Influence of Ruthenium Doping on the Crystal Structure and Magnetic Properties of Pr0.67Ba0.33Mn1–xRuxO3 Manganites
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Analysis
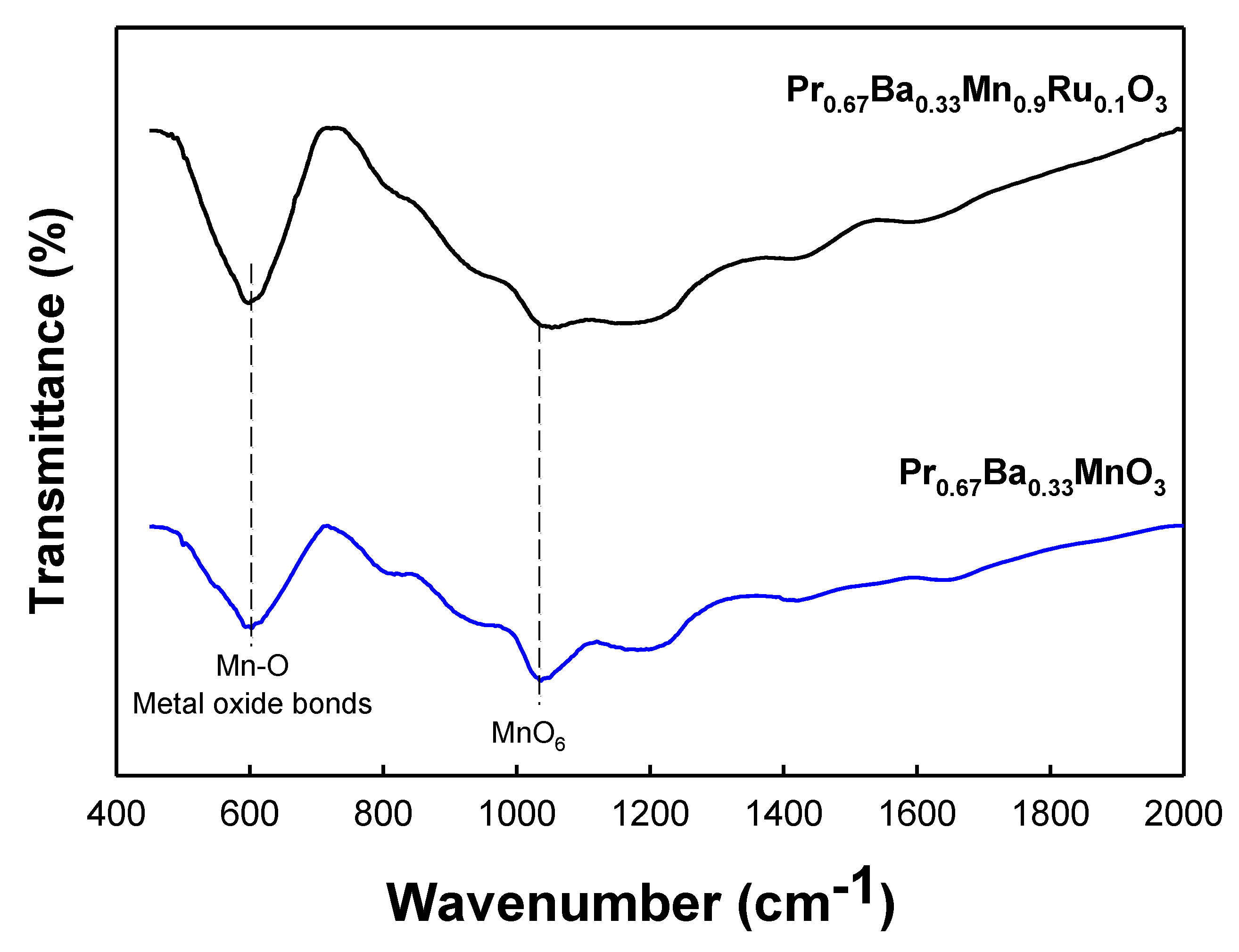
3.2. FTIR Spectra
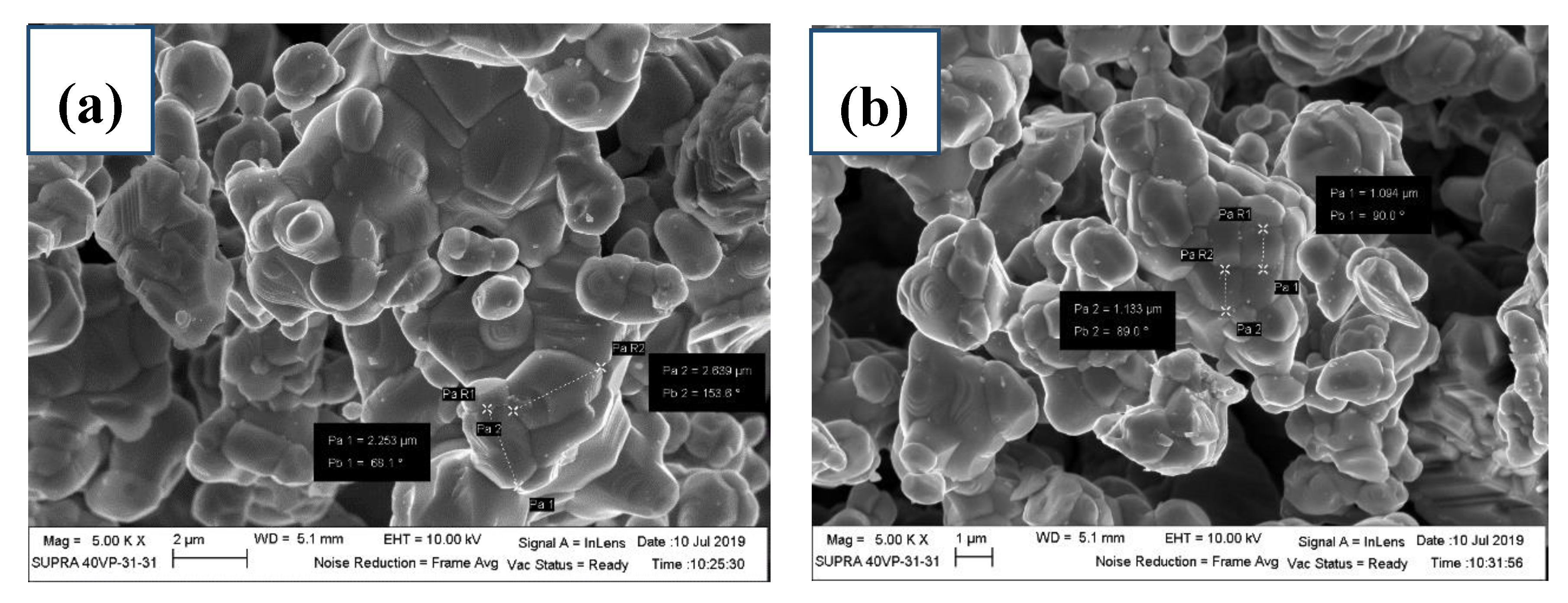
3.3. Morphology
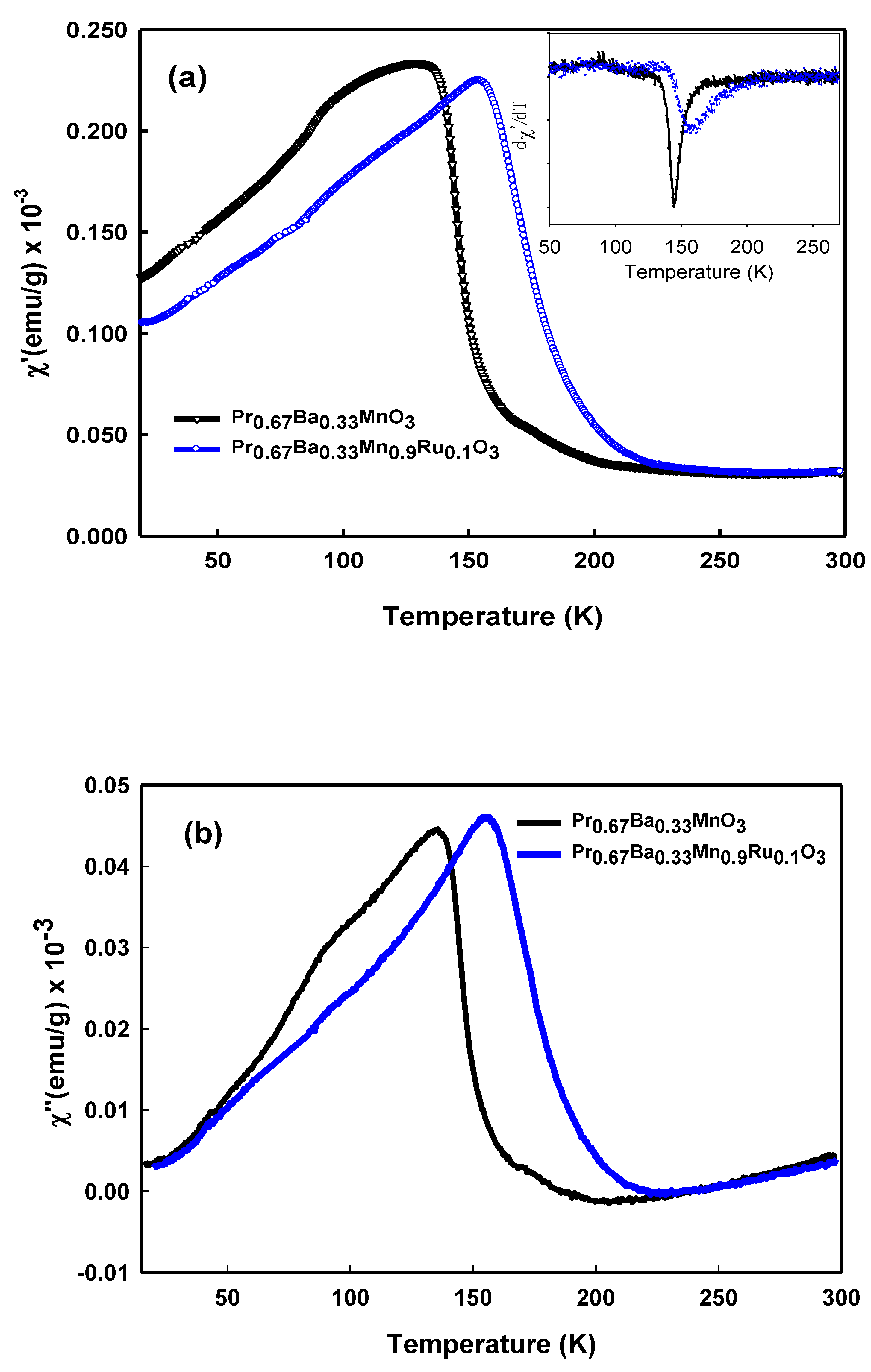
3.4. AC Susceptibility Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemaa, F.B.; Mahmood, S.; Ellouze, M.; Hlil, E.K.; Halouani, F.; Bsoul, I.; Awawdeh, M. Structural, magnetic and magnetocaloric properties of La0.67Ba0.22Sr0.11Mn1 − xFexO3 nanopowders. Solid State Sci. 2014, 37, 121–130. [Google Scholar]
- Czajkowski, J.; Kinnunen, P.; Haapanen, K.; Niinimäki, J.; Fabritius, T. Simultaneous magnetic actuation and observation with ferromagnetic sensors. Meas. Sci Technol. 2016, 27, 025301. [Google Scholar] [CrossRef]
- Jin, S.; Tiefel, T.H.; McComack, M.; Fastnacht, R.A.; Ramesh, R.; Chen, L.H. Thousand-fold change in resistivity in magnetoresistive La-Ca-Mn-O films. Science 1994, 264, 413. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.; Eom, T.W.; Dai, N.V.; Lee, Y.P. Magnetic properties and Griffiths singularity in La0.45Sr0.55 Mn1−xCoxO3. J. Magn. Magn. Mater. 2011, 323, 100–194. [Google Scholar] [CrossRef]
- Mahjoub, S.; Baazaoui, M.; M’nassri, R.; Rahmouni, H.; Boudjada, N.C.; Oumezzine, M. Effect of iron substitution on the structural, magnetic and magnetocaloric properties of Pr0.6Ca0.1Sr0.3Mn1−xFexO3 (0 ≤ x ≤ 0.075) manganites. J. Alloy. Comp. 2014, 608, 191–196. [Google Scholar] [CrossRef]
- Xiao, X.; Yuan, S.; Mia, J.; Ren, G.; Yu, G.; Wang, Y.; Yin, S. Tuning colossal magnetoresistance response at roomtemperature by La2/3+ySr1/3−yMn1−yCryO3. Mater. Lett. 2007, 61, 2315–2318. [Google Scholar] [CrossRef]
- Mohamed, Z.; Ibrahim, N.; Ghani, M.A.; Safian, S.D.; Mohamed, S.N. Structural and electrical transport properties of (La0.7-xYx)Ca0.3MnO3 manganites. Results Phys. 2019, 12, 861–866. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751. [Google Scholar] [CrossRef]
- Vanitha, P.V.; Rao, C.N.R. An investigation of the re-entrant ferromagnetic transition in rare earth manganates in the regime of competing charge-ordering and ferromagnetic interactions. J. Phys. Condens. Matter. 2001, 13, 11707. [Google Scholar] [CrossRef]
- Rørmark, L.; Wiik, K.; Stølen, S.; Grande, T. Oxygen Stoichiometry and Structural Properties of La1−xAxMnO3±δ (A = Ca or Sr and 0 ≤ x ≤ 1). J. Mater. Chem. 2002, 12, 1058–1067. [Google Scholar] [CrossRef]
- Elyana, E.; Mohamed, Z.; Kamil, S.A.; Supardan, S.N.; Chen, S.K.; Yahya, A.K. Revival of ferromagnetic behavior in charge-ordered Pr0.75Na0.25MnO3 manganite by ruthenium doping at Mn site and its MR effect. J. Solid State Chem. 2018, 258, 191–200. [Google Scholar]
- Fertman, E.; Syrkin, E.; Lykah, V.; Beznosov, V.D.A.; Pal-Val, P.; Pal-Val, L.; Fedorchenko, A.; Khalyavin, D.; Feher, A. Structural phase transition in La2/3Ba1/3MnO3 perovskite: Elastic, magnetic, and lattice anomalies and microscopic mechanism. AIP Adv. 2015, 5, 077189. [Google Scholar] [CrossRef]
- Larson, C.; Dreele, R.B.V. General Structure Analysis System (GSAS) (Report LAUR 86-748); Los Alamos National Laboratory: Los Alamos, NM, USA, 1994; pp. 86–748. [Google Scholar]
- Toby, H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272. [Google Scholar] [CrossRef]
- Rozilah, R.; Yaakob, M.K.; Mohamed, Z.; Yahya, A.K. Effects of on-site Coulomb interaction (U) on the structural and electronic properties of half-metallic ferromagnetic orthorhombic Pr0.75Na0.25MnO3 manganite: A LDA + U calculation and experimental study. Mater. Res. Express 2017, 4, 066103. [Google Scholar] [CrossRef]
- M’Nassri, R.; Khelifi, M.; Rahmouni, H.; Selmi, A.; Khirouni, K.; Chniba-Boudjada, N.; Cheikhrouhou, A. Study of physical properties of cobalt substituted Pr0.7Ca0.3MnO3. Ceram. Int. 2016, 42, 6145–6153. [Google Scholar] [CrossRef]
- Maignan, A.; Martin, C.; Hervieu, M.; Raveau, B. Ferromagnetism and metallicity in the CaMn1−xRuxO3 perovskites: A highly inhomogeneous system. Solid State Commun. 2001, 117, 377–382. [Google Scholar] [CrossRef]
- Rozilah, R.; Ibrahim, N.; Mohamed, Z.; Yahya, A.K.; Khan, N.A.; Khan, M.N. Inducement of ferromagnetic-metallic phase in intermediate-doped charge-ordered Pr0.75Na0.25MnO3 manganite by K+ substitution. Physical B 2017, 521, 281–294. [Google Scholar] [CrossRef]
- Sahu, R.K.; Manoharan, S.S. A Zener pair effect in lanthanum rutheno manganite. J. Appl. Phys. 2002, 91, 7517. [Google Scholar] [CrossRef]
- Jethva, S.; Katba, S.; Udeshi, M.; Kuberkar, D.G. Structural, transport and magnetotransport properties of Ru-doped La0.5Sr0.5Mn1−xRuxO3 (x = 0.0 & 0.05) manganite. Phys. B Condens. Matter. 2017, 520, 13–20. [Google Scholar]
- Sagdeo, P.; Anwar, S.; Lalla, N. Powder X-ray diffraction and Rietveld analysis of La1−xCaxMnO3 (0 < X < 1). Powder Diffr. 2006, 21, 40. [Google Scholar]
- Ganguly, R.; Martin, C.; Maignan, A.; Hervieu, M.; Raveau, B. Ruthenium doping of the layered charge ordered manganites, La0.5Sr1.5MnO4 and LaSr2Mn2O7. Solid State Commun. 2001, 120, 9–10, 363–368. [Google Scholar] [CrossRef]
- Maneesha, G.; Wasi, K.; Poonam, Y.; Kotnala, R.K.; Azam, A.; Naqvi, A.H. Synthesis and evolution of magnetic properties of Ni doped La2/3Sr1/3Mn1−xNixO3 nanoparticles. J. Appl. Phys. 2012, 111, 093706. [Google Scholar]
- Habib, I.; Sajawal, A.; Murtaza, G.; Ishfaq, M.; Muhammad, N.; Farid, G.; Sharif, S.; Akhtar, M.N.; Salim, S. Effect of co-doping of Fe and Gd on the structural, morphological and dielectric properties of LaMnO3 nanocrystallites using Sol-Gel technique. Mater. Res. Express 2018, 5, 075018. [Google Scholar] [CrossRef]
- Balagurov, A.M.; Bushmeleva, S.N.; Pomjakushin, V.Y.; Sheptyakov, D.V.; Amelichev, V.A.; Gorbenko, O.Y.; Kaul, A.R.; Gan’shina, E.A.; Perkins, N.B. Magnetic structure of NdMnO3 consistently doped with Sr and Ru. Phys. Rev. B 2004, 70, 014427. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Khan, M.; Pal, S. Magnetization reversal and ferrimagnetism in Pr1−xNdxMnO3. Mater. Sci. 2014, 37, 809–813. [Google Scholar] [CrossRef]
- Kumar, V.S.; Mahendiran, R. Effect of impurity doping at the Mn-site on magnetocaloric effect in Pr0.6Ca0.4Mn0.96B0.04O3 (B = Al, Fe, Cr, Ni, Co and Ru). J. Appl. Phys. 2011, 109, 023903. [Google Scholar] [CrossRef]
- Ulyanov, A.N.; Quang, H.D.; Lee, K.W.; Yu, S.C.; Sinh, N.H.; Kang, Y.M.; Yoo, S.I. Magnetic Relaxation Behavior in Nd0.5Sr0.5MnO3: Observation of negative imaginary component of ac magnetic suceptibility. IEEE Trans. Magn. 2008, 44, 11. [Google Scholar] [CrossRef]
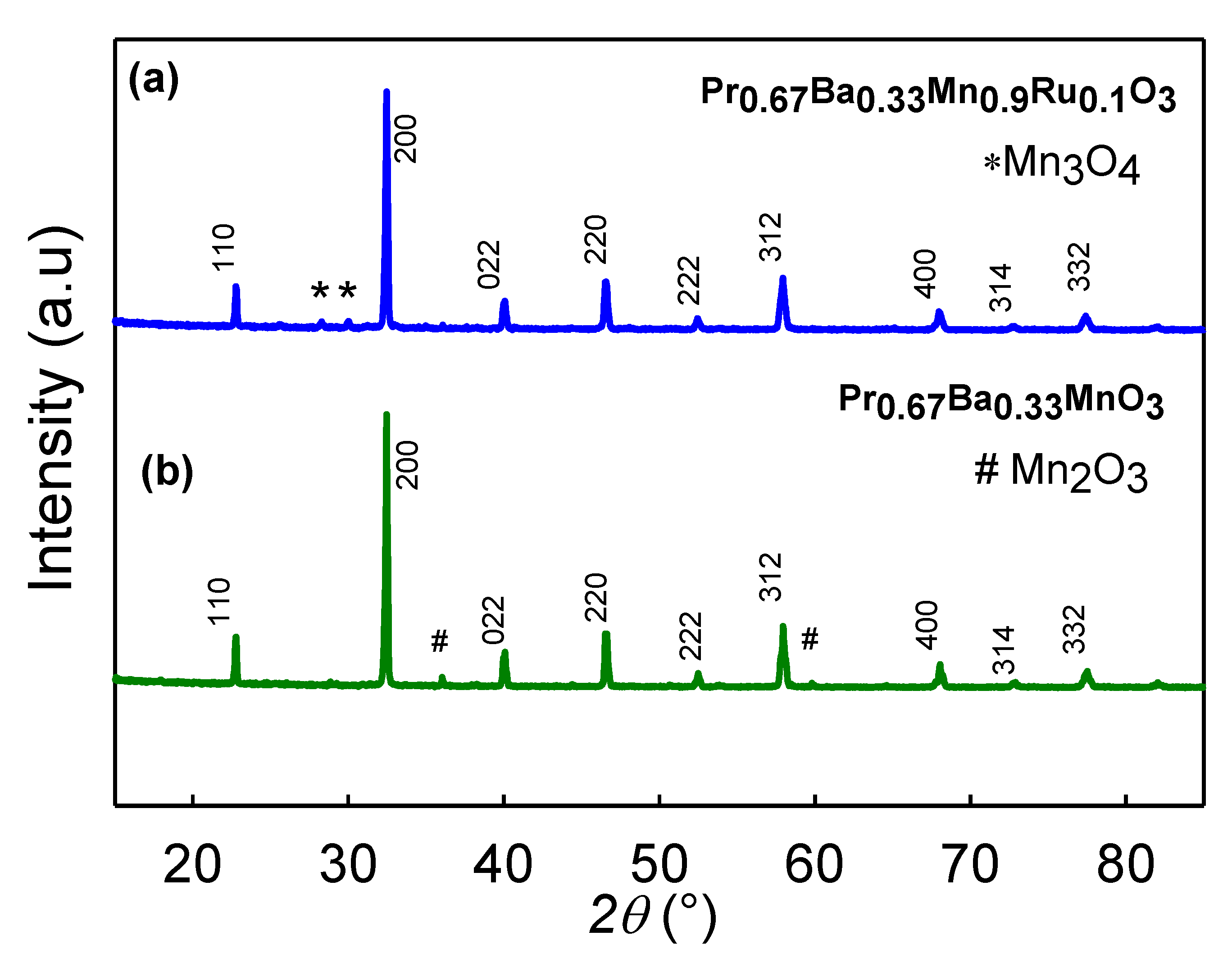
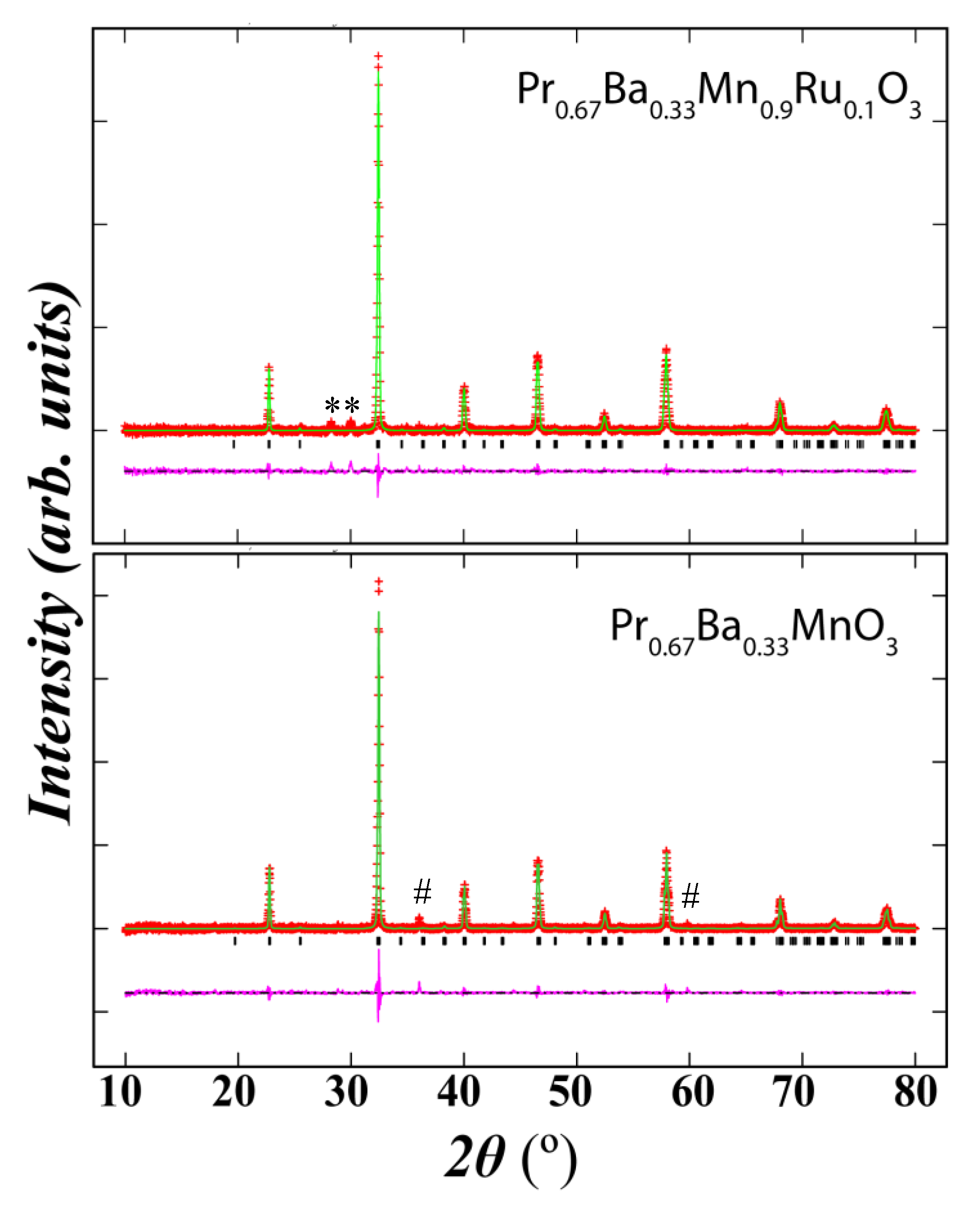
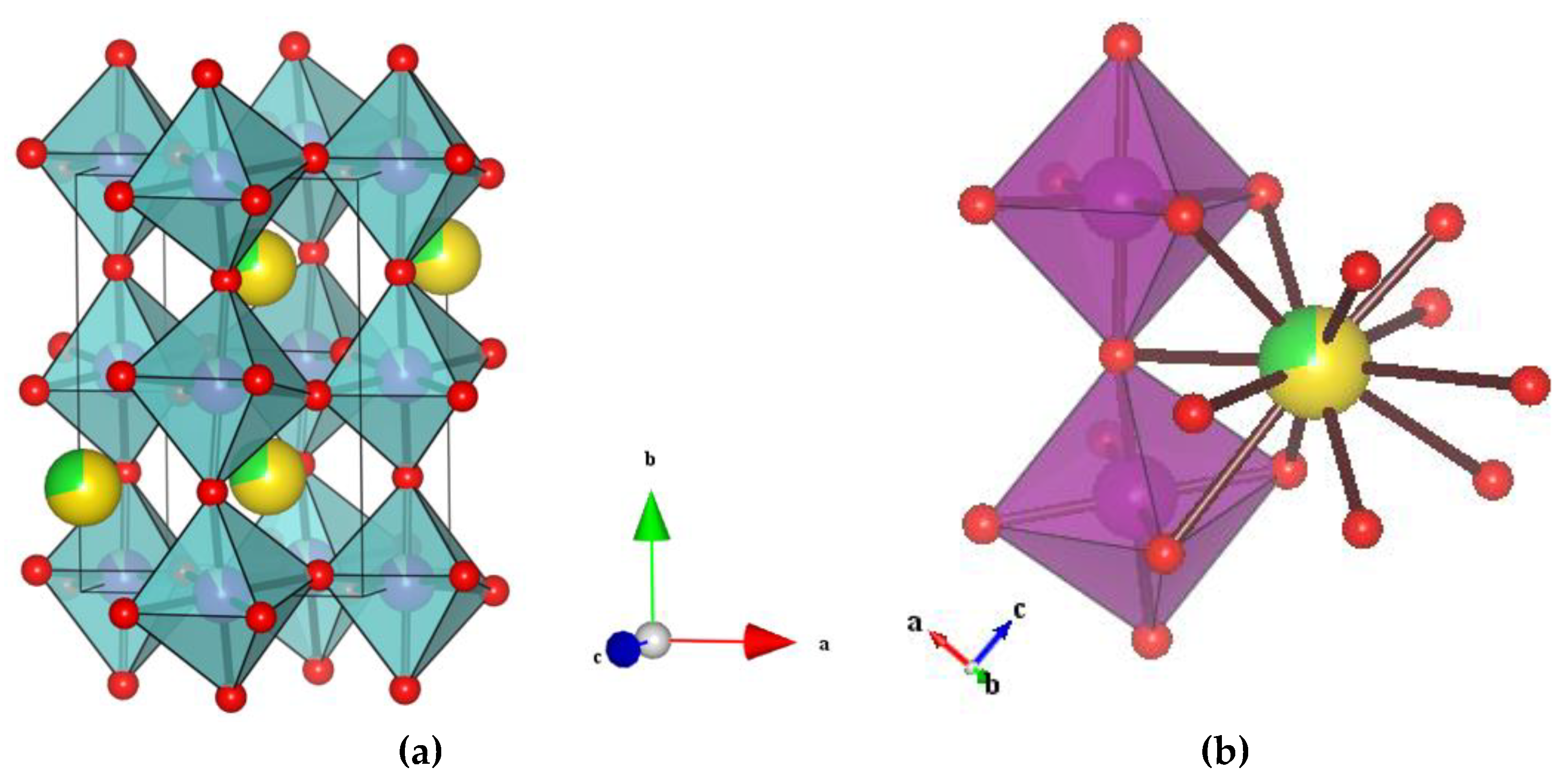
| Atom | x | y | z | g | biso (å2) | Site |
|---|---|---|---|---|---|---|
| Mn | 0.000000 | 0.000000 | 0.500000 | 0.9000 | 0.651 | 4b |
| Pr | 0.022580 | 0.250000 | 0.995214 | 0.6700 | 0.270 | 4c |
| Ba | −0.011257 | 0.250000 | 1.006197 | 0.3300 | 0.214 | 4c |
| O1 | 0.490000 | 0.250000 | 0.060800 | 1.0000 | 0.531 | 4c |
| O2 | 0.275900 | 0.034400 | 0.723900 | 1.0000 | 0.540 | 8d |
| Ru | 0.000000 | 0.000000 | 0.500000 | 0.1000 | 0.260 | 4b |
| Parameters | Pr0.67Ba0.33MnO3 | Pr0.67Ba0.33Mn0.9Ru0.1O3 |
|---|---|---|
| Symmetry | Orthorhombic | Orthorhombic |
| Space Group | Pnma | Pnma |
| a (Å) | 5.5216 | 5.5040 |
| b (Å) | 7.7720 | 7.7812 |
| c (Å) | 5.4971 | 5.5288 |
| A = β = γ | 90 | 90 |
| Unit cell volume (V) | 235.91 | 236.78 |
| χ2 | 1.017 | 1.030 |
| RF (%) | 8.58 | 11.96 |
| RP (%) | 10.45 | 11.74 |
| RWP (%) | 14.22 | 15.07 |
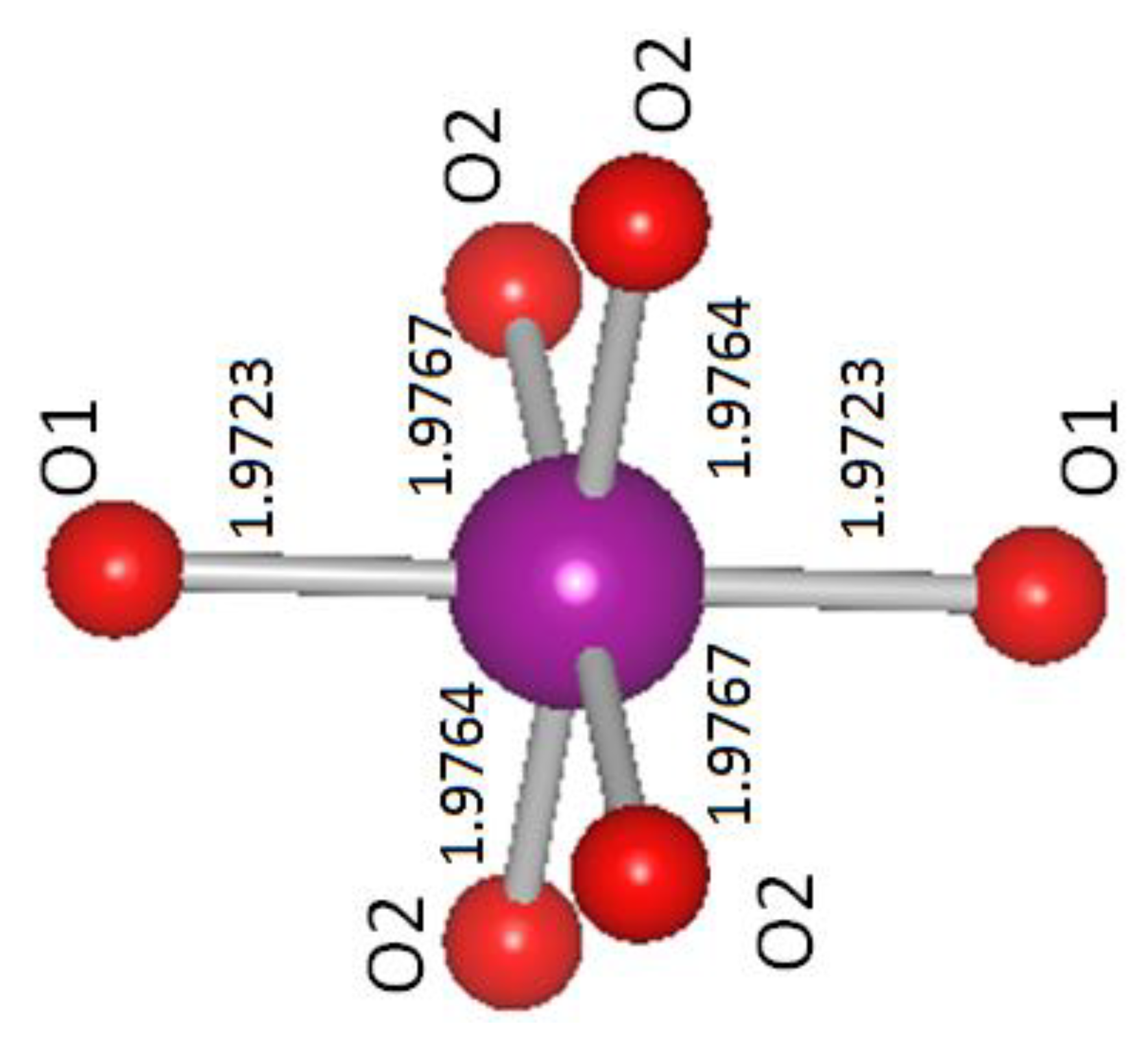
| Distances | Pr0.67Ba0.33MnO3 | Pr0.67Ba0.33Mn0.9Ru0.1O3 |
|---|---|---|
| 2 × Mn-O1 (Å) | 1.9723 | 1.9749 |
| 2 × Mn-O2 (Å) | 1.9767 | 1.9774 |
| 2 × Mn-O2 (Å) | 1.9764 | 1.9807 |
| <Mn-O>(Å) | 1.9751 | 1.9777 |
| Mn-O1-Mn (˚) | 158.6 | 158.5 |
| Mn-O2-Mn (˚) | 157.5 | 159.2 |
| <Mn-O-Mn> (˚) | 158.1 | 158.8 |
| (Ba/Pr1)-O1 (Å) | 2.9124 | 2.8976 |
| (Ba/Pr1)-O1 (Å) | 2.6037 | 2.6496 |
| (Ba/Pr1)-O1 (Å) | 3.0416 | 3.0944 |
| (Ba/Pr1)-O1 (Å) | 2.4523 | 2.4401 |
| 2× (Ba/Pr1)-O2 (Å) | 2.6925 | 2.6864 |
| 2× (Ba/Pr1)-O2 (Å) | 2.4499 | 2.4544 |
| 2× (Ba/Pr1)-O2 (Å) | 3.1145 | 3.1269 |
| 2× (Ba/Pr1)-O2 (Å) | 2.7912 | 2.7937 |
| <(Ba/Pr1)-O> (Å) | 2.7634 | 2.7671 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, Z.; Shahron, I.S.; Ibrahim, N.; Maulud, M.F. Influence of Ruthenium Doping on the Crystal Structure and Magnetic Properties of Pr0.67Ba0.33Mn1–xRuxO3 Manganites. Crystals 2020, 10, 295. https://doi.org/10.3390/cryst10040295
Mohamed Z, Shahron IS, Ibrahim N, Maulud MF. Influence of Ruthenium Doping on the Crystal Structure and Magnetic Properties of Pr0.67Ba0.33Mn1–xRuxO3 Manganites. Crystals. 2020; 10(4):295. https://doi.org/10.3390/cryst10040295
Chicago/Turabian StyleMohamed, Zakiah, Intan Syazwani Shahron, Norazila Ibrahim, and Mohd Fauzi Maulud. 2020. "Influence of Ruthenium Doping on the Crystal Structure and Magnetic Properties of Pr0.67Ba0.33Mn1–xRuxO3 Manganites" Crystals 10, no. 4: 295. https://doi.org/10.3390/cryst10040295
APA StyleMohamed, Z., Shahron, I. S., Ibrahim, N., & Maulud, M. F. (2020). Influence of Ruthenium Doping on the Crystal Structure and Magnetic Properties of Pr0.67Ba0.33Mn1–xRuxO3 Manganites. Crystals, 10(4), 295. https://doi.org/10.3390/cryst10040295




