Hydrogen Production Improvement on Water Decomposition Through Internal Interfacial Charge Transfer in M3(PO4)2-M2P2O7 Mixed-Phase Catalyst (M = Co, Ni, and Cu)
Abstract
:1. Introduction
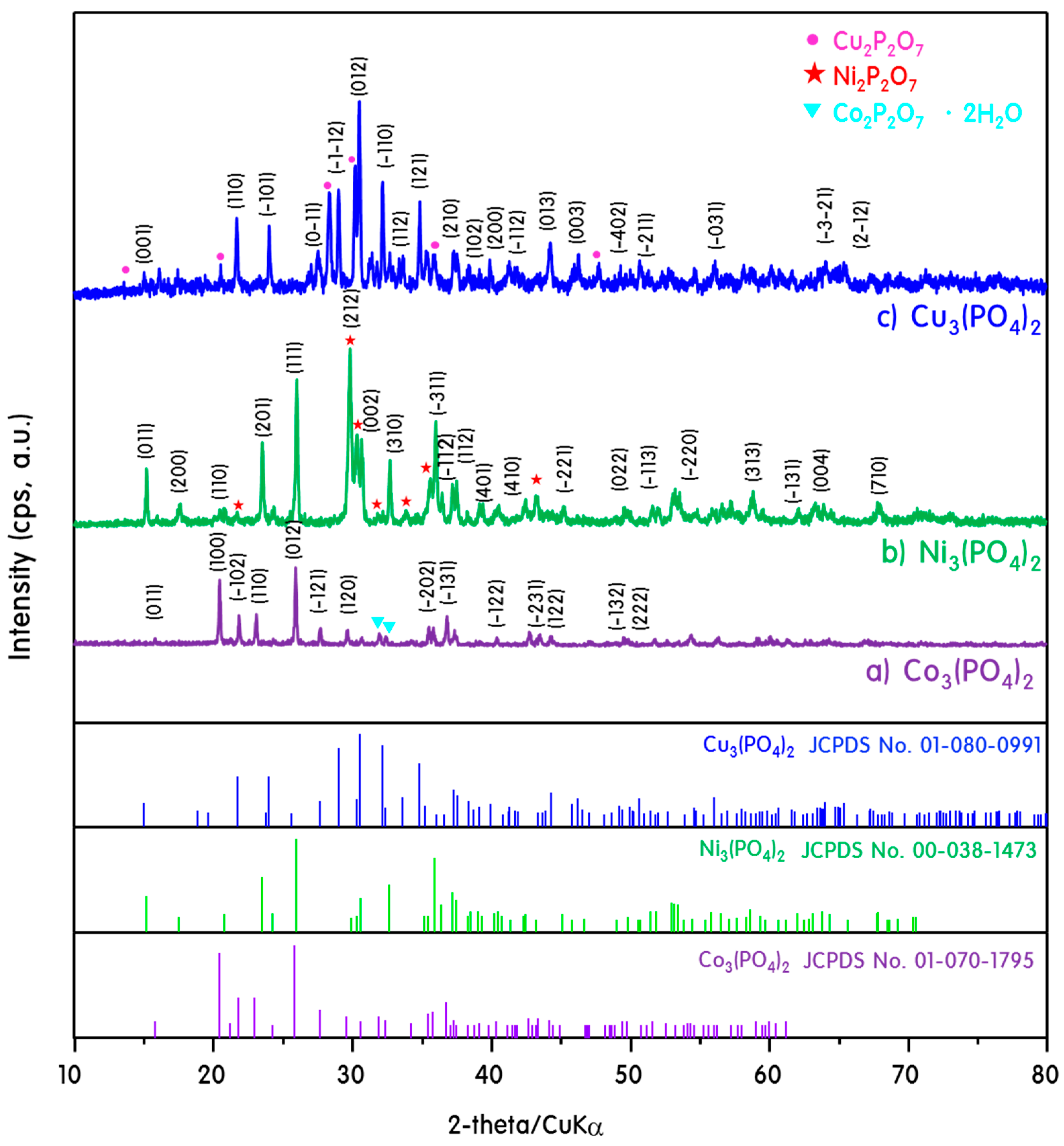
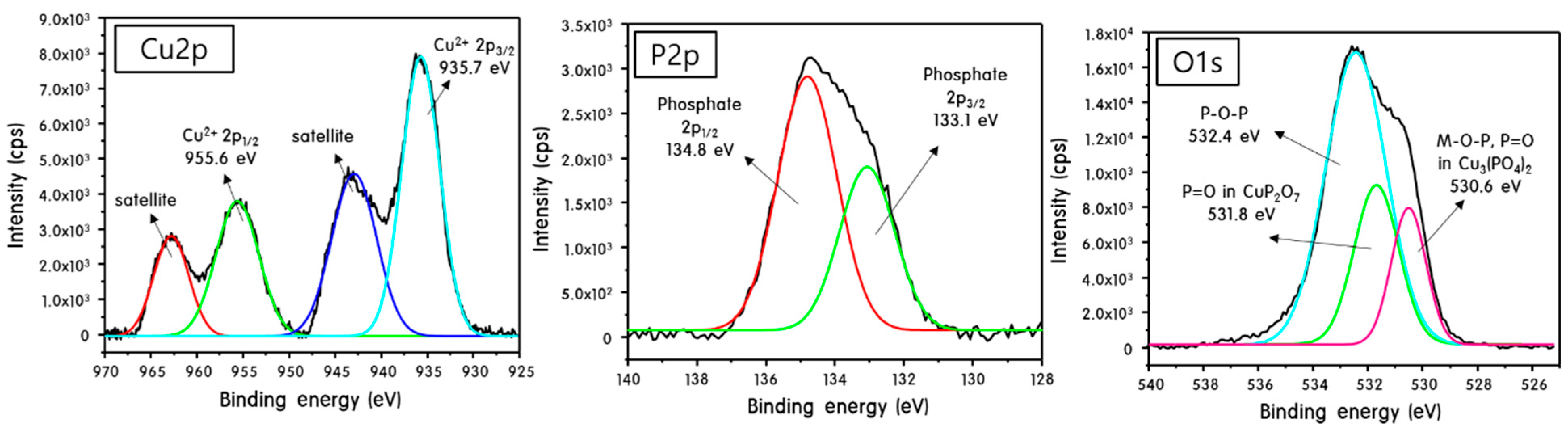
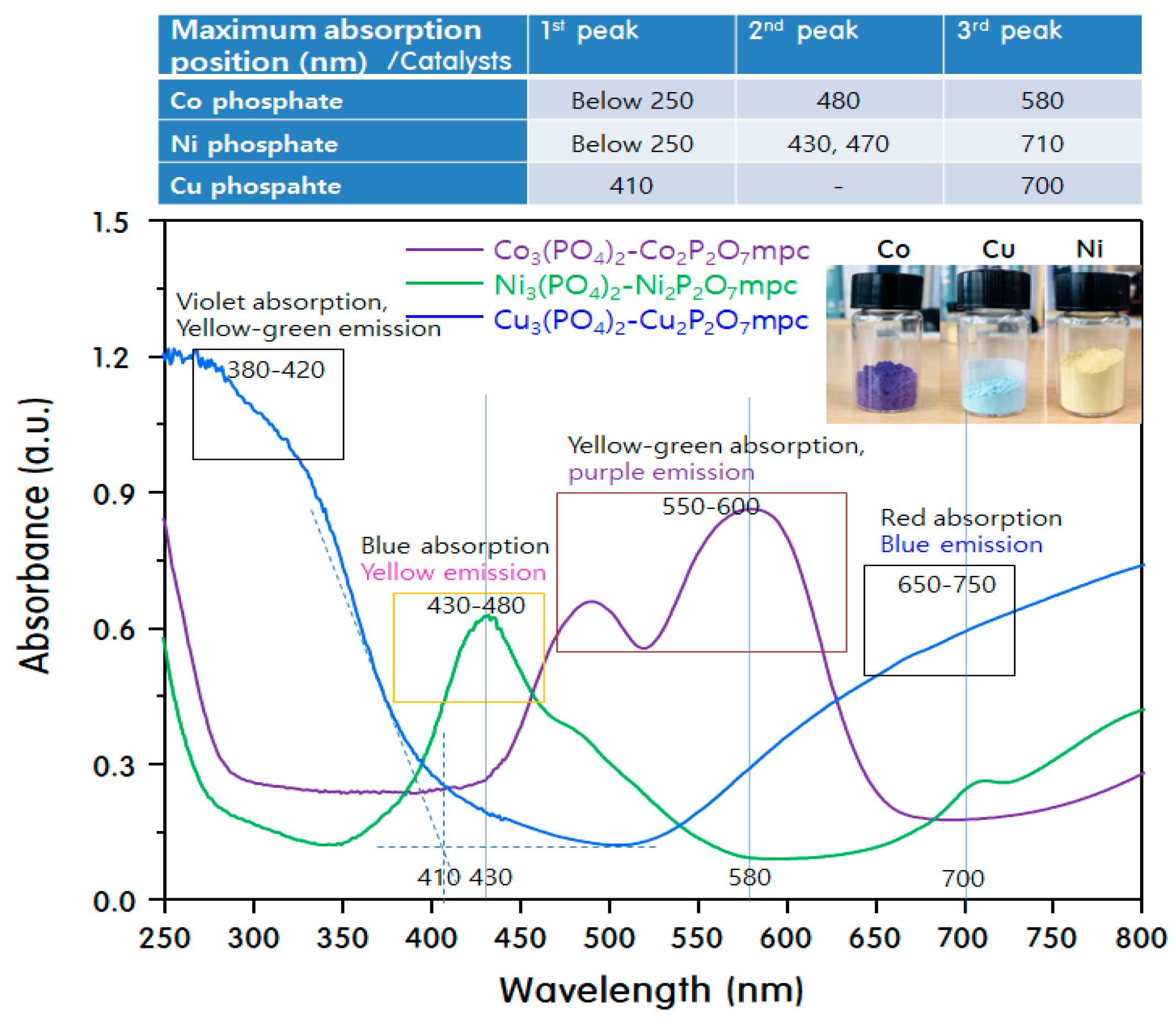
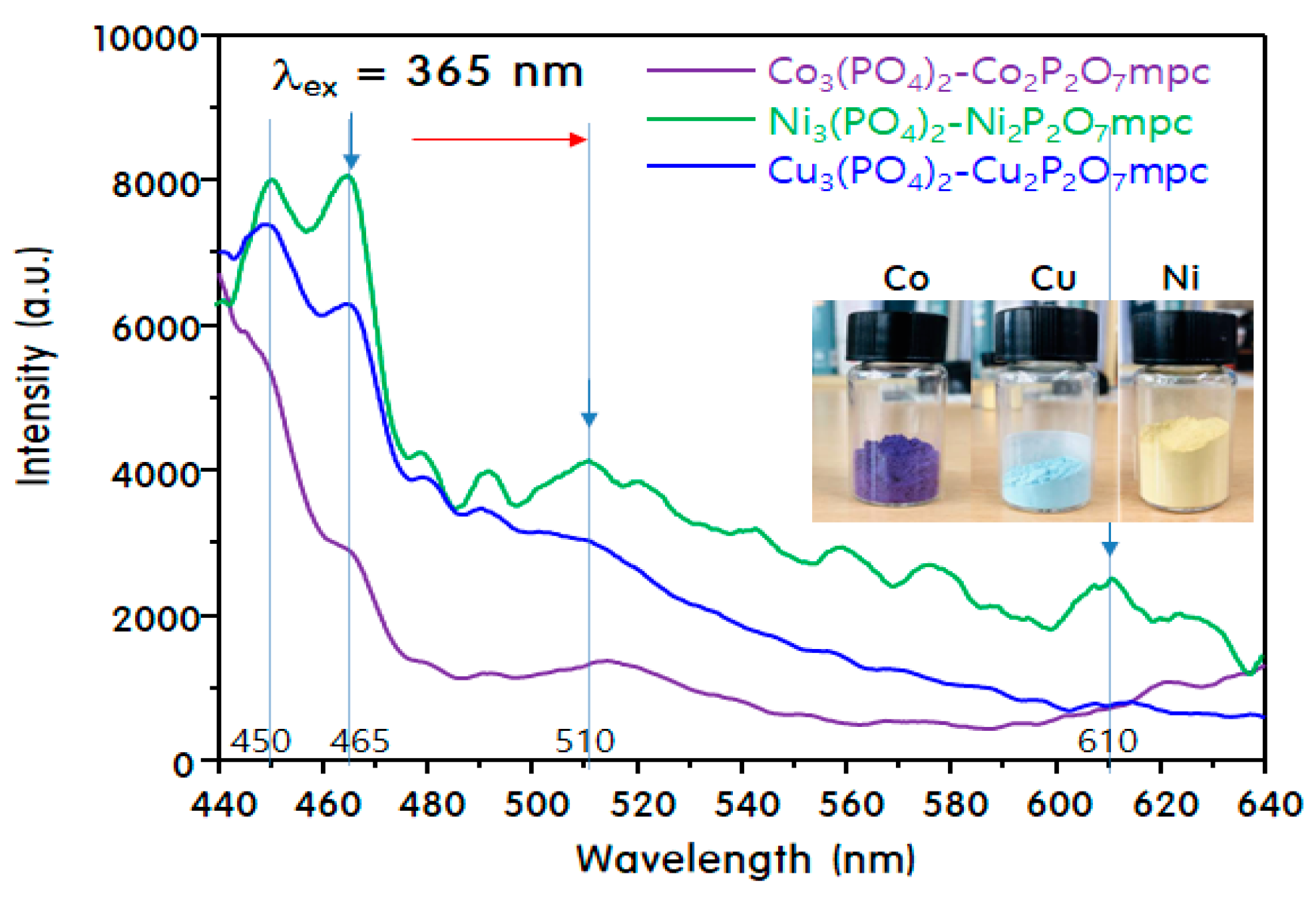
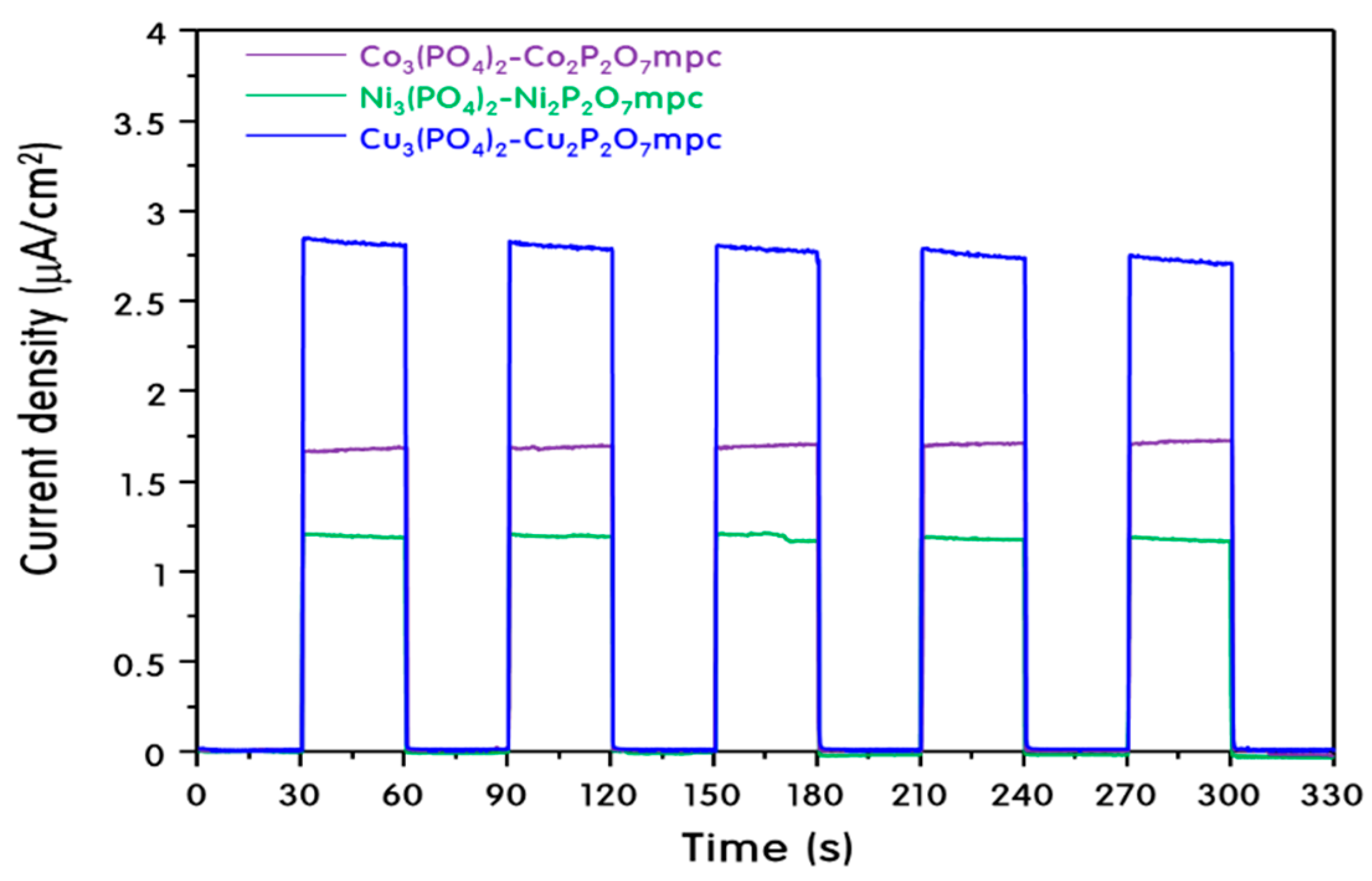
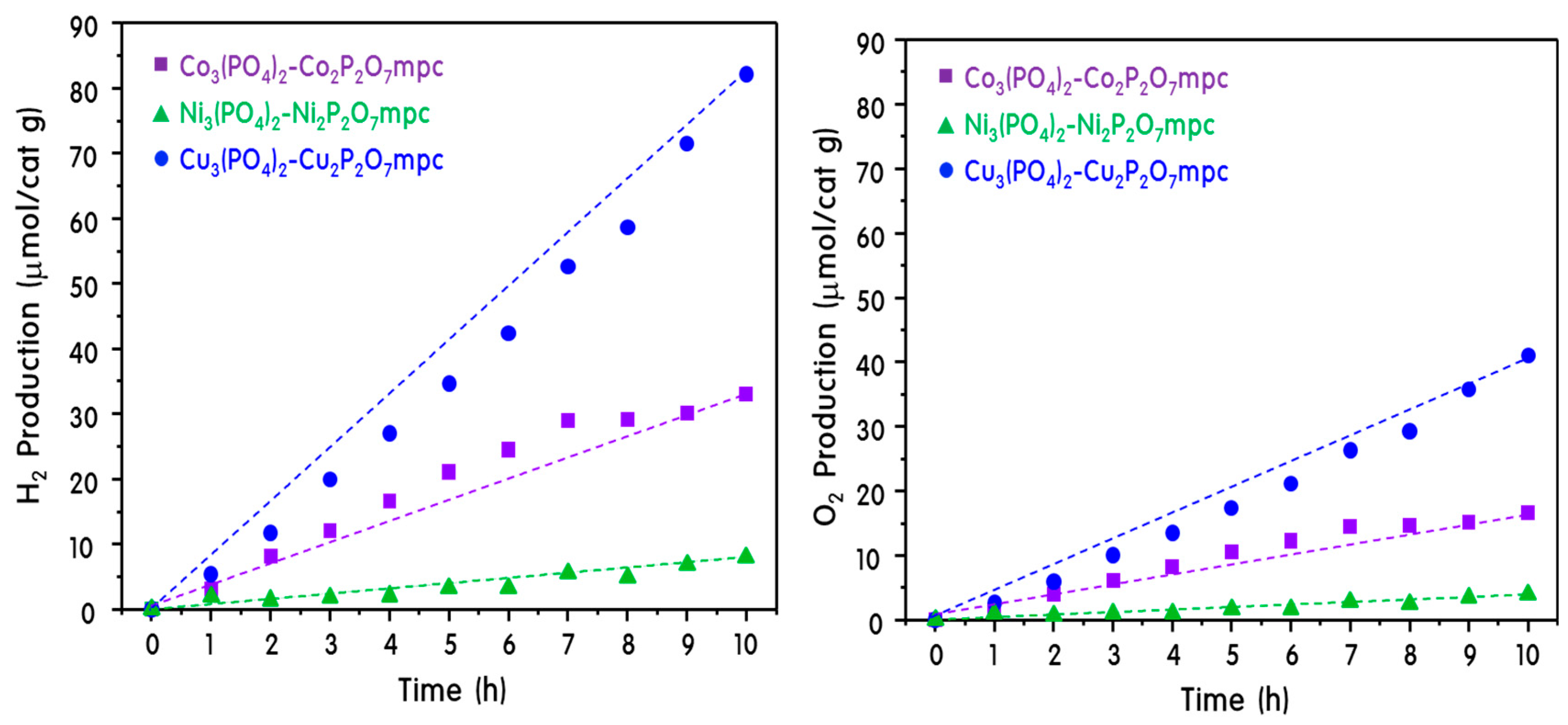
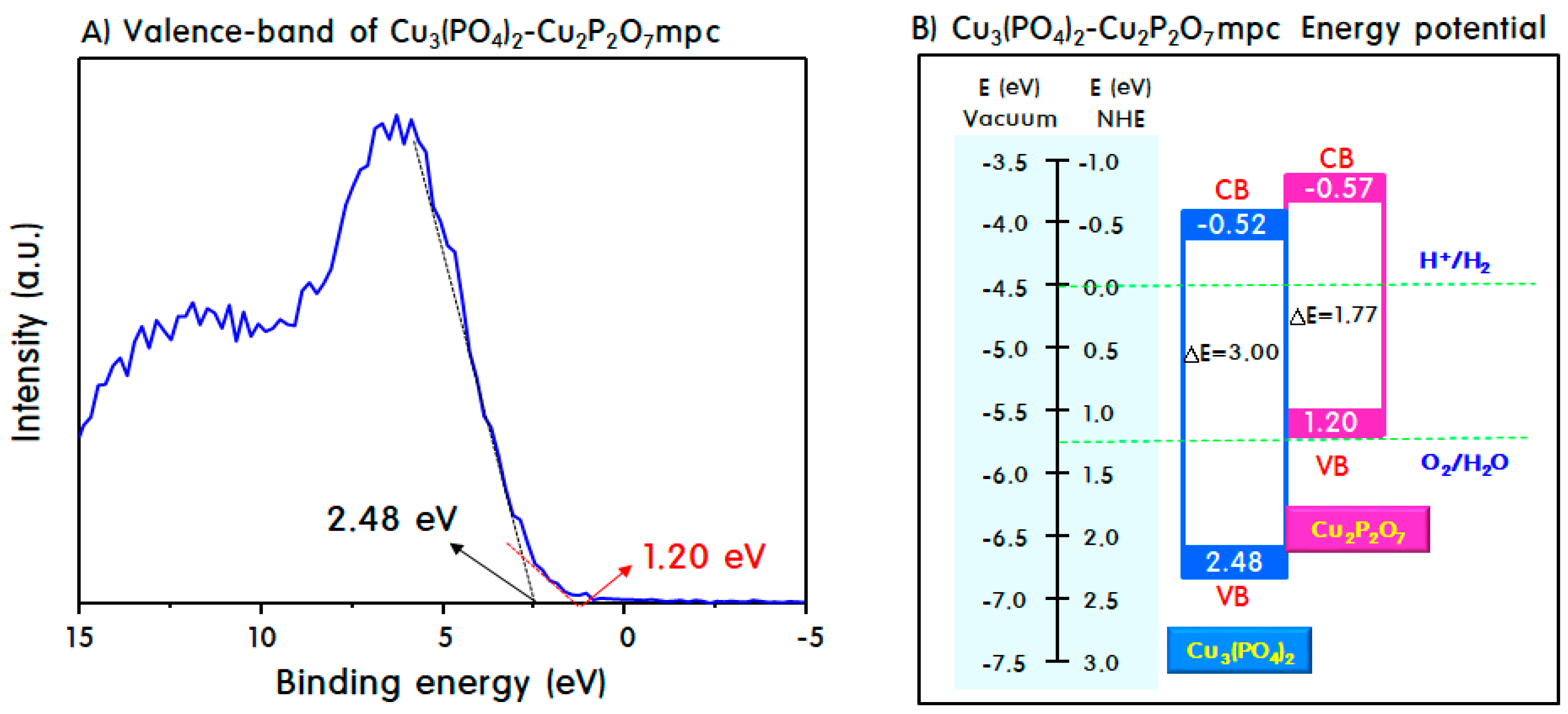
2. Results and Discussion
Characteristics of M3(PO4)2-M2P2O7 Mixed-Phase Catalysts
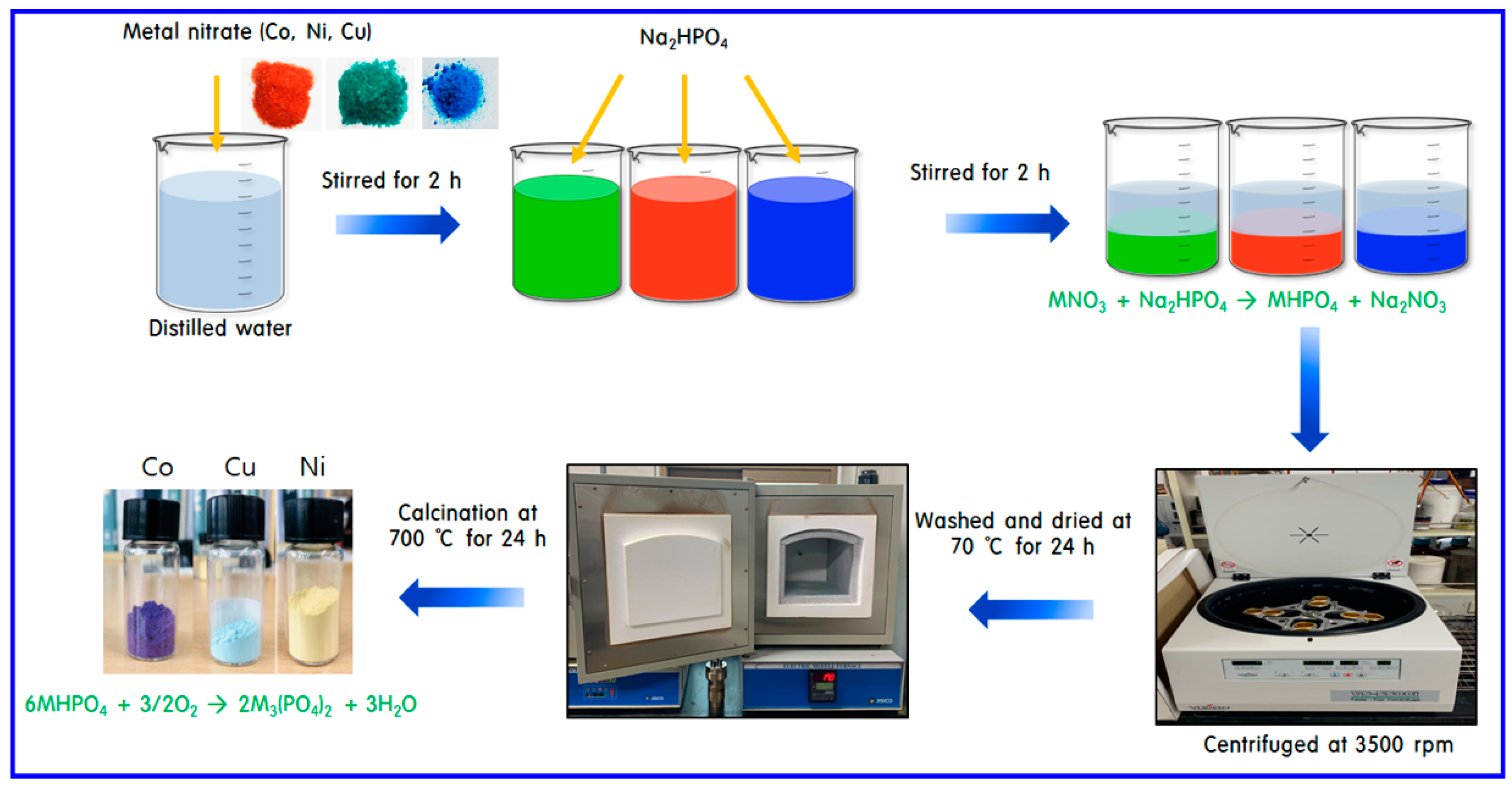
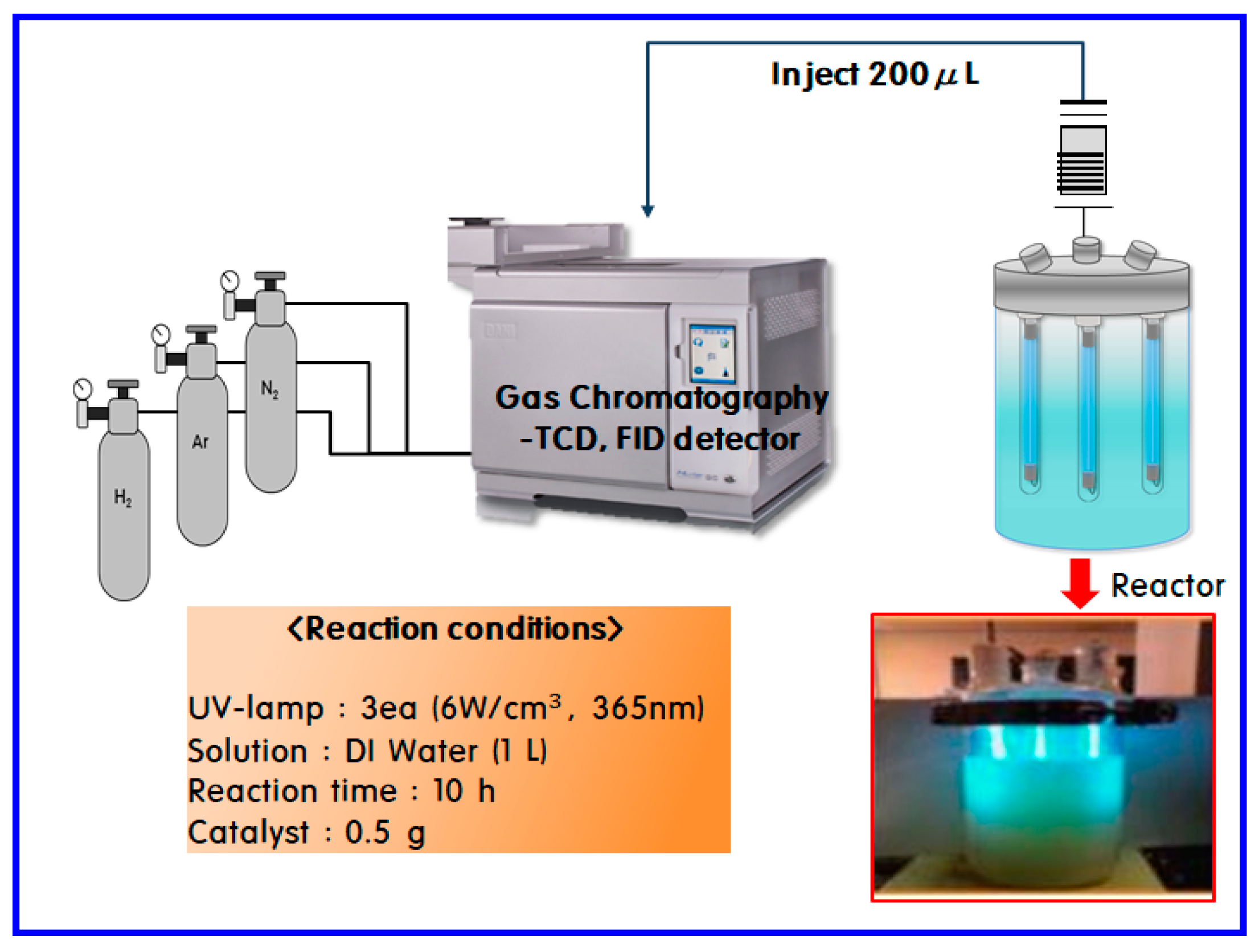
3. Experimental
3.1. Synthesis of Catalysts
3.2. Characterizations
3.3. Hydrogen Production through Water Photo Splitting
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H.; Huang, X.; Bi, Y. Photo-controlled bond changes on Pt/TiO2 for promoting overall water splitting and restraining hydrogen–oxygen recombination. J. Mater. Chem. 2019, 7, 5938–5942. [Google Scholar] [CrossRef]
- Li, J.; Cai, L.; Shang, J.; Yu, Y.; Zhang, L. Giant Enhancement of Internal Electric Field Boosting Bulk Charge Separation for Photocatalysis. Adv. Mater. 2016, 28, 4059–4064. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, T.; Yu, R.; Yu, H.; Yu, J. Highly efficient TiO2 single-crystal photocatalyst with spatially separated Ag and F bi-cocatalysts: Orientation transfer of photogenerated charges and their rapid interfacial reaction. J. Mater. Chem. A 2016, 4, 8682–8689. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Wen, F.; Yang, J.; Zong, X.; Ma, B.; Wang, D.; Li, C. Photocatalytic H2 production on hybrid catalyst system composed of inorganic semiconductor and cobaloximes catalysts. J. Catal. 2011, 281, 318–324. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Jiang, G.; Zhao, Z.; Xu, C.; Wei, Y.; Duan, A.; Liu, Z.; Gao, J. A photonic crystal-based CdS–Au–WO3 heterostructure for efficient visible-light photocatalytic hydrogen and oxygen evolution. RSC Adv. 2014, 4, 15689–15694. [Google Scholar] [CrossRef]
- Qiu, B.; Zhu, Q.; Du, M.; Fan, L.; Xing, M.; Zhang, J. Efficient Solar Light Harvesting CdS/Co9S8 Hollow Cubes for Z-Scheme Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 2684–2688. [Google Scholar] [CrossRef]
- Brito, J.; Tavella, F.; Genovese, C.; Ampelli, C.; Zanoni, M.; Centi, G.; Perathoner, S. Role of CuO in the modification of the photocatalytic water splitting behavior of TiO2 nanotube thin films. Appl. Catal. B Environ. 2018, 224, 136–145. [Google Scholar] [CrossRef]
- Scire, R.; Compagnini, L.; Bellardita, M.; Palmisano, L. Efficient H2 production by photocatalytic water splitting under UV or solar light over variously modified TiO2-based catalysts. Int. J. Hydrog. Energy 2019, 44, 14796–14807. [Google Scholar]
- Liu, J.; Ke, J.; Li, Y.; Liu, B.; Wang, L.; Xiao, H.; Wang, S. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2018, 236, 396–403. [Google Scholar] [CrossRef]
- Yan, X.; Yuan, K.; Lu, N.; Xu, H.; Zhang, S.; Takeuchi, N.; Kobayashi, H.; Li, R. The interplay of sulfur doping and surface hydroxyl in band gap engineering: Mesoporous sulfur-doped TiO2 coupled with magnetite as a recyclable, efficient, visible light active photocatalyst for water purification. Appl. Catal. B Environ. 2017, 218, 20–31. [Google Scholar] [CrossRef]
- Clatworthy, E.B.; Yick, S.; Murdock, A.T.; Allison, M.C.; Bendavid, A.; Masters, A.F.; Maschmeyer, T. Enhanced Photocatalytic Hydrogen Evolution with TiO2−TiN Nanoparticle Composites. J. Phys. Chem. C 2019, 123, 3740–3749. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Gu, S.; Li, H.; Liu, X.; Wang, M. Fabrication of FeWO4@ZnWO4/ZnO Heterojunction Photocatalyst: Synergistic Effect of ZnWO4/ZnO and FeWO4@ZnWO4/ZnO Heterojunction Structure on the Enhancement of Visible-Light Photocatalytic Activity. Chem. Eng. 2016, 4, 6288–6298. [Google Scholar] [CrossRef]
- Zhu, M.; Han, M.; Zhu, C.; Hu, L.; Huang, H.; Liu, Y.; Kang, Z. Strong coupling effect at the interface of cobalt phosphate-carbon dots boost photocatalytic water splitting. J. Colloid Interface Sci. 2018, 530, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Voronina, N.; Jo, J.H.; Choi, J.U.; Jo, C.-H.; Kim, J.; Myung, S.-T. Nb-Doped titanium phosphate for sodium storage: Electrochemical performance and structural insights. J. Mater. Chem. A 2019, 7, 5748–5759. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Rao, L.; Rambabu, G.; Kumar, B.; Radha, V.; Vithal, M. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Hong, H. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Guin, M.; Tietz, F.; Guillon, O. New promising NASICON material as solid electrolyte for sodium-ion batteries: Correlation between composition, crystal structure and ionic conductivity of Na3+xSc2SixP3−xO12. Solid State Ionics 2016, 293, 18–26. [Google Scholar] [CrossRef]
- El-Shinawi, H.; Regoutz, A.; Payne, D.; Cussen, E.; Corr, S. NASICON LiM2(PO4)3 electrolyte (M = Zr) and electrode (M = Ti) materials for all solid-state Li-ion batteries with high total conductivity and low interfacial resistance. J. Mater. Chem. A 2018, 6, 5296–5303. [Google Scholar] [CrossRef]
- Losilla, E.; Aranda, M.; Bruque, S. Sodium Mobility in the NASICON Series, Na1+xZr2-xInx(PO4)3. Chem. Mater. 2000, 12, 2134–2142. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L. Efficient Hydrogen Production by a Composite CdS/Mesoporous Zirconium Titanium Phosphate Photocatalyst under Visible Light. Phys. Chem. 2007, 111, 13437–13441. [Google Scholar] [CrossRef]
- Fu, J. Photocatalytic activity of glass ceramics containing Nasicon-type crystals. Mater. Res. Bull. 2013, 48, 70–73. [Google Scholar] [CrossRef]
- Palla, S.; Ravi, G.; Velchuri, R.; Veldurthi, N.; Reddy, J.; Muga, V. Photocatalytic degradation of organic dyes with Sn2+- and Ag+-substituted K3Nb3WO9(PO4)2 under visible light irradiation. J. Sol-Gel Sci. Technol. 2015, 75, 224–234. [Google Scholar] [CrossRef]
- Wang, M.; Luo, H.-B.; Liu, S.-X.; Zou, Y.; Tian, Z.-F.; Li, L.; Liu, J.-L.; Ren, X.-M. Water assisted high proton conductance in a highly thermally stable and superior water-stable open-framework cobalt phosphate. Dalton Trans. 2016, 45, 19466–19472. [Google Scholar] [CrossRef] [PubMed]
- Onoda, H.; Mori, D.; Kojima, K.; Nariai, H. Mechanochemical Effects on the Formation and Properties of Various Nickel Phosphates. Inorg. Mater. 2005, 41, 1089–1096. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kasuga, T.; Nogami, M. An oxygen sensor based on copper(I)- conducting CuTi2(PO4)3 glass ceramics. Appl. Phys. Lett. 1998, 73, 1–3. [Google Scholar] [CrossRef]
- Weimann, I.; Jahn, T.; Feller, J.; Zak, Z. Synthesis, Crystal Structure and Characterization of the Copper Iron Phosphate Cu8Fe2O5(PO4)4. Z. Anorg. Allg. Chem. 2014, 640, 219–223. [Google Scholar] [CrossRef]
- Jain, A.; Hautier, G.; Moore, C.; Kang, B.; Lee, J.; Chen, H.; Twu, N.; Ceder, G. A Computational Investigation of Li9M3(P2O7)3(PO4)2 (M = V, Mo) as Cathodes for Li Ion Batteries. J. Electrochem. Soc. 2012, 159, 622–633. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Chen, Y.; Chen, G.; Liao, C.; Liang, B.; Zhang, J.; Li, A.; Yang, B.; Zheng, Z.; et al. Ni2P2O7 Nanoarrays with Decorated C3N4 Nanosheets as Efficient Electrode for Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 2016–2023. [Google Scholar] [CrossRef]
- Khan, Z.; Senthilkumar, B.; Lim, S.; Shanker, R.; Kim, Y.; Ko, H. Redox-Additive-Enhanced High Capacitance Supercapacitors Based on Co2P2O7 Nanosheets. Adv. Mater. Interfaces 2017, 4, 1700059. [Google Scholar] [CrossRef]
- Chen, X.; Kuo, D.; Saragih, A.D.; Wu, Z.-Y.; Abdullah, H.; Lin, J. The effect of the Cu+/Cu2+ ratio on the redox reactions by nanoflower CuNiOS catalysts. Chem. Eng. Sci. 2019, 194, 105–115. [Google Scholar] [CrossRef]
- Luo, W.; Zemlyanov, D.Y.; Milligan, C.A.; Du, Y.; Yang, Y.; Wu, Y.; Ye, P.D. Surface chemistry of black phosphorus under a controlled oxidative environment. Nanotechnology 2016, 27, 434002–434011. [Google Scholar] [CrossRef]
- Shih, P.Y.; Yung, S.W.; Chin, T.S. FT-IR and XPS studies of P2O5-Na2O-CuO glasses. J. Non-Cryst. Solids 1999, 244, 211–222. [Google Scholar] [CrossRef]
- Wekliem, H.A. Optical Spectra of NiH, COH, and CUH in Tetrahedral Sites in Crystals. J. Chem. Phys. 1962, 36, 2117–2139. [Google Scholar] [CrossRef]
- Kaewkhao, J.; Rhianphumikarakit, S.; Udomkan, N. ESR and Optical Absorption Spectra of Copper (II) Ions in Glasses. Adv. Mater. Res. 2008, 55, 849–852. [Google Scholar] [CrossRef]
- Lin, A.; Kim, B.; Moon, D.; Chung, Y.; Han, W. Cu2+-doped germano-silicate glass fiber with high resonant nonlinearity. Opt. Express 2007, 15, 3665–3672. [Google Scholar] [CrossRef]
- Rashid, M. Electric Renewable Energy Systems; Academic Press: Cambridge, MA, USA, 2016; p. 458. [Google Scholar]
- He, G.; Hu, W.; Li, C. Spontaneous interfacial reaction between metallic copper and PBS to form cupric phosphate nanoflower and its enzyme hybrid with enhanced activity. Colloids Surf. B 2015, 135, 613–618. [Google Scholar] [CrossRef]
- Szyk, E.; Szymañska, I.; Wrzeszcz, G.; Rozpoch, F. New Polymeric Copper(II) Complexes with Triphenyl Phosphite and Perfluorinated Carboxylates. Pol. J. Chem. 2000, 74, 909–918. [Google Scholar]
- Bates, T. Ligand field theory and absorption spectra of transition metal ions in glasses. Mod. Asp. Vitr. State 1962, 2, 195–254. [Google Scholar]
- Duan, X.; Yuan, D.; Cheng, X.; Wang, Z.; Sun, Z.; Luan, C. Absorption and photoluminescence characteristics of Co2+P:MgAl2O4 nanocrystals embedded in sol–gel derived SiO2-based glass. Opt. Mater. 2004, 25, 65–69. [Google Scholar] [CrossRef]
- Mishra, D.; Qi, X. Energy levels and photoluminescence properties of nickel-doped bismuth ferrite. J. Alloys Compds. 2010, 504, 27–31. [Google Scholar] [CrossRef]
- Srinivasulu, K.; Omkaram, I.; Obeid, H.; Kumar, A.; Rao, J. Spectral studies on Cu2+ ions in sodium–lead borophosphate glasses. Phys. B 2012, 407, 4741–5474. [Google Scholar] [CrossRef]
- Wu, M.-C.; Chen, C.-H.; Huang, W.-K.; Hsiao, K.-C.; Lin, T.-H.; Chan, S.-H.; Wu, P.-Y.; Lu, C.-F.; Chang, Y.-H.; Lin, T.-F.; et al. Improved Solar-Driven Photocatalytic Performance of Highly Crystalline Hydrogenated TiO2 Nanofibers with Core-Shell Structure. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Mir, N.A.; Qutub, N.; Mehraj, O.; Sabir, S.; Muneer, M. Synthesis, characterization and optimization of photocatalytic activity of TiO2/ZrO2 nanocomposite heterostructures. Mater. Sci. Eng. B 2015, 193, 137–145. [Google Scholar] [CrossRef]
- Lei, W.; Mi, Y.; Feng, R.; Liud, P.; Hu, S.; Yu, J.; Liu, X.; Rodriguez, J.A.; Wang, J.; Zheng, L.; et al. Hybrid 0D–2D black phosphorus quantum dots–graphitic carbon nitride nanosheets for efficient hydrogen evolution. Nano Energy 2018, 50, 552–561. [Google Scholar] [CrossRef]
- Abbood, H.A.; Huang, K. Photocatalysis of several organic dyes by a hierarchical Ag2V4O11 micro–nanostructures. J. Mater. Sci. Mater. Electron. 2018, 29, 8068–8077. [Google Scholar] [CrossRef]
- Ghiringhelli, G.; Matsubara, M.; Dallera, C.; Fracassi, F.; Gusmeroli, R.; Piazzalunga, A.; Tagliaferri, A.; Brookes, N.B.; Kotani, A.; Braicovich, L. NiO as a test case for high resolution resonant inelastic soft x-ray scattering. J. Phys. Condens. Matter 2005, 17, 5397–5412. [Google Scholar] [CrossRef]
- Ye, C.; Wang, M.Q.; Li, L.J.; Luo, H.Q.; Li, N.B. Fabrication of Pt/Cu3(PO4)2 ultrathin nanosheet heterostructure for photoelectrochemical microRNA sensing using novel G-wire-enhanced strategy. Nanoscale 2017, 9, 7526–7532. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Sun, Y.; Xiao, T.; Tu, W.; Yuan, X.; Zeng, G.; Li, S.; Chew, J. Photogenerated charge transfer via interfacial internal electric field for significantly improved photocatalysis in direct Z-scheme oxygen-doped carbon nitrogen/CoAl-layered double hydroxide heterojunction. Appl. Catal. B Environ. 2018, 227, 530–540. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Heo, J.N.; Do, J.Y.; Yoon, S.J.; Kim, Y.; Kang, M. Hydrogen Production Improvement on Water Decomposition Through Internal Interfacial Charge Transfer in M3(PO4)2-M2P2O7 Mixed-Phase Catalyst (M = Co, Ni, and Cu). Catalysts 2019, 9, 602. https://doi.org/10.3390/catal9070602
Kim J, Heo JN, Do JY, Yoon SJ, Kim Y, Kang M. Hydrogen Production Improvement on Water Decomposition Through Internal Interfacial Charge Transfer in M3(PO4)2-M2P2O7 Mixed-Phase Catalyst (M = Co, Ni, and Cu). Catalysts. 2019; 9(7):602. https://doi.org/10.3390/catal9070602
Chicago/Turabian StyleKim, Junyeong, Jun Neoung Heo, Jeong Yeon Do, Seog Joon Yoon, Youngsoo Kim, and Misook Kang. 2019. "Hydrogen Production Improvement on Water Decomposition Through Internal Interfacial Charge Transfer in M3(PO4)2-M2P2O7 Mixed-Phase Catalyst (M = Co, Ni, and Cu)" Catalysts 9, no. 7: 602. https://doi.org/10.3390/catal9070602
APA StyleKim, J., Heo, J. N., Do, J. Y., Yoon, S. J., Kim, Y., & Kang, M. (2019). Hydrogen Production Improvement on Water Decomposition Through Internal Interfacial Charge Transfer in M3(PO4)2-M2P2O7 Mixed-Phase Catalyst (M = Co, Ni, and Cu). Catalysts, 9(7), 602. https://doi.org/10.3390/catal9070602





