Oxygen Vacancy Enhanced Photoreduction Cr(VI) on Few-Layers BiOBr Nanosheets
Abstract
:1. Introduction
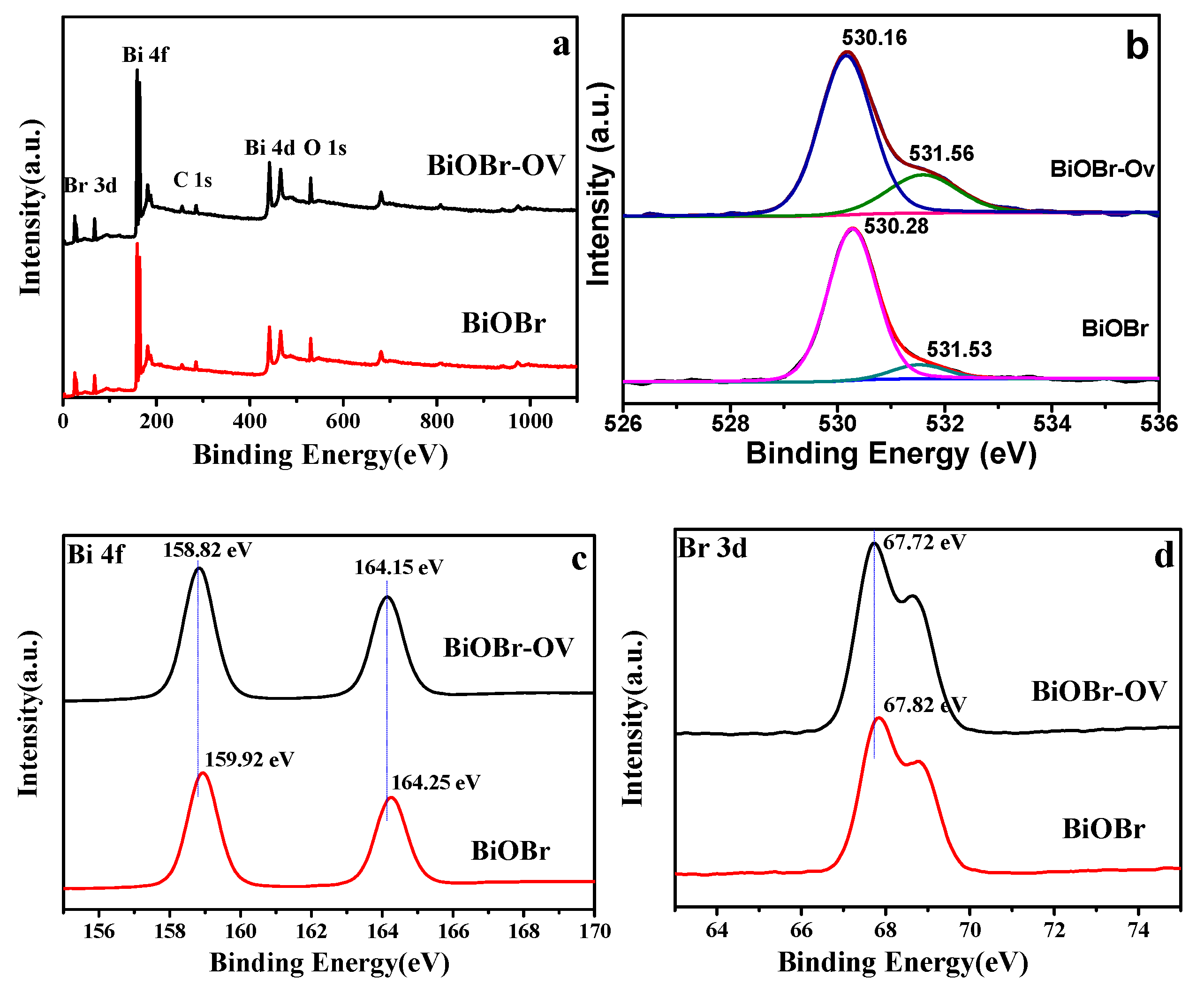
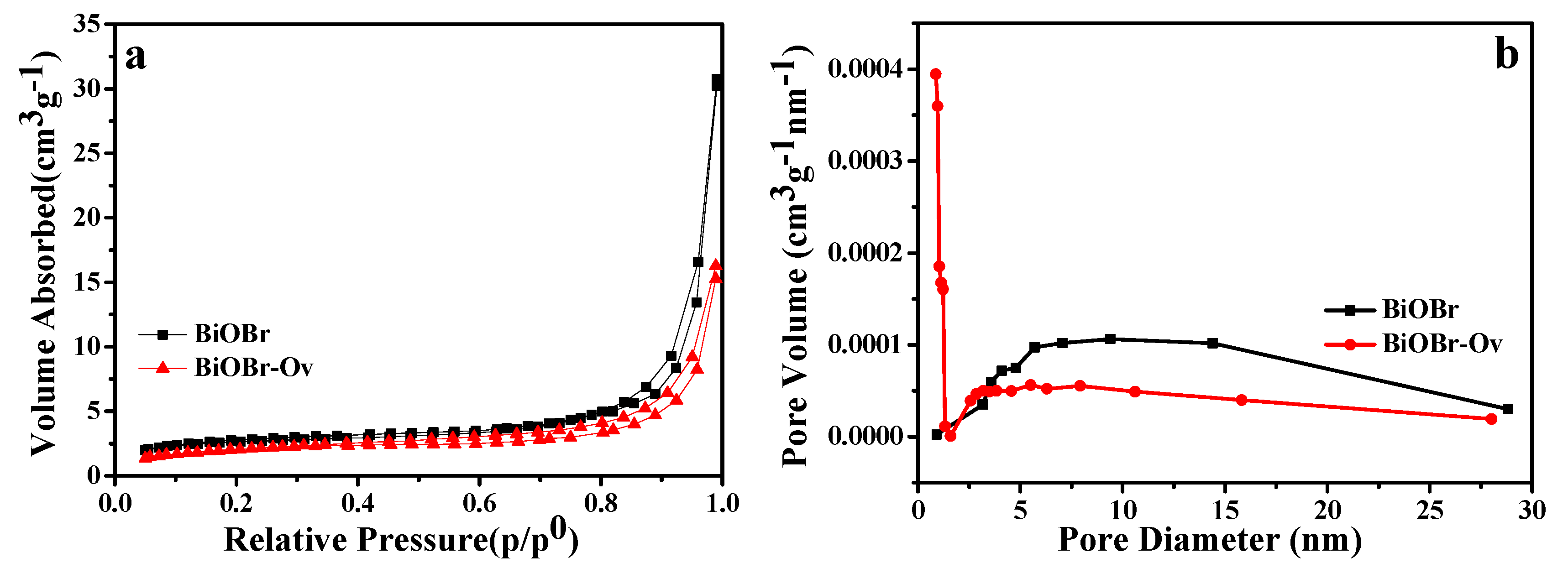
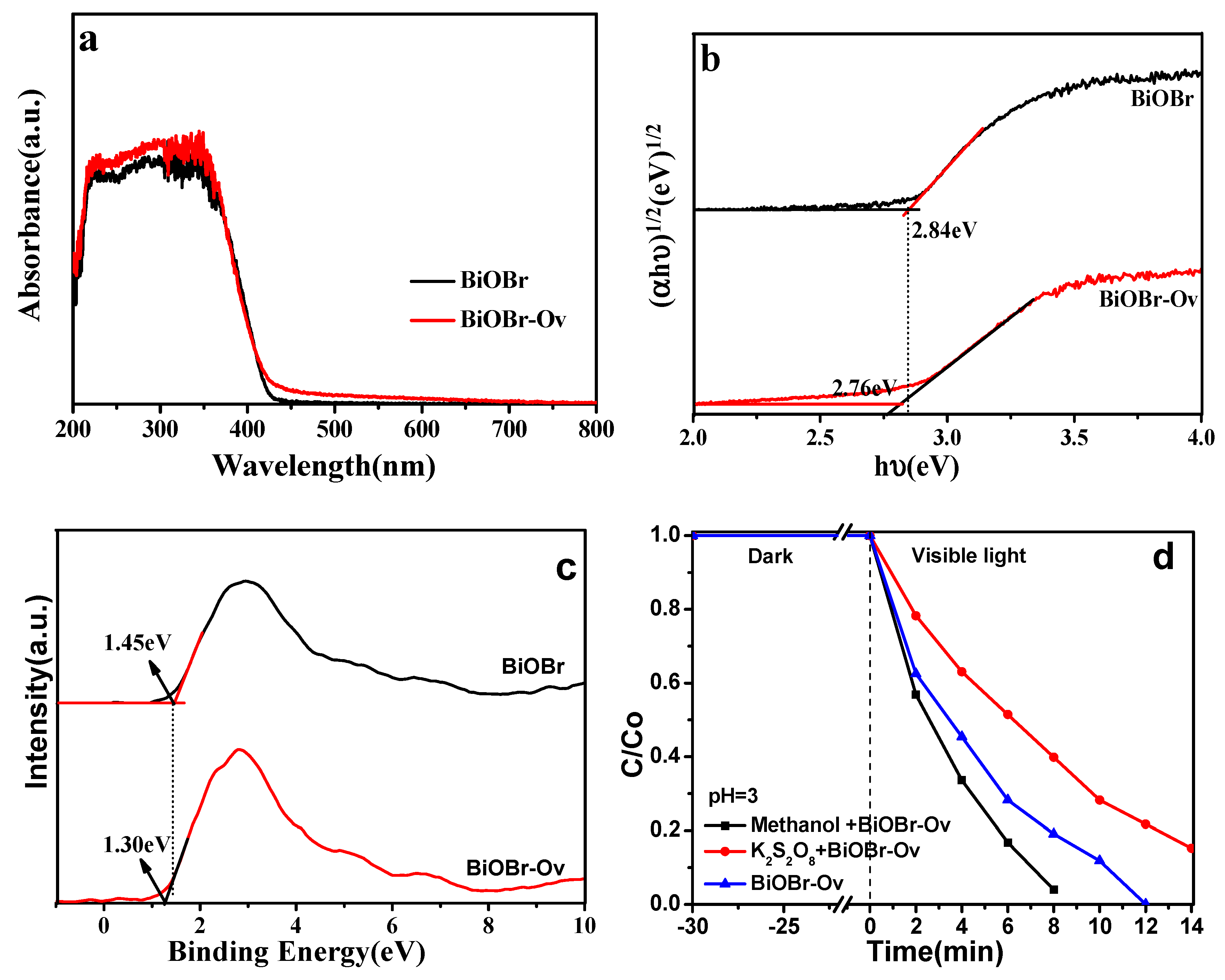
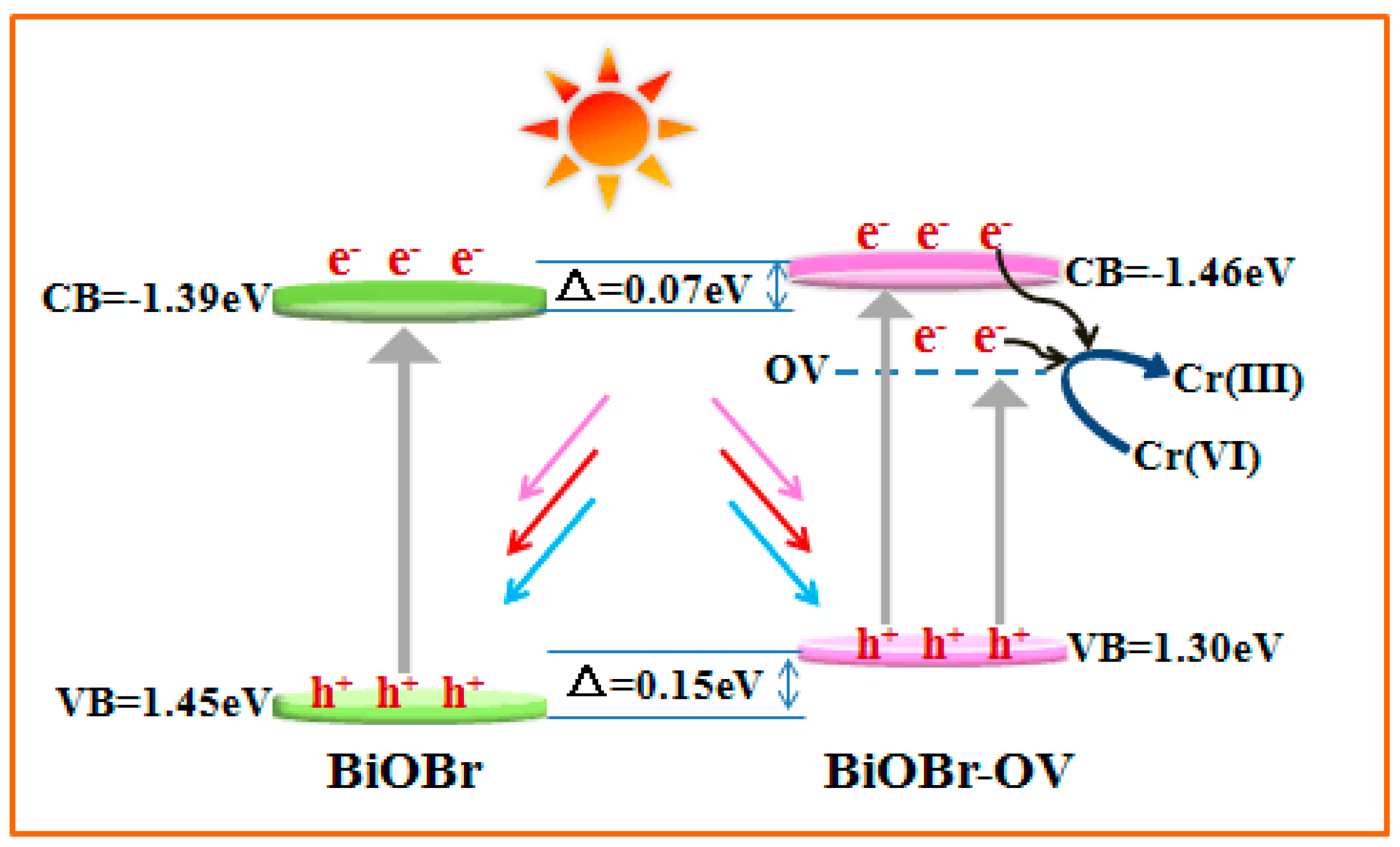
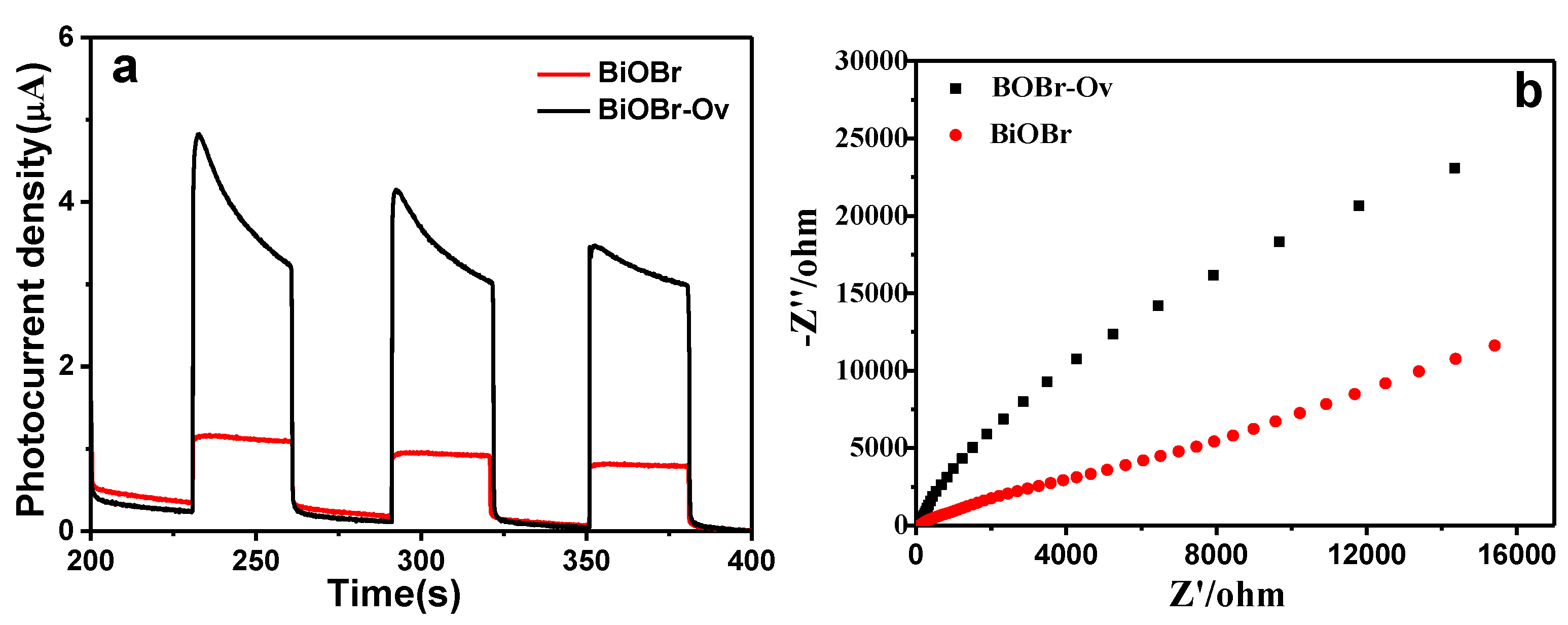
2. Results and Discussion
3. Experimental Section
3.1. Photocatalyst Synthesis
3.2. Photocatalytic Activity Assessment
3.3. Electrochemical Impedance Spectroscopy (EIS) and Photoelectrochemical Measurements
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiang, Q.J.; Cheng, B.; Yu, J.G. Graphene-based photocatalysts for solar-fuel generation. Angew. Chem. Int. Ed. 2015, 54, 11350–11366. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ye, L.; Xie, H.; Chen, G. Bismuth-rich bismuth oxyhalides for environmental and energy photocatalysis. Coord. Chem. Rev. 2017, 349, 84–101. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity Enhancement in Heterogeneous Photocatalytic Transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Li, J.; Cai, L.; Shang, J.; Yu, Y.; Zhang, L. Giant enhancement of internal electric field boosting bulk charge separation for photocatalysis. Adv. Mater. 2016, 28, 4059–4064. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of hierarchical V2O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.-M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, K.K.; Liu, T.; Xu, J.; Xu, B. Synthesis of one-dimensional Bi2O3-Bi2O2.33 heterojunctions with high interface quality for enhanced visible light photocatalysis in degradation of high-concentration phenol and MO dyes. Appl. Catal. B-Environ. 2017, 203, 946–954. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interf. 2012, 4, 4024–4030. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, M.; Chen, Q.G.; Fan, C.M.; Zhou, H.Y.; Xu, A.W. Novel onedimensional Bi2O3-Bi2WO6 p-n hierarchical heterojunction with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8517–8524. [Google Scholar] [CrossRef]
- Mi, Y.; Li, H.; Zhang, Y.; Du, N.; Hou, W. Synthesis and photocatalytic activity of BiOBr nanosheets with tunable crystal facets and sizes. Catal. Sci.Technol. 2018, 8, 2588–2597. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Zhang, Q.; Yan, W.; Pan, B.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved Molecular oxygen activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tang, Z. Ultrathin two-dimensional layered metal hydroxides: an emerging platform for advanced catalysis, energy conversion and storage. Chem. Soc. Rev. 2016, 45, 4873–4891. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Nasilowski, M.; Mahler, B.; Lhuillier, E.; Ithurria, S.; Dubertret, B. Two-Dimensional Colloidal Nanocrystals. Chem. Rev. 2016, 116, 10934–10982. [Google Scholar] [CrossRef]
- Gao, S.; Gu, B.; Jiao, X.; Sun, Y.; Zu, X.; Yang, F.; Zhu, W.; Wang, C.; Feng, Z.; Ye, B.; et al. Highly efficient and exceptionally durable CO2 photoreduction to methanol over freestanding defective single-unit-cell bismuth vanadate layers. J. Am. Chem. Soc. 2017, 139, 3438–3445. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Li, H.; Liu, Z. Freestanding atomically-thin two-dimensional materials beyond graphene meeting photocatalysis: Opportunities and challenges. Nano Energy 2017, 35, 79–91. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, C.; Li, Z.; Xie, Y. Vacancy engineering for tuning electron and phonon structures of two-dimensional materials. Adv. Energy Mater. 2016, 6, 1600436. [Google Scholar] [CrossRef]
- Li, X.D.; Shi, W.; Ling, P.Q.; Sun, Y.F.; Jiao, X.C.; Gao, S.; Liang, L.; Xu, J.Q.; Yan, W.S.; Wang, C.M.; et al. Efficient visible-light-driven CO2 reduction mediated by defect- engineered BiOBr atomic layers. Angew. Chem. Int. Ed. 2018, 130, 8855–8859. [Google Scholar] [CrossRef]
- Lei, F.; Sun, Y.; Liu, K.; Gao, S.; Liang, L.; Pan, B.; Xie, Y. Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shang, J.; Ai, Z.H.; Zhang, L.Z. Efficient visible light nitrogen fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Y.; Ng, B.J.; Tan, K.H.; Chen, X.F.; Wang, H.T.; Mohamed, A.R.; Chai, S.P. Simultaneous generation of oxygen vacancies on ultrathin BiOBr nanosheets during visible-light-driven CO2 photoreduction evoked superior activity and long-term stability. Catal. Today 2018, 314, 20–27. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Chen, H.; Hu, Y.; Ding, Q.; Liu, Z.; Ma, L.; Zhu, G.; Zhang, W.; Yu, Q.; et al. Oxygen Vacancy Engineering Promoted Photocatalytic Ammonia Synthesis on Ultrathin Two-Dimensional Bismuth Oxybromide Nanosheets. Nano Lett. 2018, 18, 7372–7377. [Google Scholar] [CrossRef]
- Shi, X.; Wang, P.Q.; Wang, L.; Bai, Y.; Xie, H.Q.; Zhou, Y.; Wang, J.A.; Li, Z.J.; Qu, L.B.; Shi, M.J.; et al. Few layered BiOBr with expanded interlayer spacing and oxygen vacancies for efficient decomposition of real oil Field produced wastewater. ACS Sustain. Chem. Eng. 2018, 6, 13739–13746. [Google Scholar] [CrossRef]
- Hua, C.H.; Dong, X.L.; Wang, Y.; Zheng, N.; Ma, H.C.; Zhang, X.F. Bi-modified 3D BiOBr microsphere with oxygen vacancies for efficient visible-light photocatalytic performance. J. Mater. Sci. 2019, 54, 9397–9413. [Google Scholar] [CrossRef]
- McLean, J.E.; McNeill, L.S.; Edwards, M.; Parks, J.L. Factors controlling micropollutant removal during riverbank filtration. J. Am. Water Works Assoc. 2012, 104, 35–36. [Google Scholar]
- Mishra, S.; Bharagava, R.N. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Health C 2016, 34, 1–32. [Google Scholar] [CrossRef]
- Blowes, D. Tracking hexavalent Cr in groundwater. Science 2002, 295, 2024. [Google Scholar] [CrossRef]
- Vaiopoulou, E.; Gikas, P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: A review. Water Res. 2012, 46, 549–570. [Google Scholar] [CrossRef]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Hsu, L.C.; Wang, S.L.; Lin, Y.C.; Wang, M.K.; Chiang, P.N.; Liu, J.C.; Kuan, W.H.; Chen, C.C.; Tzou, Y.M. Cr(VI) removal on fungal biomass of neurospora crassa: the importance of dissolved organic carbons derived from the biomass to Cr(VI) reduction. Environ. Sci. Technol. 2010, 44, 6202–6208. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Yashwanthraj, M. Sequestration of toxic Cr(VI) ions from industrial wastewater using waste biomass: A review. Desalin Water Treat. 2017, 68, 245–266. [Google Scholar]
- Qiu, B.; Xu, C.X.; Sun, D.Z.; Wang, Q.; Gu, H.B.; Zhang, X.; Weeks, B.L.; Hopper, J.; Ho, T.C.; Guo, Z.H.; et al. Polyaniline coating with various substrates for hexavalent chromium removal. Appl. Surf. Sci. 2015, 334, 7–14. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Pakade, V.; Chimuka, L. Polymeric sorbents for removal of Cr(VI) from environmental samples. Pure Appl. Chem. 2013, 85, 2145–2160. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Liu, Z.F.; Zeng, G.M.; Liu, Y.J.; Shao, B.B.; Li, Z.G.; Liu, Y.; Zhang, W.; He, Q.Y. Polyaniline-based adsorbents for removal of hexavalent chromium from aqueous solution: a mini review. Environ. Sci. Pollut. Res. Int. 2018, 25, 6158–6174. [Google Scholar] [CrossRef]
- Fernandez, P.M.; Vinarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef]
- Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Du, X.D.; Li, J.; Guo, X.X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B-Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Y.B.; Zhai, W.; Qiu, L.; Li, H.; Hoffman, M.R. Facet-dependent performance of BiOBr for photocatalytic reduction of Cr(VI). RSC Adv. 2016, 6, 2028–2031. [Google Scholar] [CrossRef]
- Han, J.L.; Zhu, G.Q.; Hojamberdiev, M.; Peng, J.H.; Zhang, X.; Liu, Y.; Ge, B.; Liu, P. Rapid adsorption and photocatalytic activity for Rhodamine B and Cr(VI) by ultrathin BiOI nanosheets with highly exposed {001} facets. New J. Chem. 2015, 39, 1874–1882. [Google Scholar] [CrossRef]
- Shang, J.; Hao, W.; Lv, X.; Wang, T.; Wang, X.; Du, Y.; Dou, S.; Xie, T.; Wang, D.; Wang, J. Bismuth oxybromide with reasonable photocatalytic reduction activity under visible light. ACS Catal. 2014, 4, 954–961. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Chen, T.; Wang, P.; Wang, L.; Shi, X.; Wong, P.K. Synthesis of hierarchical bismuth-rich Bi4O5BrxI2-x solid solutions for enhanced photocatalytic activities of CO2 conversion and Cr(VI) reduction under visible light. Appl. Catal. B Environ. 2017, 203, 633–640. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L. Oxygen vacancy induced selective silver deposition on the {001} facets of BiOCl single-crystalline nanosheets for enhanced Cr(VI) and sodium pentachlorophenate removal under visible light. Nanoscale 2014, 6, 7805–7810. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Xiong, X.Y.; Ding, S.P.; Liu, X.F.; Jiang, Q.Q.; Hu, J.C. In-situ topotactic synthesis and photocatalytic activity of plate-like BiOCl/2D networks Bi2S3 heterostructures. Appl. Catal. B-Environ. 2018, 220, 570–580. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Jiang, Y.F.; Xiong, X.Y.; Ding, S.P.; Shi, Y.Q.; Liu, X.F.; Liu, Y.; Huang, Z.X.; Hu, J.C. Synthesis and characterization of single-crystalline Bi19Cl3S27 nanorods. Catal. Sci. Technol. 2017, 7, 3464–3468. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, X.; Ding, S.; Liu, X.; Jiang, Q.; Hu, J. In-situ topotactic synthesis and photocatalytic activity of plate-like BiOCl/2D networks Bi2S3 heterostructures. Appl. Catal. B Environ. 2018, 220, 570–580. [Google Scholar] [CrossRef]
- Peng, Y.; Mao, Y.G.; Kan, P.F.; Liu, J.Y.; Fang, Z. Controllable synthesis and photoreduction performance towards Cr(vi) of BiOCl microrods with exposed (110) crystal facets. New J. Chem. 2018, 42, 16911–16918. [Google Scholar] [CrossRef]
- Chen, J.; Guan, M.; Cai, W.; Guo, J.; Xiao, C.; Zhang, G. The dominant {001} facet-dependent enhanced visible-light photoactivity of ultrathin BiOBr nanosheets. Phys. Chem. Chem. Phys. PCCP 2014, 16, 20909–20914. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Zhao, K.; Zhang, L. Sustainable molecular oxygen activation with oxygen vacancies on the {001} facets of BiOCl nanosheets under solar light. Nanoscale 2014, 6, 14168–14173. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M. TiO2/Ti1-xSnxO2 heterojunction nanowires: characterization, formation, and gas sensing performance. J. Mater. Chem. A 2011, 21, 14048–14055. [Google Scholar] [CrossRef]
- Lee, J.; Ham, S.; Choi, D.; Jang, D.-J. Facile fabrication of porous ZnS nanostructures with a controlled amount of S vacancies for enhanced photocatalytic performances. Nanoscale 2018, 10, 14254–14263. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Oxygen vacancies in ZnO. Appl. Phys. Lett. 2005, 87, 122102. [Google Scholar] [CrossRef]
- Gao, S.; Sun, Z.; Liu, W.; Jiao, X.; Zu, X.; Hu, Q.; Sun, Y.; Yao, T.; Zhang, W.; Wei, S.; et al. Atomic layer confined vacancies for atomic-level insights into carbon dioxide electroreduction. Nat. Commun. 2017, 8, 14503–14511. [Google Scholar] [CrossRef]
- Ye, L.; Zan, L.; Tian, L.; Peng, T.; Zhang, J. The {001} facets-dependent high photoactivity of BiOCl nanosheets. Chem. Commun. 2011, 47, 6951–6953. [Google Scholar] [CrossRef]
- Ghasimi, S.; Prescher, S.; Wang, Z.J.; Landfester, K.; Yuan, J.; Zhang, K.A.I. Heterophase photocatalysts from water-soluble conjugated polyelectrolytes: an example of self-initiation under visible light. Angew. Chem. Int. Ed. 2015, 54, 14549–14553. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.B.; Zheng, L.; Zhong, Y.; Hu, Y.; Lou, X.W. Formation of mesoporous heterostructured BiVO4/Bi2S3 hollow discoids with enhanced photoactivity. Angew. Chem. Int. Ed. 2014, 126, 6027–6031. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Meng, X.; Liu, H.; Ye, J. An amine-functionalized iron(III) metal–organic framework as efficient visible-light photocatalyst for Cr(VI) reduction. Adv. Sci. 2015, 2, 1500006. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, X.; Zhao, Y.; Zhang, X.; An, L.; Huang, M.; Chen, G.; Zhang, R. Engineering the band gap states of the rutile TiO2(110) surface by modulating the active heteroatom. Angew. Chem. Int. Ed. 2018, 130, 8686–8690. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr(VI) to Cr(III) using carbon dots coupled TiO2 mesocrystals. Appl. Catal. B Environ. 2018, 226, 213–219. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Zhu, Z.; Yan, M.; Zhou, Y.; Wang, J.; Liu, Y.; Wang, J. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
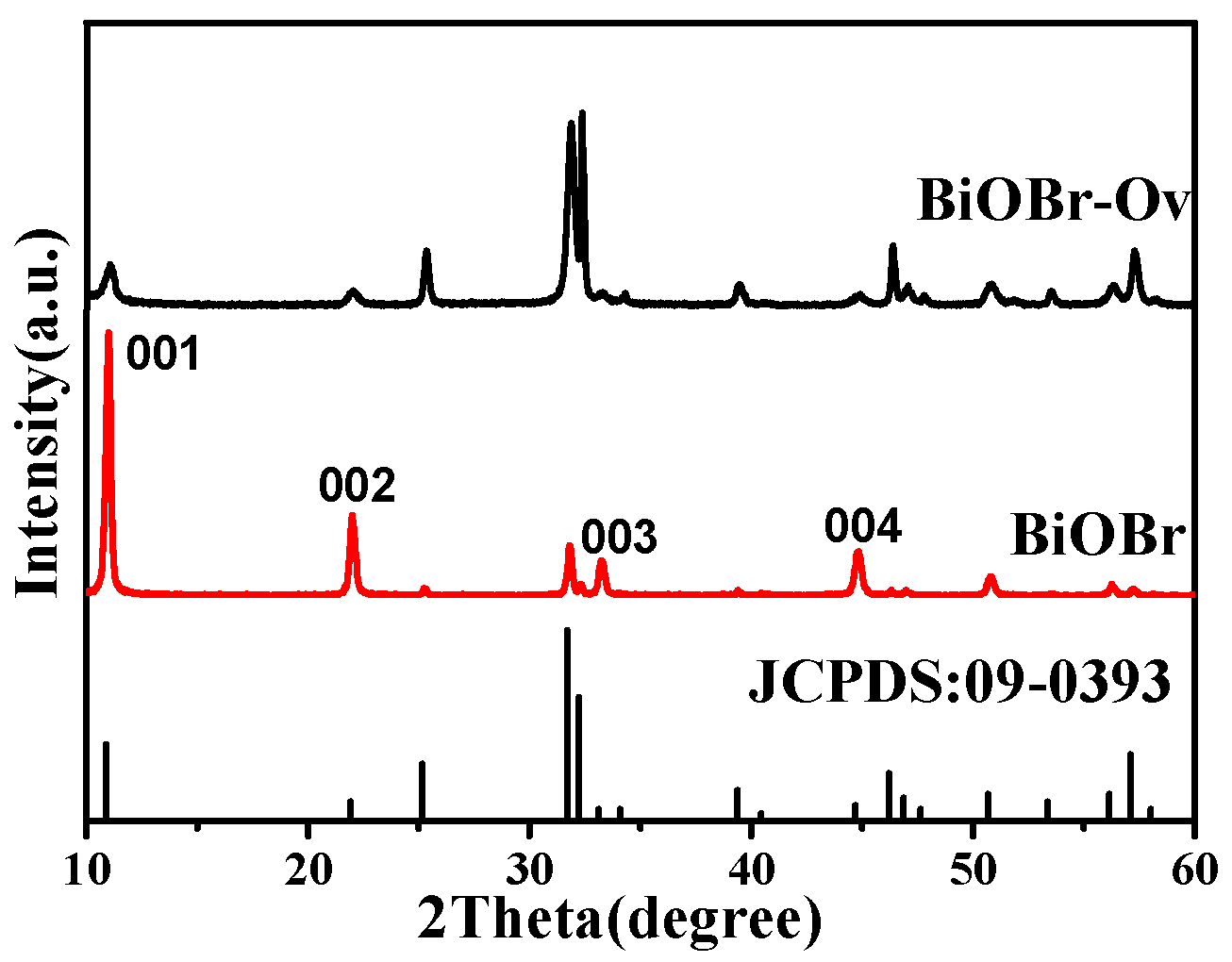
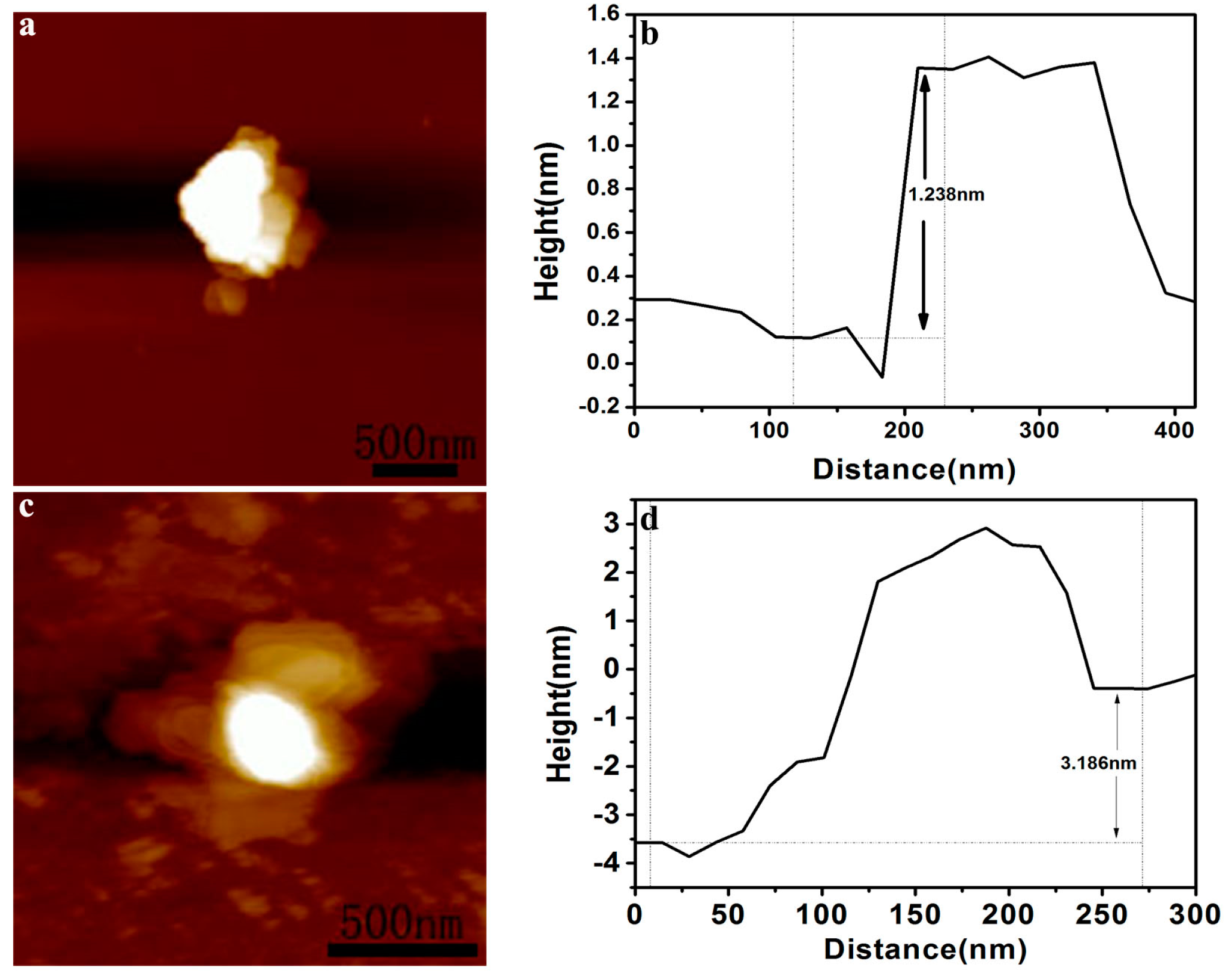
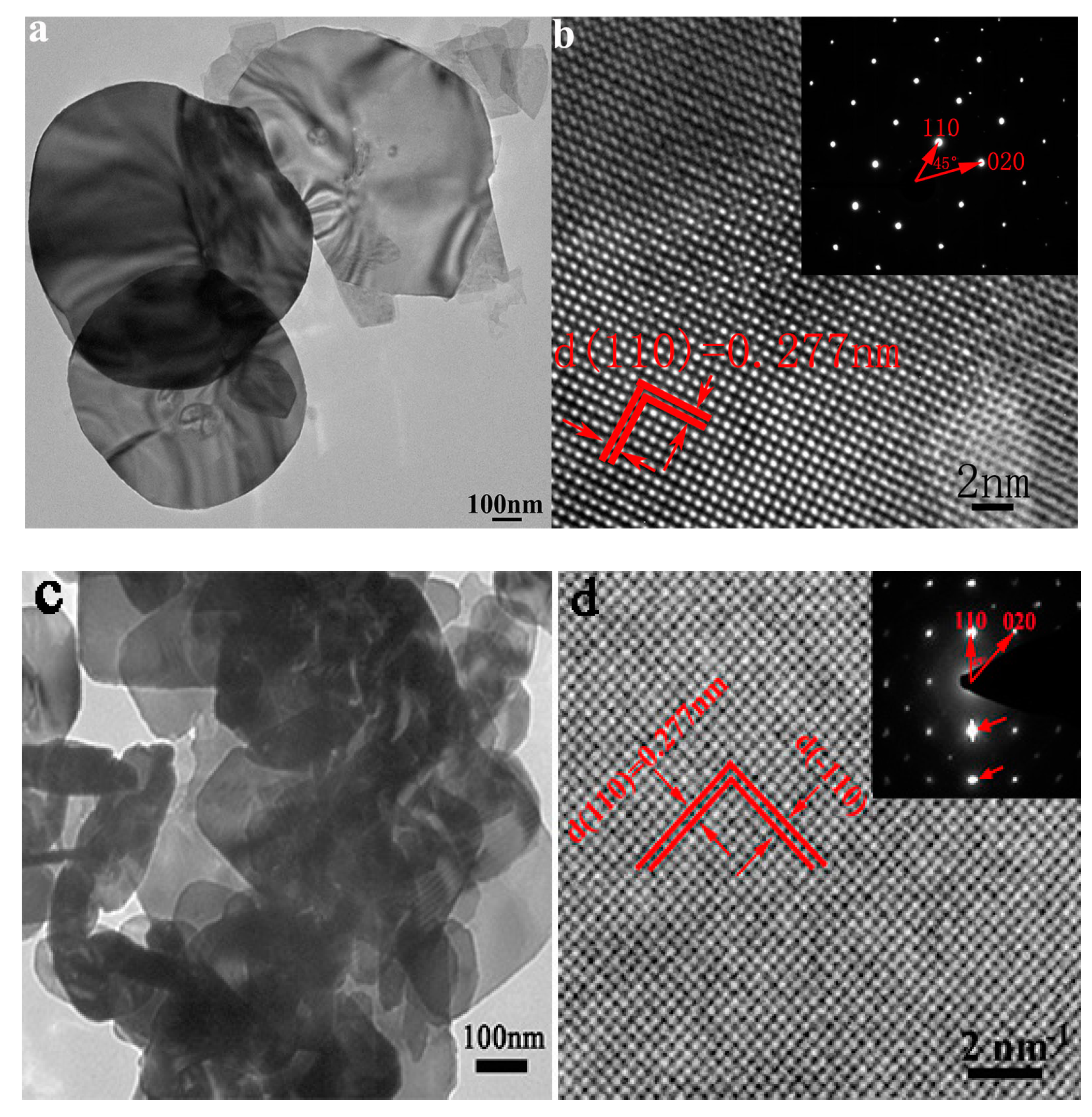
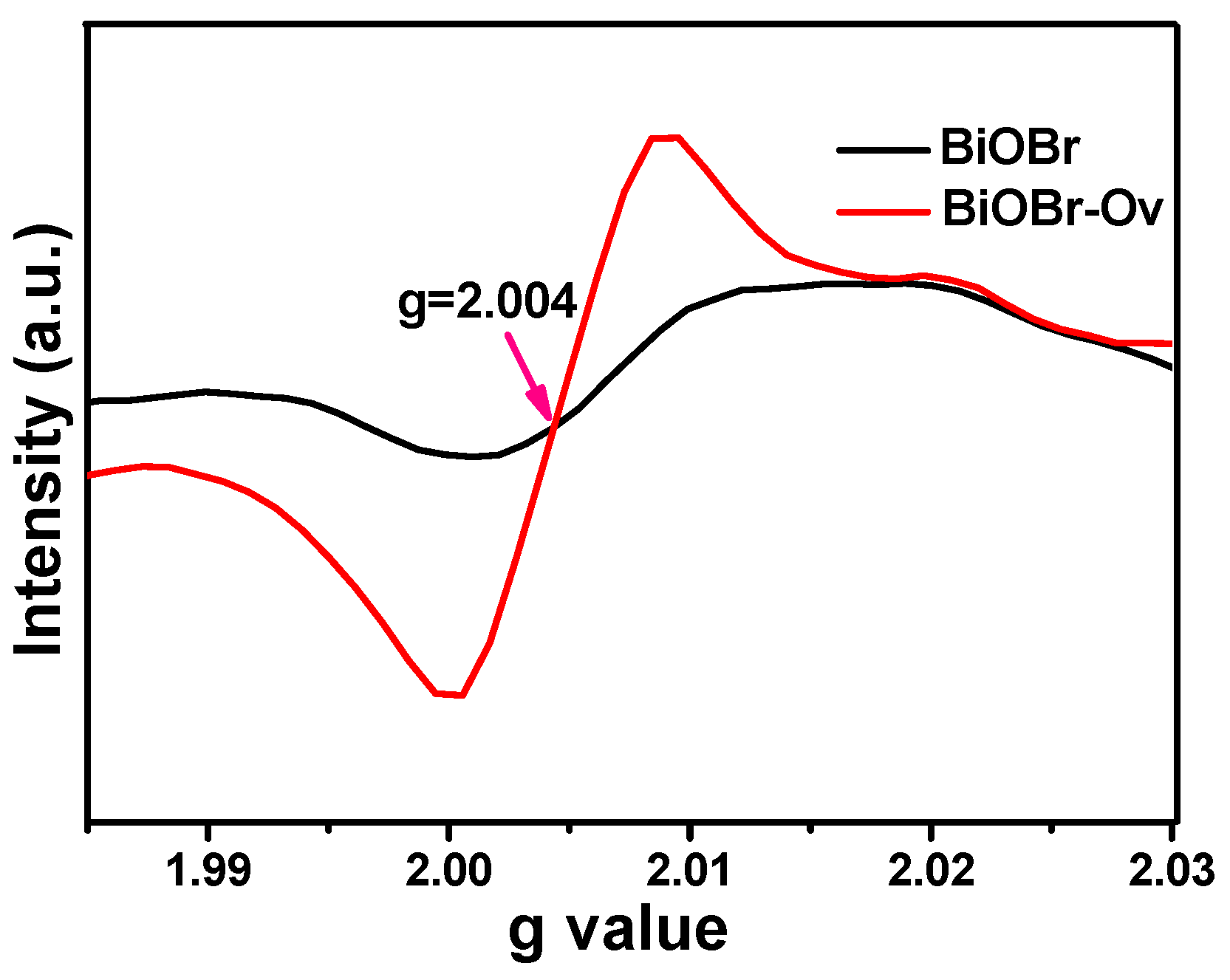

| No. | Material | Reaction Condition | Time (min) | Redcution Rate (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Catalyst (mg) | Volume (mL) | Cr(VI) (mg/L) | |||||
| 1 | Conjugatedpolyelectrolyte photocatalyst (P-FL-BT-3) | 1 | 5 | 25 | 120 | 100 | [60] |
| 2 | BiVO4/Bi2S3 | 10 | 20 | 10 | 60 | 92 | [61] |
| 3 | Metal–organic framework (NH2–MIL-88B (Fe)) | 20 | 40 | 8 | 40 | 100 | [62] |
| 4 | R-TiO2:N | 25 | 100 | 10 | 10 | 100 | [63] |
| 5 | Bi24O31Br10 | 50 | 50 | 50 | 40 | 100 | [47] |
| 6 | Carbon dots coupled TiO2 mesocrystals | 20 | 20 | 10 | 20 | 100 | [64] |
| 7 | Bi4O5BrxI2−x solid solutions | 20 | 50 | 60 | 60 | 100 | [48] |
| 8 | P doped-g-C3N4 | 50 | 50 | 20 | 60 | 100 | [65] |
| 9 | BiOBr-Ov | 40 | 40 | 20 | 12 | 100 | Our work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Kan, P.; Zhang, Q.; Zhou, Y. Oxygen Vacancy Enhanced Photoreduction Cr(VI) on Few-Layers BiOBr Nanosheets. Catalysts 2019, 9, 558. https://doi.org/10.3390/catal9060558
Peng Y, Kan P, Zhang Q, Zhou Y. Oxygen Vacancy Enhanced Photoreduction Cr(VI) on Few-Layers BiOBr Nanosheets. Catalysts. 2019; 9(6):558. https://doi.org/10.3390/catal9060558
Chicago/Turabian StylePeng, Yin, Pengfei Kan, Qian Zhang, and Yinghua Zhou. 2019. "Oxygen Vacancy Enhanced Photoreduction Cr(VI) on Few-Layers BiOBr Nanosheets" Catalysts 9, no. 6: 558. https://doi.org/10.3390/catal9060558
APA StylePeng, Y., Kan, P., Zhang, Q., & Zhou, Y. (2019). Oxygen Vacancy Enhanced Photoreduction Cr(VI) on Few-Layers BiOBr Nanosheets. Catalysts, 9(6), 558. https://doi.org/10.3390/catal9060558




