol | 3.7 |
| 2 | EASC | 10 | 400 | 20 | 30 | 5 μmol | 7.1 |
| 3 | EASC | 10 | 400 | 20 | 30 | 5 μmol | 8.7 |
| 4 | EASC | 10 | 400 | 20 | 30 | 5 μmol | 2.4 |
| 5 | EASC | 10 | 400 | 20 | 30 | 5 μmol | 2.5 |
| 6 | EASC | 10 | 800 | 20 | 30 | 3 μmol | 7.18 |
| 7 | EASC | 10 | 800 | 20 | 30 | 3 μmol | 6.22 |
| 8 | EASC | 10 | 800 | 20 | 30 | 3 μmol | 4.71 |
| 9 | EASC | 10 | 800 | 20 | 30 | 3 μmol | 6.85 |
| 10 | EASC | 10 | 800 | 20 | 30 | 3 μmol | 5.65 |
| Complex 3 | Experiment | Singlet | Triplet |
|---|---|---|---|
| Bond Length[Å] | |||
| Ni(1)-N(1) | 2.077 | 1.969 | 2.047 |
| Ni(1)-N(2) | 2.054 | 1.944 | 2.028 |
| Ni(1)-Cl(1) | 2.278 | 2.168 | 2.201 |
| Ni(1)-Cl(2) | 2.324 | 2.158 | 2.201 |
| Ni(2)-C(9) | 1.292 | 1.320 | 1.301 |
| N(2)-C(10) | 1.460 | 1.439 | 1.444 |
| N(1)-C(1) | 1.327 | 1.343 | 1.333 |
| N(1)-C(5) | 1.379 | 1.379 | 1.363 |
| δ | 3.490 | 1.958 | |
| Bond Angles[°] | |||
| N(2)-Ni(1)-N(1) | 79.8 | 82.43 | 80.93 |
| N(2)-Ni(1)-Cl(1) | 102.5 | 96.35 | 108.7 |
| N(1)-Ni(1)-Cl(1) | 89.4 | 95.71 | 81.73 |
| N(2)-Ni(1)-Cl(2) | 107.6 | 96.45 | 111.7 |
| N(1)-Ni(1)-Cl(2) | 89.6 | 97.20 | 94.3 |
| Cl(1)-Ni(1)-Cl(2) | 149.2 | 94.25 | 133.2 |
| δ | 16.94 | 7.32 | |
| ΔE [kcal·mol−1] | 4.83 | 0 | |
| Complex No. | Descriptors | Activity (106 g·mol−1·h−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| F | Q [e] | θ [°] | β [°] | ΔE [kcal/mol] | Δε1 [kcal/mol] | Δε2 [kcal/mol] | ||
| 1 | 0.47 | 0.489 | 245.8 | 80.6 | 1.45 | 65.87 | 100.71 | 3.7 |
| 2 | 0.45 | 0.483 | 243.5 | 80.8 | 2.67 | 66.42 | 100.74 | 7.1 |
| 3 | 0.53 | 0.482 | 230.4 | 80.9 | 4.38 | 66.48 | 100.95 | 8.7 |
| 4 | 0.45 | 0.493 | 245.6 | 80.6 | 1.38 | 66.63 | 98.96 | 2.4 |
| 5 | 0.43 | 0.489 | 243.3 | 80.8 | 2.60 | 66.88 | 99.00 | 2.5 |
| Four Descriptors | Correlation Coefficient (R2) | Three Descriptors | Correlation Coefficient (R2) | Two Descriptors | Correlation Coefficient (R2) | Single Descriptor | Correlation Coefficient (R2) |
|---|---|---|---|---|---|---|---|
| F, Q,θ, β | 1.00 | F,ΔE,β | 0.998 | Δε1,Δε2 | 0.980 | Q | 0.866 |
| ΔΕ, Q,θ, β | 1.00 | F,Δε1,Δε2 | 0.987 | Q,θ | 0.961 | β | 0.605 |
| Δε1, Q,θ, β | 1.00 | ΔΕ, Q,θ | 0.985 | Q,Δε2 | 0.955 | Δε2 | 0.563 |
| Δε2, Q,θ, β | 1.00 | ΔE,Δε1,β | 0.963 | Q,Δε1 | 0.951 | ΔE | 0.549 |
| Complex No. | Descriptors | Activity (106 g·mol−1·h−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| F | Q [e] | θ [°] | β [°] | ΔE [kcal/mol] | Δε1 [kcal/mol] | Δε2 [kcal/mol] | ||
| 6 | 0.08 | 0.396 | 253.2 | 80.1 | 1.09 | 70.59 | 96.20 | 7.18 |
| 7 | 0.06 | 0.398 | 251.0 | 80.2 | 0.86 | 70.91 | 96.13 | 6.22 |
| 8 | 0.14 | 0.403 | 244.2 | 80.3 | 0.00 | 70.53 | 95.88 | 4.71 |
| 9 | 0.06 | 0.403 | 253.2 | 80.2 | 1.04 | 71.54 | 95.32 | 6.85 |
| 10 | 0.04 | 0.402 | 248.4 | 80.2 | 0.82 | 71.79 | 95.19 | 5.65 |
| Four Descriptors | Correlation Coefficient (R2) | Three Descriptors | Correlation Coefficient (R2) | Two Descriptors | Correlation Coefficient (R2) | Single Descriptor | Correlation Coefficient (R2) |
|---|---|---|---|---|---|---|---|
| F, Q,θ,β | 1.00 | F,ΔE,β | 0.998 | F,ΔΕ | 0.997 | θ | 0.964 |
| Δε1, Q,θ,β | 1.00 | F, Q,θ | 0.993 | Δε1,θ | 0.991 | ΔΕ | 0.814 |
| Δε2, Q,θ,β | 1.00 | ΔE,Δε1,β | 0.984 | Q,θ | 0.989 | β | 0.789 |
| F,Δε1,θ,β | 1.00 | F,Δε1,β | 0.969 | Δε2,θ | 0.988 | Δε1 | 0.317 |
| Complex No. | Descriptors | ΔAct. (106 g·mol−1·h−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| F | Q [e] | θ [°] | β [°] | ΔE [kcal/mol] | Δε1 [kcal/mol] | Δε2 [kcal/mol] | ||
| 1–6 | 0.39 | 0.093 | −7.37 | 0.49 | 0.36 | −4.72 | 4.51 | −3.48 |
| 2–7 | 0.39 | 0.085 | −7.57 | 0.61 | 1.81 | −4.49 | 4.61 | 0.88 |
| 3–8 | 0.39 | 0.079 | −13.83 | 0.62 | 4.38 | −4.05 | 5.07 | 3.99 |
| 4–9 | 0.39 | 0.090 | −7.59 | 0.41 | 0.35 | −4.91 | 3.64 | −4.45 |
| 5–10 | 0.39 | 0.087 | −5.10 | 0.52 | 1.79 | −4.91 | 3.81 | −3.15 |
| Four Descriptors | Correlation Coefficient (R2) | Three Descriptors | Correlation Coefficient (R2) | Two Descriptors | Correlation Coefficient (R2) | Single Descriptor | Correlation Coefficient (R2) |
|---|---|---|---|---|---|---|---|
| F, Q,θ, β | 1.00 | Δε2, Q,θ | 0.995 | Q,Δε2 | 0.995 | Δε1 | 0.910 |
| Δε1, F, Q, β | 1.00 | F, Q,Δε2 | 0.995 | Q,Δε1 | 0.988 | β | 0.817 |
| F,ΔΕ,θ, β | 1.00 | Q,θ, β | 0.994 | θ,β | 0.989 | ΔE | 0.779 |
| F,Δε2,θ, β | 1.00 | ΔΕ,θ, β | 0.989 | Q,θ | 0.938 | Q | 0.778 |
| Systems | Standardized Values | |||
|---|---|---|---|---|
| Complex | Q | θ | Activity | |
| Ni1 system | 1 | 0.39 | 0.63 | −0.41 |
| 2 | −0.91 | 0.27 | 0.77 | |
| 3 | −1.12 | −1.75 | 1.33 | |
| 4 | 1.25 | 0.59 | −0.86 | |
| 5 | 0.39 | 0.24 | −0.83 | |
| Ni2 system | 6 | −1.37 | 0.84 | 1.07 |
| 7 | −0.74 | 0.27 | 0.09 | |
| 8 | 0.81 | −1.53 | −1.43 | |
| 9 | 0.81 | 0.83 | 0.73 | |
| 10 | 0.49 | −0.42 | −0.47 | |
| Ni1–Ni2 system | 1–6 | 1.16 | 0.28 | −0.62 |
| 2–7 | −0.33 | 0.22 | 0.59 | |
| 3–8 | −1.46 | −1.69 | 1.46 | |
| 4–9 | 0.60 | 0.21 | −0.90 | |
| 5–10 | 0.03 | 0.97 | −0.53 | |
| Complex System | Q [e] | θ [°] |
|---|---|---|
| Ni1 System | 80.98 | 19.02 |
| Ni2 System | 11.23 | 88.77 |
| Ni1–Ni2 analogue | 65.26 | 34.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, A.A.; Yang, W.; Ma, Z.; Sun, W.-H. The Catalytic Activities of Carbocyclic Fused Pyridineimine Nickel Complexes Analogues in Ethylene Polymerization by Modeling Study. Catalysts 2019, 9, 520. https://doi.org/10.3390/catal9060520
Malik AA, Yang W, Ma Z, Sun W-H. The Catalytic Activities of Carbocyclic Fused Pyridineimine Nickel Complexes Analogues in Ethylene Polymerization by Modeling Study. Catalysts. 2019; 9(6):520. https://doi.org/10.3390/catal9060520
Chicago/Turabian StyleMalik, Arfa Abrar, Wenhong Yang, Zhifeng Ma, and Wen-Hua Sun. 2019. "The Catalytic Activities of Carbocyclic Fused Pyridineimine Nickel Complexes Analogues in Ethylene Polymerization by Modeling Study" Catalysts 9, no. 6: 520. https://doi.org/10.3390/catal9060520
APA StyleMalik, A. A., Yang, W., Ma, Z., & Sun, W.-H. (2019). The Catalytic Activities of Carbocyclic Fused Pyridineimine Nickel Complexes Analogues in Ethylene Polymerization by Modeling Study. Catalysts, 9(6), 520. https://doi.org/10.3390/catal9060520