Hydrogen Bonds with BF4− Anion as a Proton Acceptor
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
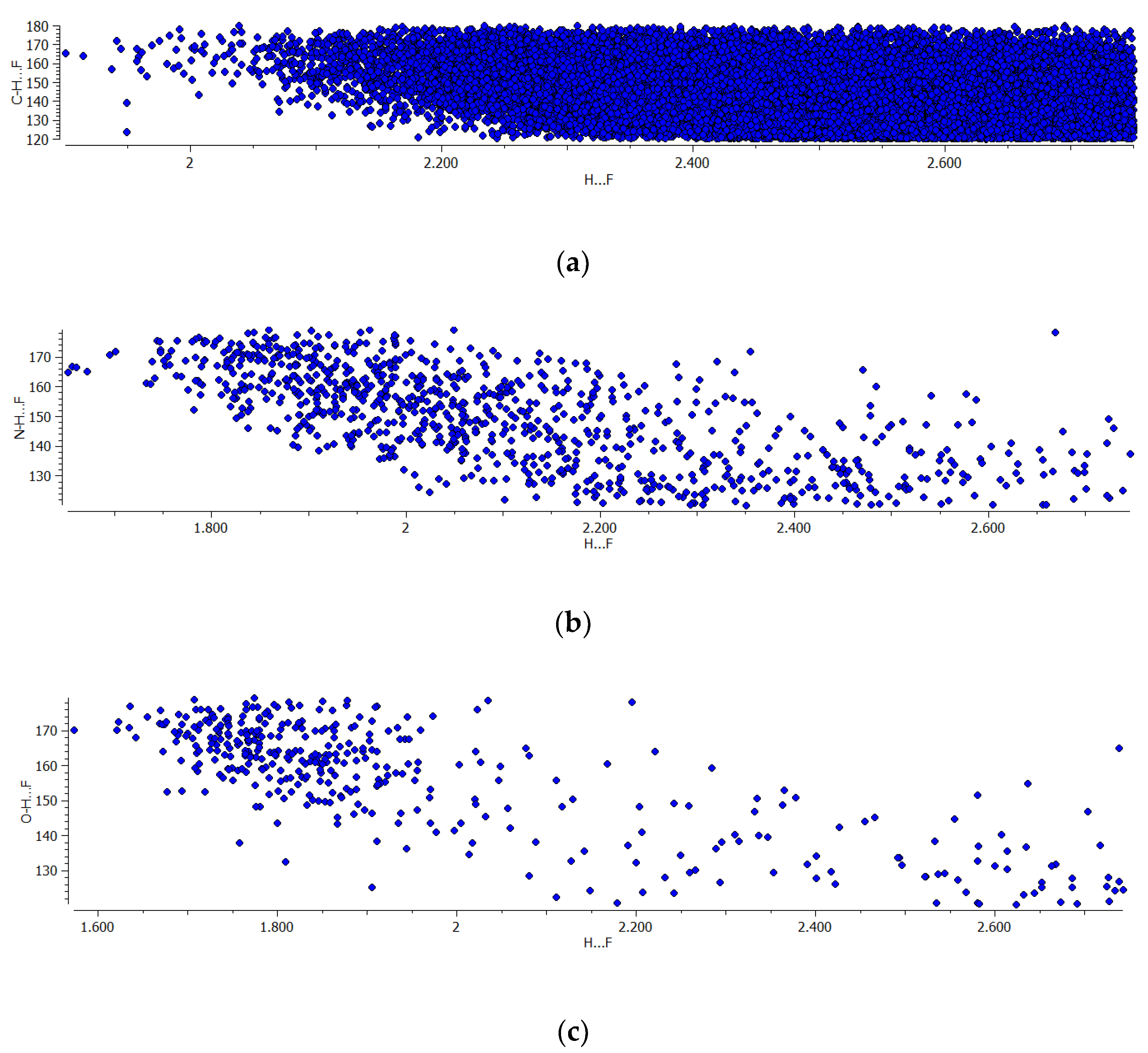
3.1. Crystal Structures with BF4− Anion as Proton Acceptor in Hydrogen Bonds
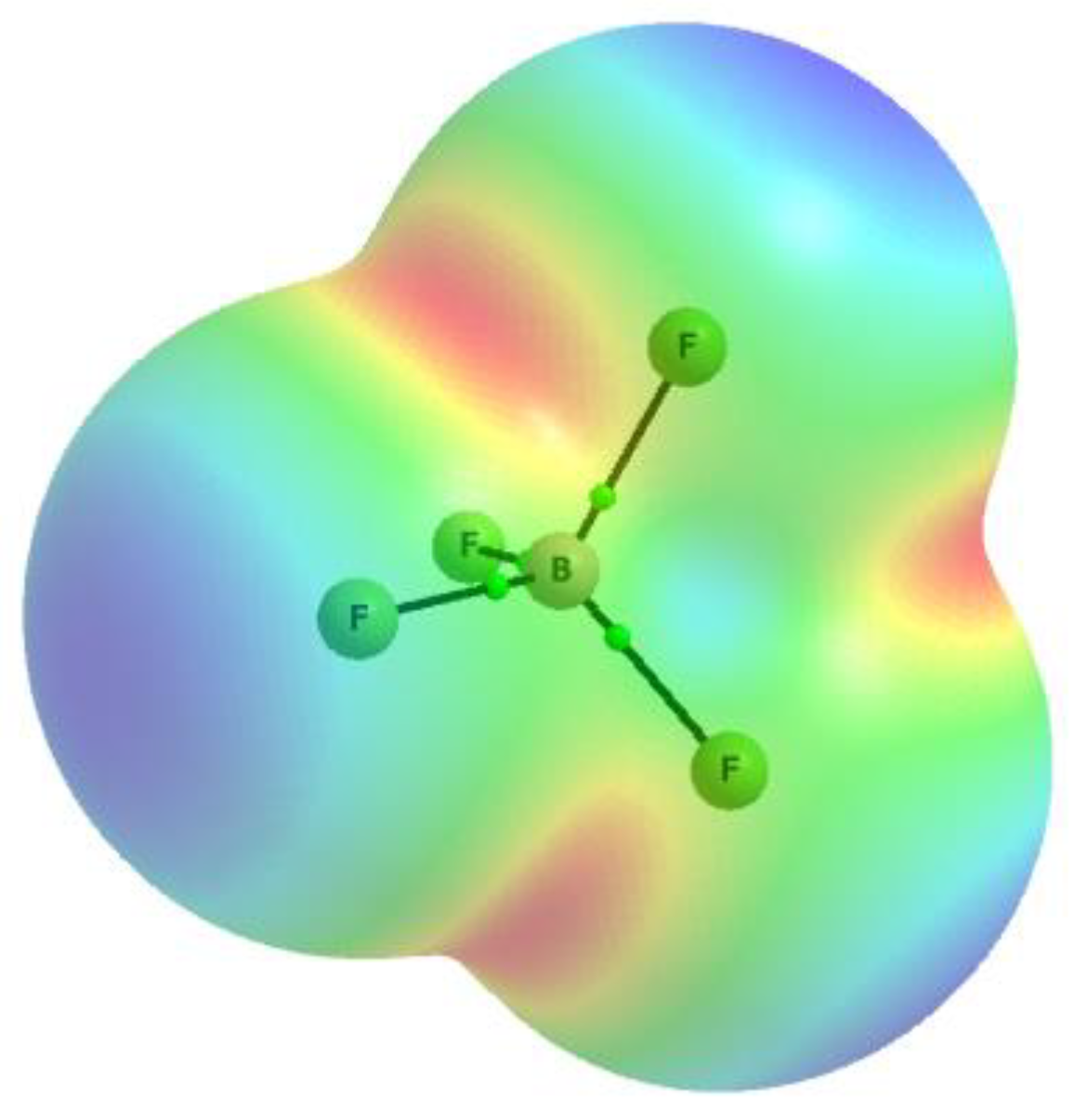
3.2. Theoretical Analysis of the Lewis Base Properties of the BF4− Anion
4. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, W.; Sünkel, K. Metal Complexes of Weakly Coordinating Anions. Precursors of Strong Cationic Organometallic Lewis Acids. Chem. Rev. 1988, 88, 1405–1421. [Google Scholar] [CrossRef] [Green Version]
- Garrison, J.C.; Youngs, W.J. Ag(I) N-Heterocyclic Carbene Complexes: Synthesis, Structure, and Application. Chem. Rev. 2005, 105, 3978–4008. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Riera, L. Organometallic complexes as anion hosts. Chem. Commun. 2008, 5, 533–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krossing, I.; Raabe, I. Noncoordinating Anions–fact or Fiction? A Survay of Likely Candidates. Angew. Chem. Int. Ed. 2004, 43, 2066–2090. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R.J.; Hamilton, G.L.; Toste, F.D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614. [Google Scholar] [CrossRef]
- Rosenthal, M.R. The Myth of the Non-Coordinating Anion. J. Chem. Educ. 1973, 50, 331–335. [Google Scholar] [CrossRef]
- Farooq, O.; Tiers, G.V.D. Alkali metal salts of perfluorinated complex anions. Effective reagents for nucleophilic fluorination. J. Org. Chem. 1994, 59, 2122–2124. [Google Scholar] [CrossRef]
- Cresswell, A.J.; Davies, S.G.; Roberts, P.M.; Thomson, J.E. Beyond the Balz-Schiemann Reaction: The Utility of Tetrafluoroborates and Boron Trifluoride as Nucleophilic Fluoride Sources. Chem. Rev. 2015, 115, 566–611. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C. Valency and Bonding, A Natural Bond Orbital Donor—Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005; pp. 306–351, 593–660. [Google Scholar]
- Hirao, H.; Omoto, K.; Fujimoto, H. Lewis Acidity of Boron Trihalides. J. Phys. Chem. A 1999, 103, 5807–5811. [Google Scholar] [CrossRef]
- Jacobsen, H. Hypovalency—A kinetic energy density description of 4c-2e bond. Dalton Trans. 2009, 21, 4252–4258. [Google Scholar] [CrossRef]
- Grabowski, S.J. Boron and other Triel Lewis Acid Centers: From Hypovalency to Hypervalency. ChemPhysChem 2014, 15, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. π-Hole Bonds: Boron and Aluminium Lewis Acid Centers. ChemPhysChem 2015, 18, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Allen, F.H.; Willett, P. The scientific impact of the Cambridge Structural Database: A citation-based study. J. Appl. Cryst. 2010, 43, 811–824. [Google Scholar] [CrossRef] [Green Version]
- Reed, C.A. The Strongest Acid. Chem. N. Z. 2011, 174–179. [Google Scholar]
- Marcellus, C.G.; Oakley, R.T.; Cordes, A.W.; Pennington, W.T. The preparation and the crystal and molecular structure of S3N2NH2+BF4−; a molecular orbital study of the protonation of the trisulphur trinitride anion. Can. J. Chem. 1984, 62, 1822–1827. [Google Scholar] [CrossRef]
- Ishida, H.; Kashino, S.; Ikeda, R. Crystal Structure of Trimethylammonium Tetrafluoroborate in Three Solid Phases. Z. Naturforsch. A Phys. Sci. 2000, 55, 765–768. [Google Scholar] [CrossRef]
- Wang, N.-N.; Zhang, Q.-G.; Wu, F.-G.; Li, Q.-Z.; Yu, Z.-W. Hydrogen Bonding Interactions between a Representative Pyridinium-Based Ionic Liquid [BuPy][BF4] and Water/Dimethyl Sulfoxide. J. Phys. Chem. B 2010, 114, 8689–8700. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Wang, N.-N.; Luo, J.-J.; Zhou, Y.; Yu, Z.-W. Hydrogen-bonding interactions between [BMIM][BF4] and acetonitrile. Phys. Chem. Chem. Phys. 2013, 15, 18055–18064. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Polito, M.; Grepioni, F. Hydrogen Bonding Interactions Between Ions: A Powerful Tool in Molecular Crystal Engineering. In Supramolecular Assembly via Hydrogen Bonds II; Springer: Berlin/Heidelberg, Germany, 2004; Volume 111, pp. 1–32. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Matta, C.; Boyd, R.J. Quantum Theory of Atoms in Molecules: Recent Progress in Theory and Application; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Keith, T.A. AIMAll, Version 13.05.06; TK Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Reed, E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- ADF2017, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: http://www.scm.com (accessed on 18 May 2020).
- Morokuma, K.J. Molecular Orbital Studies of Hydrogen Bonds. III. C=O···H–O Hydrogen Bond in H2CO···H2O and H2CO···2 H2O. J. Chem. Phys. 1971, 55, 1236. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: New York, NY, USA, 1960; pp. 88–102. [Google Scholar]
- Emge, T.J.; Wang, H.H.; Beno, M.A.; Williams, J.M.; Whangbo, M.-H.; Evain, M. Effect of anion ordering on the H-anion interactions and band electronic structure of (TMTSF)2BF4 at 20 K. J. Am. Chem. Soc. 1986, 108, 8215–8223. [Google Scholar] [CrossRef]
- Albinati, A.; Cesarotti, E.; Mason, S.A.; Rimoldi, I.; Rizzato, S.; Zerla, D. Histidine and deuterium-labelled histidine by asymmetric catalytic reduction and assignment of the absolute stereochemistry by neutron diffraction. Tetrahedron Assym. 2010, 21, 1162–1165. [Google Scholar] [CrossRef]
- Politzer, P.; Truhlar, D.G. Chemical Applications od Atomic and Molecular Electrostatic Potentials; Plenum; Springer Science & Business Media: New York, NY, USA, 1981. [Google Scholar]
- Pingale, S.S.; Gadre, S.R.; Bartolotti, L.J. Electrostatic insights into molecular hydration process: A case study of crown ethers. J. Phys. Chem. A 1998, 102, 9987–9992. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7758. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–561. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Sokalski, W.A. Diffrent types of hydrogen bonds: Correlation analysis of interaction energy components. J. Phys. Org. Chem. 2005, 18, 779–784. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Correlation Problem in Many-Electron Quantum Mechanics I. Review of Different Approaches and Discussion of Some Current Ideas. Adv. Chem. Phys. 1959, 2, 207–322. [Google Scholar]
- Grabowski, S.J. What is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 11, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO, 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013; Available online: http://nbo6.chem.wisc.edu/ (accessed on 18 May 2020)(this web-side refers nowadays to NBO 7.0 version, all pre-NBO7 versions are cease).
- Grabowski, S.J. Clusters of Ammonium Cation–Hydrogen Bond versus σ-Hole Bond. ChemPhysChem 2014, 15, 289–297. [Google Scholar] [CrossRef]
- Grabowski, S.J. Lewis acid–Lewis base interactions: From NFH3+...NCH and NF4+…NCH complexes to NFH3+...(NCH)n and NF4+…(NCH)n clusters. Comput. Theor. Chem. 2015, 1053, 876–884. [Google Scholar] [CrossRef]
- Dovgaliuk, I.; Møller, K.T.; Robeyns, K.; Louppe, V.; Jensen, T.R.; Filinchuk, Y. Complexation of Ammonia Boranes with Al3+. Inorg. Chem. 2019, 58, 4753–4760. [Google Scholar] [CrossRef]





| Energy | Nonlinear | Linear |
|---|---|---|
| ΔEint (HF) | −15.9 | −15.6 |
| ΔEint (MP2) | −17.2 | −16.2 |
| ΔEcorr | −1.3 | −0.6 |
| ΔEbin(MP2) | −16.8 | −15.7 |
| ΔEdef (MP2) | 0.4 | 0.5 |
| ΔEbin(MP2,BSSE) | −16.2 | −15.3 |
| BSSE | 0.6 | 0.4 |
| Energy | Nonlinear | Linear |
|---|---|---|
| ΔEPauli | 9.77 | 11.06 |
| ΔEelstat | −17.93 | −17.87 |
| ΔEorb | −7.40 | −8.26 |
| ΔEdisp | −1.87 | −0.88 |
| ΔEint | −17.42 | −15.96 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, S.J. Hydrogen Bonds with BF4− Anion as a Proton Acceptor. Crystals 2020, 10, 460. https://doi.org/10.3390/cryst10060460
Grabowski SJ. Hydrogen Bonds with BF4− Anion as a Proton Acceptor. Crystals. 2020; 10(6):460. https://doi.org/10.3390/cryst10060460
Chicago/Turabian StyleGrabowski, Sławomir J. 2020. "Hydrogen Bonds with BF4− Anion as a Proton Acceptor" Crystals 10, no. 6: 460. https://doi.org/10.3390/cryst10060460
APA StyleGrabowski, S. J. (2020). Hydrogen Bonds with BF4− Anion as a Proton Acceptor. Crystals, 10(6), 460. https://doi.org/10.3390/cryst10060460





