WO3–V2O5 Active Oxides for NOx SCR by NH3: Preparation Methods, Catalysts’ Composition, and Deactivation Mechanism—A Review
Abstract
:1. Introduction
1.1. NOx Problem, Control Regulations, and Removal Technologies
1.2. SCR Application for After-Treatment of Fossil Fueled Engines in Transportation
2. WO3–V2O5-Based SCR Catalysts
2.1. Reasons for the Use of WO3–V2O5-Based Catalysts
2.2. Effect of Preparation Method on Activity and Selectivity
2.3. Effect of the Support on Catalytic Activity
2.3.1. Metal Oxides as Carriers
2.3.2. Molecular Sieves or Filters as the Carrier
2.4. Effect of Chemical Composition on Catalytic Activity
2.5. Positive/Negative Effects of Different Doping Agents on Catalytic Activity and Selectivity
3. Deactivation or Poisoning Mechanism
4. SO2 and H2O Effects
5. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shaddick, G.; Thomas, M.L.; Amini, H.; Broday, D.; Cohen, A.; Frostad, J.; Green, A.; Gumy, S.; Liu, Y.; Martin, R.V. Data integration for the assessment of population exposure to ambient air pollution for global burden of disease assessment. Env. Sci. Technol. 2018, 52, 9069–9078. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Lee, J.M. Conventional and new materials for selective catalytic reduction (SCR) of NOx. Chem. Cat. Chem. 2018, 10, 1499–1511. [Google Scholar]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O–MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A Review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Bergmeijer, P. The International Convention for the Prevention of Pollution from Ships. In Ports as Nodal Points in a Global Transport System; Elsevier: Amsterdam, The Netherlands, 1992; pp. 259–270. [Google Scholar]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total. Env. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Fu, M.; Li, C.; Lu, P.; Qu, L.; Zhang, M.; Zhou, Y.; Yu, M.; Fang, Y. A review on selective catalytic reduction of NOx by supported catalysts at 100–300 °C-catalysts, mechanism, kinetics. Catal. Sci. Technol. 2014, 4, 14–25. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Miyamoto, A. Removal technology for nitrogen oxides and sulfur oxides from exhaust gases. Catal. Today 1991, 10, 21–31. [Google Scholar] [CrossRef]
- Sorrels, J.L.; Randall, D.D.; Fry, C.R.; Schaffner, K.S. Selective Noncatalytic Reduction; U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
- Radojevic, M. Reduction of Nitrogen Oxides in Flue Gases. In Nitrogen, the Confer-Ns; Elsevier: Amsterdam, The Netherlands, 1998; pp. 685–689. [Google Scholar]
- Liu, J.; Li, X.; Li, R.; Zhao, Q.; Ke, J.; Xiao, H.; Wang, L.; Liu, S.; Tadé, M.; Wang, S. Facile synthesis of tube-shaped Mn-Ni-Ti solid solution and preferable Langmuir-Hinshelwood mechanism for selective catalytic reduction of NOx by NH3. Appl. Catal. A 2018, 549, 289–301. [Google Scholar] [CrossRef]
- Crocker, M.; Shi, C. Non-Thermal Plasma Nox Storage-reduction. NOx Trap Catal. Technol. 2018, 384–406. [Google Scholar]
- Zhang, R.; Yang, W.; Luo, N.; Li, P.; Lei, Z.; Chen, B. Low-Temperature NH3-SCR of NO by Lanthanum Manganite Perovskites: Effect of A-/B-Site Substitution and TiO2/CeO2 Support. Appl. Catal. B 2014, 146, 94–104. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and Mechanistic Aspects of the Selective Catalytic Reduction of NOx by Ammonia over Oxide Catalysts: A Review. Appl. Catal. B 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Hirata, K.; Masaki, N.; Ueno, H.; Akagawa, H. Development of Urea-SCR System for Heavy-Duty Commercial Vehicles. SAE Trans. 2005, 678–685. [Google Scholar]
- Magnusson, M.; Fridell, E.; Ingelsten, H.H. The Influence of Sulfur Dioxide and Water on the Performance of a Marine Scr Catalyst. Appl. Catal. B 2012, 111, 20–26. [Google Scholar] [CrossRef]
- Guo, M.; Fu, Z.; Ma, D.; Ji, N.; Song, C.; Liu, Q. A Short Review of Treatment Methods of Marine Diesel Engine Exhaust Gases. Procedia Eng. 2015, 121, 938–943. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the Latest Developments in Modified Vanadium-Titanium-Based SCR Catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Tsuji, K.; Shiraishi, I. Combined desulfurization, denitrification and reduction of air toxics using activated coke. 2. Process applications and performance of activated coke. Fuel 1997, 76, 555–560. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhong, Q. Promotional Effect of F-Doped V2O5-WO3/TiO2 Catalyst for NH3-SCR of NO at Low-Temperature. Appl. Catal. A 2012, 435, 156–162. [Google Scholar] [CrossRef]
- Aguilar-Romero, M.; Camposeco, R.; Castillo, S.; Marín, J.; Rodríguez-González, V.; García-Serrano, L.A.; Mejía-Centeno, I. Acidity, Surface Species, and Catalytic Activity Study on V2O5-WO3/TiO2 Nanotube Catalysts for Selective NO Reduction by NH3. Fuel 2017, 198, 123–133. [Google Scholar] [CrossRef]
- Kompio, P.G.; Brückner, A.; Hipler, F.; Auer, G.; Löffler, E.; Grünert, W. A New View on the Relations between Tungsten and Vanadium in V2O5-WO3/TiO2 Catalysts for the Selective Reduction of NO with NH3. J. Catal. 2012, 286, 237–247. [Google Scholar] [CrossRef]
- Yu, W.; Wu, X.; Si, Z.; Weng, D. Influences of impregnation procedure on the SCR activity and alkali resistance of V2O5-WO3/TiO2 catalyst. Appl. Surf. Sci. 2013, 283, 209–214. [Google Scholar] [CrossRef]
- Dong, G.-J.; Zhang, Y.-F.; Yuan, Z.; Yang, B. Effect of the pH value of precursor solution on the catalytic performance of V2O5-WO3/TiO2 in the low temperature NH3-SCR of NOx. J. Fuel Chem. Technol. 2014, 42, 1455–1463. [Google Scholar] [CrossRef]
- He, Y.; Ford, M.E.; Zhu, M.; Liu, Q.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B 2016, 193, 141–150. [Google Scholar] [CrossRef]
- Qi, C.; Bao, W.; Wang, L.; Li, H.; Wu, W. Study of the V2O5-WO3/TiO2 catalyst synthesized from waste catalyst on selective catalytic reduction of NOx by NH3. Catalysts 2017, 7, 110. [Google Scholar] [CrossRef]
- Meiqing, S.; Lili, X.; Jianqiang, W.; Chenxu, L.; Wulin, W.; Jun, W.; Yanping, Z. Effect of Synthesis Methods on Activity of V2O5/CeO2/WO3-TiO2 Catalyst for Selective Catalytic Reduction of NOx with NH3. J. Rare Earths 2016, 34, 259–267. [Google Scholar]
- Djerad, S.; Tifouti, L.; Crocoll, M.; Weisweiler, W. Effect of vanadia and tungsten loadings on the physical and chemical characteristics of V2O5-WO3/TiO2 catalysts. J. Mol. Catal. A Chem. 2004, 208, 257–265. [Google Scholar] [CrossRef]
- Reiche, M.; Buergi, T.; Baiker, A.; Scholz, A.; Schnyder, B.; Wokaun, A. Vanadia and Tungsta Grafted on TiO2: Influence of the Grafting Sequence on Structural and Chemical Properties. Appl. Catal. A 2000, 198, 155–169. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-Doped Mn/TiO2 as an Efficient Catalyst for the Low-Temperature SCR of NO with NH3: Catalytic Evaluation and Characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Mamedov, S.; Boolchand, P.; Smirniotis, P.G. Surface Characterization Studies of TiO2 Supported Manganese Oxide Catalysts for Low Temperature SCR of NO with NH3. Appl. Catal. B 2007, 76, 123–134. [Google Scholar] [CrossRef]
- Reiche, M.; Hug, P.; Baiker, A. Effect of grafting sequence on the behavior of titania-supported V2O5-WO3 catalysts in the selective reduction of NO by NH3. J. Catal. 2000, 192, 400–411. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Q. Surface characterization studies on the interaction of V2O5-WO3/TiO2 catalyst for low temperature SCR of NO with NH3. J. Solid State Chem. 2015, 221, 49–56. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Q. Promotional effect of WO3 on O2− over V2O5/TiO2 catalyst for selective catalytic reduction of NO with NH3. J. Mol. Catal. A Chem. 2013, 373, 108–113. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of tungsten oxide on SCR performance of V2O5-WO3/TiO2: Acidity, surface species and catalytic activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Li, J.; Zhang, H.; Xu, H.; Xiao, R.; Yang, L. Promotional roles of ZrO2 and WO3 in V2O5-WO3/TiO2-ZrO2 catalysts for NOx reduction by NH3: Catalytic performance, morphology, and reaction mechanism. Chin. J. Catal. 2016, 37, 1918–1930. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Disdier, J. Electrical properties of V2O5-WO3/TiO2 EUROCAT catalysts evidence for redox process in selective catalytic reduction (SCR) deNOx reaction. Catal. Today 2000, 56, 389–401. [Google Scholar] [CrossRef]
- Jung, S.M.; Grange, P. Characterization and reactivity of V2O5-WO3 supported on TiO2-SO42− catalyst for the SCR reaction. Appl. Catal. B 2001, 32, 123–131. [Google Scholar] [CrossRef]
- Sun, C.; Dong, L.; Yu, W.; Liu, L.; Li, H.; Gao, F.; Dong, L.; Chen, Y. Promotion effect of tungsten oxide on SCR of NO with NH3 for the V2O5-WO3/Ti0.5Sn0.5O2 catalyst: Experiments combined with DFT calculations. J. Mol. Catal. A Chem. 2011, 346, 29–38. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Mugica, V.; Mejía-Centeno, I.; Marín, J. Role of V2O5-WO3/H2Ti3O7-nanotube-model catalysts in the enhancement of the catalytic activity for the SCR-NH3 process. Chem. Eng. J. 2014, 242, 313–320. [Google Scholar] [CrossRef]
- Mejía-Centeno, I.; Castillo, S.; Camposeco, R.; Marín, J.; García, L.A.; Fuentes, G.A. Activity and selectivity of V2O5/H2Ti3O7, V2O5-WO3/H2Ti3O7 and Al2O3/H2Ti3O7 model catalysts during the SCR-NO with NH3. Chem. Eng. J. 2015, 264, 873–885. [Google Scholar] [CrossRef]
- Boudali, L.K.; Ghorbel, A.; Grange, P. Characterization and reactivity of V2O5-WO3 supported on sulfated titanium pillared clay catalysts for the SCR-NO reaction. C.R. Chim. 2009, 12, 779–786. [Google Scholar] [CrossRef]
- Najbar, M.; Mizukami, F.; Białas, A.; Camra, J.; Wesełucha-Birczyńska, A.; Izutsu, H.; Góra, A. Evolution of Ti–Sn-Rutile-supported V2O5-WO3 catalyst during its use in nitric oxide reduction by ammonia. Top. Catal. 2000, 11, 131–138. [Google Scholar] [CrossRef]
- Li, F.; Shen, B.; Tian, L.; Li, G.; He, C. Enhancement of SCR activity and mechanical stability on cordierite supported V2O5-WO3/TiO2 catalyst by substrate acid pretreatment and addition of silica. Powder Technol. 2016, 297, 384–391. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, J.H.; Phule, A.D. Development of high performance catalytic filter of V2O5-WO3/TiO2 supported SiC for NOx reduction. Powder Technol. 2018, 327, 282–290. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, J.-H.; Bak, Y.-C.; Amal, R.; Scott, J. Pt-V2O5-WO3/TiO2 catalysts supported on SiC filter for NO reduction at low temperature. Korean J. Chem. Eng. 2005, 22, 844–851. [Google Scholar] [CrossRef]
- Najbar, M.; Banaś, J.; Korchowiec, J.; Białas, A. Competition between NO reduction and NO decomposition over reduced V–W–O catalysts. Catal. Today 2002, 73, 249–254. [Google Scholar] [CrossRef]
- Zhao, K.; Han, W.; Tang, Z.; Zhang, G.; Lu, J.; Lu, G.; Zhen, X. Investigation of coating technology and catalytic performance over monolithic V2O5-WO3/TiO2 catalyst for selective catalytic reduction of NOx with NH3. Colloids Surf. A 2016, 503, 53–60. [Google Scholar] [CrossRef]
- Shen, B.; Wang, F.; Zhao, B.; Li, Y.; Wang, Y. The behaviors of V2O5-WO3/TiO2 loaded on ceramic surfaces for NH3-SCR. J. Ind. Eng. Chem. 2016, 33, 262–269. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Xu, T.; Weng, D.; Si, Z.; Ran, R. Effects of silica additive on the NH3-SCR activity and thermal stability of a V2O5-WO3/TiO2 catalyst. Chin. J. Catal. 2016, 37, 1340–1346. [Google Scholar] [CrossRef]
- Dong, G.-J.; Yuan, Z.; Zhang, Y.-F. Preparation and performance of V-W reparation and performance of VW/x(Mn-Ce-Ti)/y(Cu-Ce-Ti)/cordierite catalyst by impregnation method in sequence for SCR reaction with urea. J. Fuel Chem. Technol. 2014, 42, 1093–1101. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Liu, S.; Ren, X.; Ma, J.; Su, W.; Peng, Y. Ultra-hydrothermal stability of CeO2-WO3/TiO2 for NH3-SCR of NO compared to traditional V2O5-WO3/TiO2 catalyst. Catal. Today 2015, 258, 11–16. [Google Scholar] [CrossRef]
- Shi, A.; Wang, X.; Yu, T.; Shen, M. The effect of zirconia additive on the activity and structure stability of V2O5-WO3/TiO2 ammonia SCR catalysts. Appl. Catal. B 2011, 106, 359–369. [Google Scholar] [CrossRef]
- Tao, P.; Sun, M.; Qu, S.; Song, C.; Li, C.; Yin, Y.; Cheng, M. Effects of V2O5 and WO3 loadings on the catalytic performance of V2O5-WO3/TiO2 catalyst for SCR of NO with NH3. Glob. Nest J. 2017, 19, 160–166. [Google Scholar]
- Lietti, L.; Nova, I.; Forzatti, P. Selective catalytic reduction (SCR) of NO by NH3 over TiO2-supported V2O5-WO3 and V2O5-MoO3 catalysts. Top. Catal. 2000, 11, 111–122. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Gao, Y.; Lin, Q.; Hu, J.; Weng, D. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts for low-temperature NH3-SCR. Catal. Commun. 2017, 93, 33–36. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Zhong, Q. Cluster molecular modeling of strong interaction for F-doped V2O5-WO3/TiO2 supported catalyst. J. Fluor. Chem. 2013, 153, 26–32. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Zheng, C.; Cen, K.; Gao, X. Mechanistic investigation of enhanced reactivity of NH4HSO4 and NO on Nb-and Sb-doped VW/Ti SCR catalysts. Appl. Catal. A 2018, 549, 310–319. [Google Scholar] [CrossRef]
- Tian, X.; Xiao, Y.; Zhou, P.; Zhang, W.; Luo, X. Investigation on performance of V2O5-WO3/TiO2-cordierite catalyst modified with Cu, Mn and Ce for urea-SCR of NO. Mater. Res. Innov. 2014, 18, S2-202–S2-206. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. Promotional effect of Ce-doped V2O5-WO3/TiO2 with low vanadium loadings for selective catalytic reduction of NOx by NH3. J. Phys. Chem. C 2009, 113, 21177–21184. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Zhang, T.; Li, J.; Zhao, Z.; Wei, Y.; Jiang, G.; Duan, A. Effect of Ce doping of TiO2 support on NH3-SCR activity over V2O5-WO3/CeO2-TiO2 catalyst. J. Env. Sci. 2014, 26, 2106–2113. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. The Poisoning Effect of Alkali Metals Doping over Nano V2O5–WO3/TiO2 Catalysts on Selective Catalytic Reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.; Jiang, L.; Guo, F.; Ren, S.; Yao, L.; Yang, J. Property influence and poisoning mechanism of HgCl2 on V2O5-WO3/TiO2 SCR-deNOx catalysts. Catal. Commun. 2016, 85, 34–38. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Q.; Sun, J.; Liu, Q.; Zhao, D.; Gao, W.; Liu, L. Effects of mercury oxidation on V2O5-WO3/TiO2 catalyst properties in NH3-SCR process. Catal. Commun. 2015, 59, 78–82. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.; Zhu, B.; Yang, J.; Li, L.; Zhou, Q.; Ren, S. Synergy of KCl and Hgel on selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts. Chem. Eng. J. 2015, 264, 815–823. [Google Scholar] [CrossRef]
- Lei, T.; Li, Q.; Chen, S.; Liu, Z.; Liu, Q. KCl-induced deactivation of V2O5-WO3/TiO2 catalyst during selective catalytic reduction of NO by NH3: Comparison of poisoning methods. Chem. Eng. J. 2016, 296, 1–10. [Google Scholar] [CrossRef]
- Xie, X.; Lu, J.; Hums, E.; Huang, Q.; Lu, Z. Study on the deactivation of V2O5-WO3/TiO2 selective catalytic reduction catalysts through transient kinetics. Energy Fuels 2015, 29, 3890–3896. [Google Scholar] [CrossRef]
- Klimczak, M.; Kern, P.; Heinzelmann, T.; Lucas, M.; Claus, P. High-throughput study of the effects of inorganic additives and poisons on NH3-SCR catalysts—Part I: V2O5-WO3/TiO2 catalysts. Appl. Catal. B 2010, 95, 39–47. [Google Scholar] [CrossRef]
- Deng, L.; Liu, X.; Cao, P.; Zhao, Y.; Du, Y.; Wang, C.; Che, D. A study on deactivation of V2O5-WO3/TiO2 SCR catalyst by alkali metals during entrained-flow combustion. J. Energy Inst. 2017, 90, 743–751. [Google Scholar] [CrossRef]
- Li, X.; Li, X.-S.; Chen, J.; Li, J.; Hao, J. An efficient novel regeneration method for Ca-poisoning V2O5-WO3/TiO2 catalyst. Catal. Commun. 2016, 87, 45–48. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.; Wang, X.; Ren, S.; Yang, J.; Zhao, D.; Xi, W.; Yao, L. Performance impact and poisoning mechanism of arsenic over commercial V2O5-WO3/TiO2 SCR catalyst. Catal. Commun. 2015, 72, 121–126. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R. Mechanism of poisoning of the V2O5/TiO2 Catalyst for the reduction of NO by NH3. J. Catal. 1990, 125, 411–420. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R. Selective Catalytic Reduction of NO with NH3 on SO4−2/TiO2 Superacid Catalyst. J. Catal. 1993, 139, 277–288. [Google Scholar] [CrossRef]
- Ciambelli, P.; Fortuna, M.; Sannino, D.; Baldacci, A. The Influence of Sulphate on the Catalytic Properties of V2O5-TiO2 and WO3-TiO2 in the Reduction of Nitric Oxide with Ammonia. Catal. Today 1996, 29, 161–164. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Wachs, I.E.; Deo, G.; Jehng, J.-M. Reactivity of V2O5 catalysts for the Selective Catalytic Reduction of NO by NH3: Influence of Vanadia Loading, H2O, and SO2. J. Catal. 1996, 161, 247–253. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5-WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]


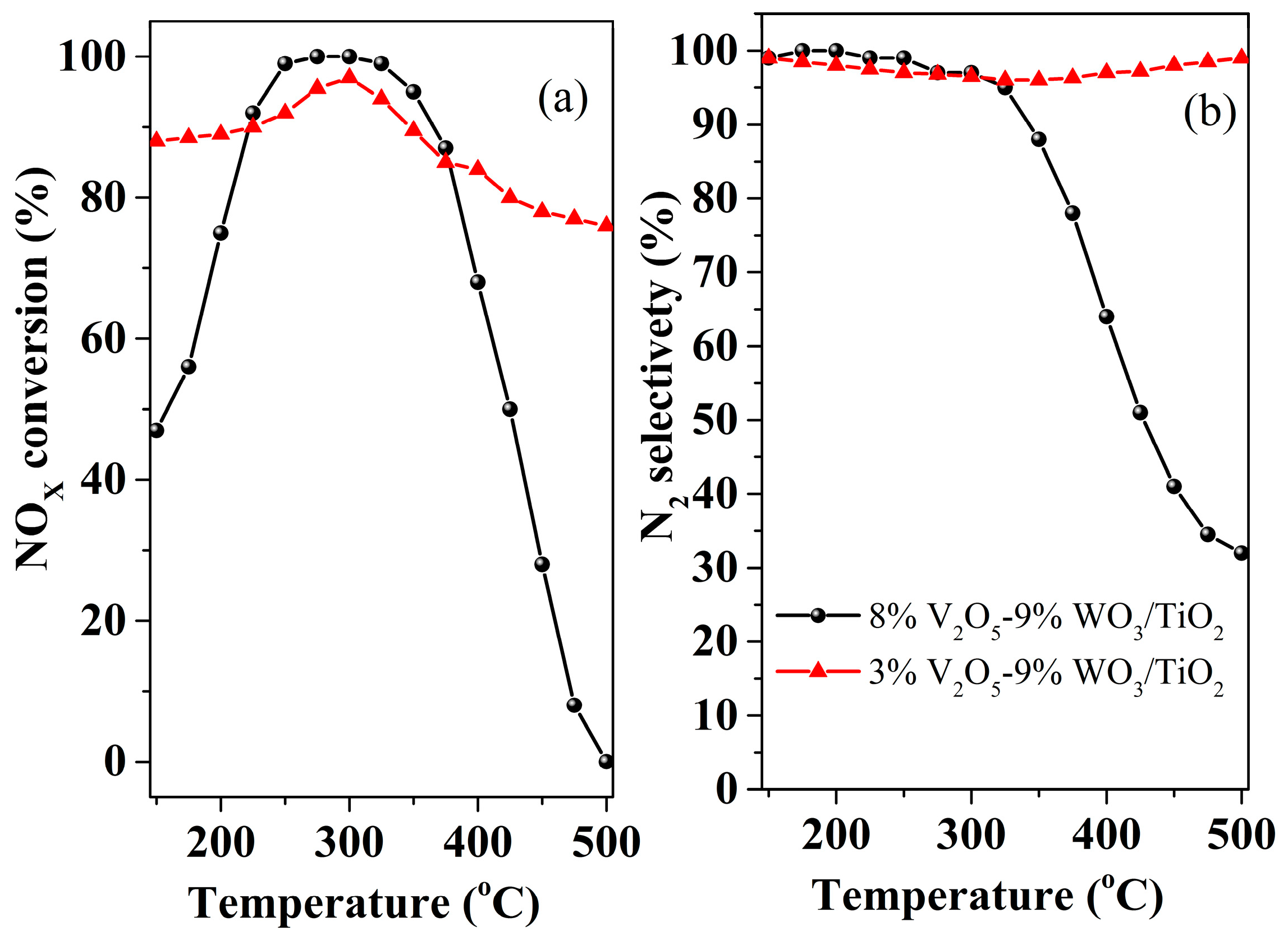






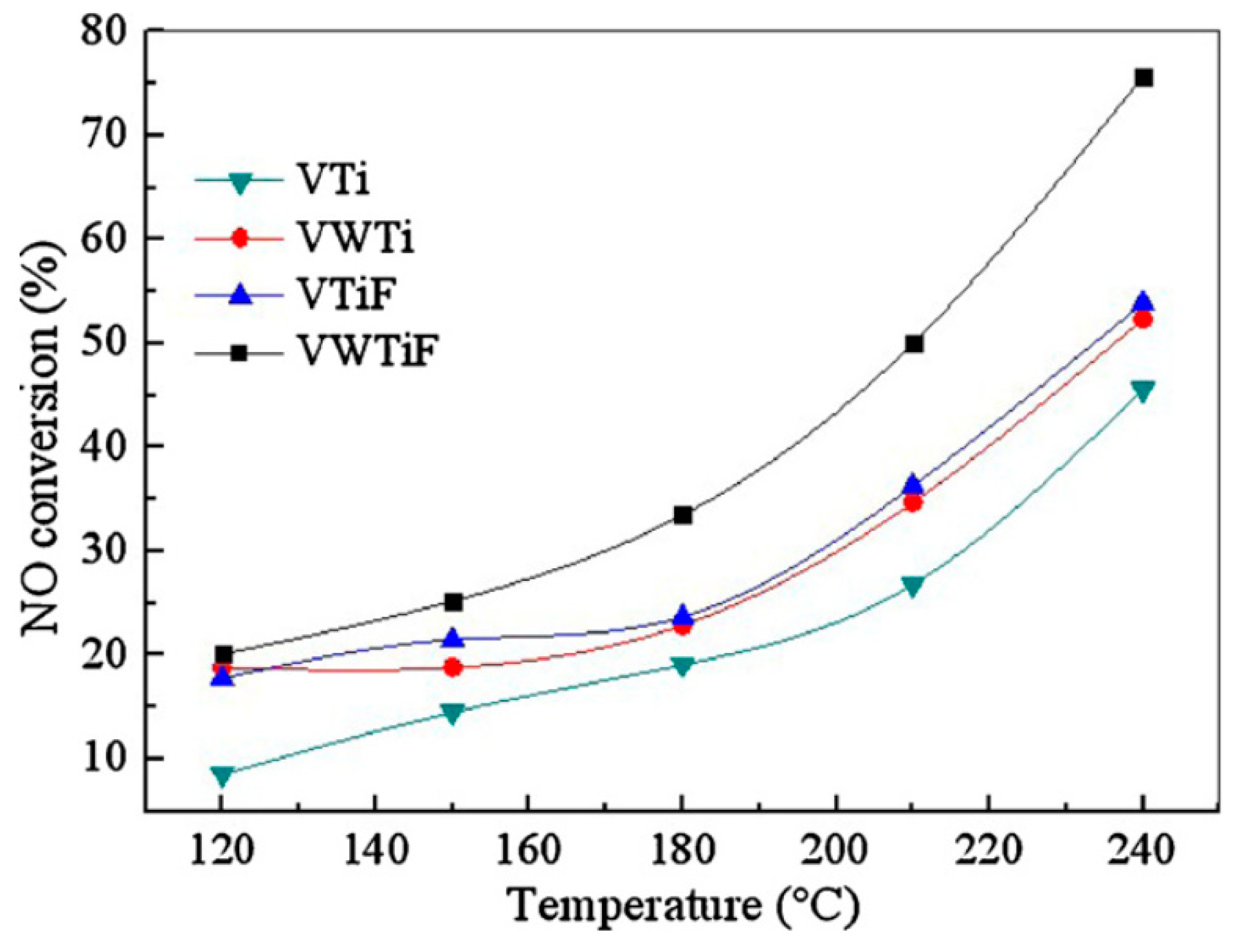



| Catalysts | Syntheses Method (Cal. Tem./°C) | The Key Facts to the Activity | Ref. |
|---|---|---|---|
| V2O5-WO3/TiO2 | Wet impregnation (450 °C) | Compared with dry-impregnated catalyst, wet-impregnated catalyst increases the intensity of Brønsted acid sites and the oxidation of the ab-NH3. | [24] |
| V2O5-WO3/TiO2 | Dry impregnation (450 °C) | ||
| V2O5-WO3/TiO2 | Wet impregnation (500 °C) | By increasing the precursor solution acidity, more polymeric vanadium species are formed on the catalyst surface; the ratio of V4+(3+)/V5+, surface acidity, and quantity of active sites are increased, whereas the activity of the catalyst is largely improved. | [25] |
| V2O5-WO3/TiO2 | Co-precipitation (500 °C) | The enhanced performance of co-precipitated compared to the impregnated catalysts was associated with the co-precipitated V2O5-WO3/TiO2 catalysts possess new surface: O = WO4 (enhanced the adsorption of NH3) and redox surface mono-oxo O = VO3 sites, because of the surface defects of the TiO2. | [26] |
| V2O5-WO3/TiO2 | Incipient wetness impregnation (500 °C) | ||
| V2O5-WO3/TiO2 | Oxalic acid leaching and impregnation (600 °C) | Oxalic acid leaching is an effective way to reduce the impurities and increase reducibility of the recovered catalyst. | [27] |
| Ce-V2O5-WO3/TiO2 | Deposition-precipitation (500 °C) | The higher NOx conversion at low temperature has origin from surface Ce species and can be assigned to more active Lewis acid sites, the weakly adsorbed NO2 and monodentate nitrate. | [28] |
| Ce-V2O5-WO3/TiO2 | Impregnation method (500 °C) | ||
| V2O5-WO3/TiO2 | Sol–gel method (400–800 °C) | Well dispersed and isolated vanadium oxide species were found to be weakly active for the SCR reaction but with a high selectivity to N2. | [29] |
| V2O5-WO3/TiO2 | Grafting (300 °C) | Grafting mode and metal oxides loadings were significantly affecting the strength of interaction of V2O5 and WO3. | [30] |
| Catalysts | Carries | NOx Conversion (Tem./°C) | Surface Active Components | the Key Facts to the Activity | Ref. |
|---|---|---|---|---|---|
| V2O5-WO3/TiO2 | TiO2 | 90–100% (225–375 °C) | V2O5 and WO3 | The well dispersed and isolated vanadium oxide species were found to be weakly active for the SCR reaction but with a high selectivity to N2. | [29] |
| V2O5-WO3/TiO2 | TiO2 | ~ | V and W species do not exist independently on the titania surface (V-O-W connectivity were present on the V2O5-WO3/TiO2 catalysts), which results in a higher activity. | [30] | |
| V2O5-WO3/TiO2 | TiO2 | ~ | [33] | ||
| V2O5-WO3/TiO2 | TiO2 | 90% (>245 °C) | [34] | ||
| V2O5-WO3/TiO2 | TiO2 | 80% (320 °C) | The promotional effect of tungsten may originate from the follows: the influence of the V species or the dispersion of V species ensembles | [23] | |
| V2O5-WO3/TiO2 | TiO2 | 90–100% (250–360 °C) | WO3 species play an important role to form superoxide ions (O2−), which improved the activity. | [35] | |
| V2O5-WO3/TiO2-ZrO2 | TiO2-ZrO2 | 90–100% (290–370 °C) | V2O5, WO3 and ZrO2 | ZrO2 mainly promotional role was to change the path-way of NOx reduction and surface acidity of catalyst. | [37] |
| V2O5-WO3/TiO2-SO42- | TiO2-SO42- | 90–98% (260–480 °C) | V2O5, WO3 and SO42- | Sulfate stability strongly depends on the loaded metal oxides in addition the reduction property of V2O5 also changed due to the V-O-W species. | [39] |
| V2O5-WO3/Ti0.5Sn0.5O2 | Ti0.5Sn0.5O2 | 90% (>300 °C) | V2O5, WO3 and TiSnO2 | Activity was closely correlated to the amounts of the Brønsted acid sites: they were in direct proportion to highly dispersed WO3. However, too much WO3 will cover the active sites and lead to the decrease of the activities; About the reduction temperature, which becomes higher due to the V-O-W species. | [40] |
| V2O5-WO3/ H2Ti3O7 | H2Ti3O7 | 90–98% (325–450 °C) | V2O5, WO3 and H2Ti3O7 | The higher activity can be explained by the following factors: (1) The excellent physics properties that titanic acid nanotubes possess tubular structure, good thermal stability (460 °C) and high specific surface area (314 m2/g). (2) TAN as carriers not only significantly increase Brønsted acid sites that light-off conversion at low temperatures, but also increase Lewis acid sites that promote the NO reduction at high temperature. | [41,42] |
| WO3-V2O5/STi-PILC | STi-PILC | 90–100% (250–400 °C) | ~ | Sulfate species seems to play a more important role for NO removal activity than WO3. | [43] |
| WO3-V2O5/Ti–Sn-rutile | Ti–Sn-rutile | 90–100% (>230 °C) | ~ | W species mainly responsible for the oxidation-induced outward vanadium segregation in the vanadia-like species, which was play an important role to improve activity. | [44] |
| WO3-V2O5/TiO2-NT | TiO2-NT | 90–92% (320–380°C) | V2O5, WO3 and H2Ti3O7 | Morphology of the nanotubes and the metal loading (increased surface Brønsted and Lewis acidity sites) play an important role for the catalyst’s activity | [22] |
| V2O5-WO3-TiO2/cordierite | Cordierite | 90–98%(300–500 °C) | V2O5, WO3 and SiO2 | For acid pre-treatment of cordierite, which enlarged the BET surface area and pore volume of the catalysts significantly; SiO2 shifted the V valence from V5+ to V4+ and increased chemisorbed oxygen. All of them enhanced the activity. | [45] |
| V2O5-WO3-TiO2/SiC | SiC | 90–99%(210–360 °C) | V2O5, WO3 and TiO2 | Smaller particle size and better dispersion of catalyst coating solution that is helpful to the catalyst go into the inside of pores of the SiC filters to improve the catalytic activity by increasing the BET surface area and exposing more active sites. | [46] |
| Pt-V2O5-WO3-TiO2/SiC | SiC | 95–100% (170–250 °C) | V2O5, WO3 and TiO2 | Pt promoted catalytic activity at low temperatures but increased ammonia oxidation properties. The promotional effect was believed to result from a high electron transfer of Pt-V2O5-WO3-TiO2/SiC. | [47] |
| V2O5-WO3-Ti(Sn)O2/monolith | Cr–Al steel monolith | 95–100% (150–200 °C) | V2O5, WO3 and TiO2 | Increase the pre-reduction temperature was in favor of improving the activity and stability in direct NO decomposition due to the tungsten cations were substituted by vanadium. | [48] |
| V2O5-WO3-TiO2/monolith | Monolith | 90–92% (420–450 °C) | V2O5, WO3 and TiO2 | It was important to facilitate the NH3-SCR reaction that XPS confirmed the co-existence of V5+ and V4+ and the BET surface area was significantly improved due to the pore structure. | [49] |
| Catalysts | NOx Conversion (Tem./°C) | the Key Factors for the Activity | Ref. |
|---|---|---|---|
| V2O5-WO3/TiO2-SiO2 | 90–98% (280–410 °C) | The introduction of SiO2 significantly increased the catalysts acidity and leaded to more V and W species were exposure on the surface of catalyst by forming Si–OK that was the result of the reaction between enriched K and Si. | [50] |
| V2O5-WO3/TiO2 | 42–70% (280–410 °C) | ||
| V2O5-WO3/TiO2-SiO2 | 90–98% (500–725 °C) | The addition of SiO2 on the one hand, inhibited the shrinkage of catalyst BET surface area due to the phase transition from anatase to rutile and the growth of TiO2 size. On the other hands, the W species remained highly dispersion. | [51] |
| V2O5-WO3/TiO2 | <58% (500–725 °C) | ||
| V2O5-WO3/TiO2 | <58% (500–725 °C) | The higher activity origin from high V4+/V5+ ratio and large amount of surface chemisorbed oxygen as well as BET surface area caused by the M and C composites. | [52] |
| V2O5-WO3/C/ ceramics | 52–99% (200–50 °C) | ||
| V2O5-WO3/C/M/ ceramics | 78–99% (200–550 °C) | ||
| V2O5-WO3/ ceramics | 10–65% (200–550 °C) | ||
| CeO2-WO3/TiO2 | 90–99% (250–550 °C) | The higher activity of CeO2-WO3/TiO2 is responsible for the well dispersion of CeO2 and W species; Ce2(WO4)3 prevent the W species crystallization and TiO2 sintering and phase transition; The existence of Ce4+-Ce3+ couple that provide more oxygen vacancies and improve the surface Lewis acid of catalyst. | [53] |
| V2O5-WO3/TiO2 | 90–95% (275–350 °C) | ||
| 1.5%V2O5-1%WO3/TiO2 | 96–100% (100–550 °C) | Catalytic activities could improve by increasing V2O5 loading less than 2 wt%. However, when V2O5 loading exceeds 2 wt%, the activity begins to decline because high V2O5 loading on TiO2 speeds up the phase transition from anatase to rutile. On other hands, NO conversion also can be significantly improved with the increase of moderate WO3 loadings; however, too much WO3 will inhibit the formation of superoxide ions. | [55] |
| 1.5%V2O5-6%WO3/TiO2 | 70–100% (100–550 °C) | ||
| 0.5%V2O5-1%WO3/TiO2 | 70–100% (100–550 °C) | ||
| 0.5%V2O5-6%WO3/TiO2 | 70–100% (100–550 °C) | ||
| V2O5-WO3/TiO2 | 90–98% (550–725 °C) | WO3 and MoO3 could promote the activity not only due to the special structural, but also to the unique chemical property; Compared with the commercial V2O5-WO3/TiO2 catalyst, V2O5- MoO3/TiO2 catalyst was more active, but less selective. | [56] |
| V2O5-MoO3/TiO2 | 90–100% (500–725 °C) | ||
| V2O5-Sb2O3/TiO2 | 90–100% (225–380 °C) | Sb2O3-based catalyst showed a higher NO conversion and broader temperature windows as well as higher SO2 and H2O resistance. The excellent performance was suppressed by the extent of NH4HSO4 accumulated on the catalyst surface | [57] |
| V2O5-WO3/TiO2 | 90–94% (275–350 °C) |
| Doping-Agents | Co/Cd (Cc) | Reaction Conditions | Deactivated/Recovered Activity (concentration of H2O and SO2) | Key Factors for Enhancing SCR Efficiency | Ref. |
|---|---|---|---|---|---|
| F | 52%/82.8% (+57%) | [NO] = [NH3] = 500 ppm, [O2] = 5 Vol % and N2 in balance, GHSV = 43,000 h−1 at 240 °C | 62%/99% ([H2O] = [SO2] = 300) | Improve the interaction of WO3 with TiO2 by oxygen vacancies; Increase the number of the reduced W species (W5+). | [21,58] |
| Na | 100%/20% (−80%) | [NO] = [NH3] = 500 ppm, [O2] = 3 Vol % and N2 in balance, GHSV = 70,000 h−1 at 450 °C | ~ | Decrease the quantity and stability of the Brønsted acid sites; Reduced catalysts surface chemisorbed oxygen; Decrease the reducibility of vanadium and tungsten species. | [63] |
| K | 100%/30% (−70%) | ||||
| Ca | 100%/53% (−47%) | ||||
| Mg | 100%/95% (−5%) | ||||
| Cu | 72%/98% (+36.1%) | exhaust gas and [NO] = 1200 ppm [O2] = 2 Vol % and N2 in balance, GHSV = 10,800 h−1 at 550 °C | ~ | Form moderate acidity and improve the V4+/V5+ ratio on the catalyst surface; Increase the redox performance of the catalyst. | [60] |
| Mn | 72%/97% (+34.7%) | ||||
| Ce | 52%/95% (+82.6%) | [NO] = [NH3] = 500 ppm, [O2] = 3 Vol % and N2 in balance, GHSV = 28,000 h−1 at 200 °C | 95%/100% (100 ppm SO2 and 10% H2O) | Increase chemisorbed oxygen; Provide stronger and more active Brønsted acid centers | [60,61,62] |
| Deactivating Species | Key Factors | Ref. |
|---|---|---|
| HgCl2 | Reduce the Brønsted acid sites (V-OH) but produce new NH3 adsorption sites (Cl-V-O-H). | [64] |
| Hg | Transform V5+ species to V4+ species and consumed the lattice oxygen. | [65] |
| KCl | KCl could react with V-OH, leading the active sites for NH3 absorption inactive. | [66] |
| Hg | The gaseous Hg adsorbed on the vanadia sites reduce the active sites. | |
| KCl and Hg | There is the competition for active sites that are partially reduced by gaseous Hg, while others can be increased by delaying the deactivation caused by KCl. | |
| KCl | Lowering the acidity at different extent by different forms of V species; NH3 adsorption temperature allows to evaluate the acid sites. | [67] |
| Alkali metal | Decrease the ability of NH3 adsorption but increase in the NH3 desorption rate. | [68] |
| P, Cr and Cu | Moderate poisoning of the catalysts due to competition between increase the N2O production and lowering the number of acid sites. | [69] |
| K, Na, Mg and Ca | Reduced capacity of ab-NH3. | [69,70] |
| Ca | Can passivate surface acid sites of fresh and bulk W species. | [71] |
| As | As2O5 dense layers derived from the ab-As2O3 oxidized of by chemisorption oxygen on catalyst surface prevent NH3 adsorption and active sites recovery. | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Qi, S.; Pantaleo, G.; Liotta, L.F. WO3–V2O5 Active Oxides for NOx SCR by NH3: Preparation Methods, Catalysts’ Composition, and Deactivation Mechanism—A Review. Catalysts 2019, 9, 527. https://doi.org/10.3390/catal9060527
Zhang W, Qi S, Pantaleo G, Liotta LF. WO3–V2O5 Active Oxides for NOx SCR by NH3: Preparation Methods, Catalysts’ Composition, and Deactivation Mechanism—A Review. Catalysts. 2019; 9(6):527. https://doi.org/10.3390/catal9060527
Chicago/Turabian StyleZhang, Weidong, Shuhua Qi, Giuseppe Pantaleo, and Leonarda Francesca Liotta. 2019. "WO3–V2O5 Active Oxides for NOx SCR by NH3: Preparation Methods, Catalysts’ Composition, and Deactivation Mechanism—A Review" Catalysts 9, no. 6: 527. https://doi.org/10.3390/catal9060527
APA StyleZhang, W., Qi, S., Pantaleo, G., & Liotta, L. F. (2019). WO3–V2O5 Active Oxides for NOx SCR by NH3: Preparation Methods, Catalysts’ Composition, and Deactivation Mechanism—A Review. Catalysts, 9(6), 527. https://doi.org/10.3390/catal9060527







