Liquid Crystals Templating
Abstract
:1. Introduction
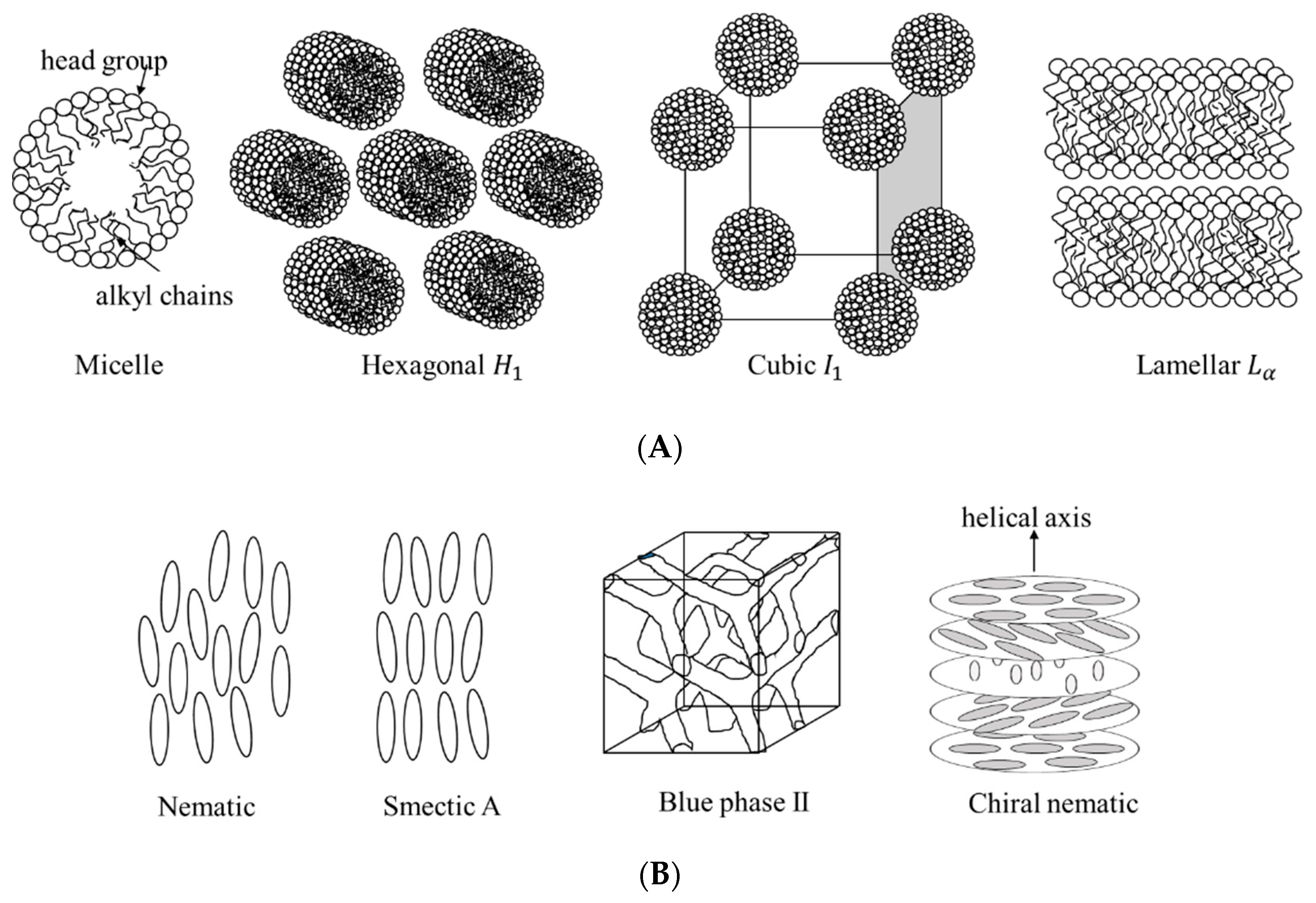
2. Templating Lyotropic Liquid Crystal Phases
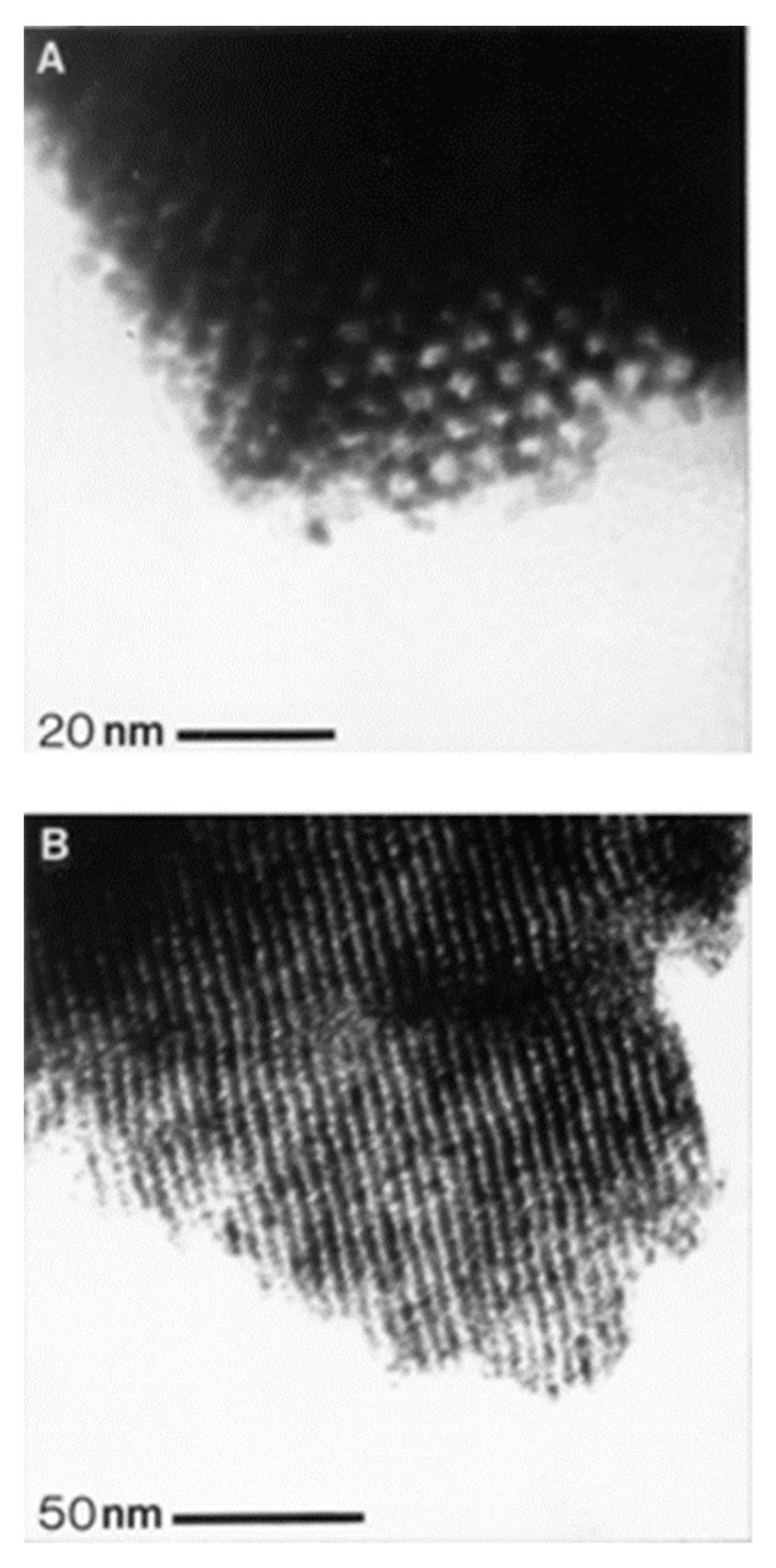
2.1. Porous and Nanostructured Materials
2.2. Polymerisation within Lyotropic Mesophases
2.3. Synthesis of 1D and 2D Materials
2.4. Electronic Applications
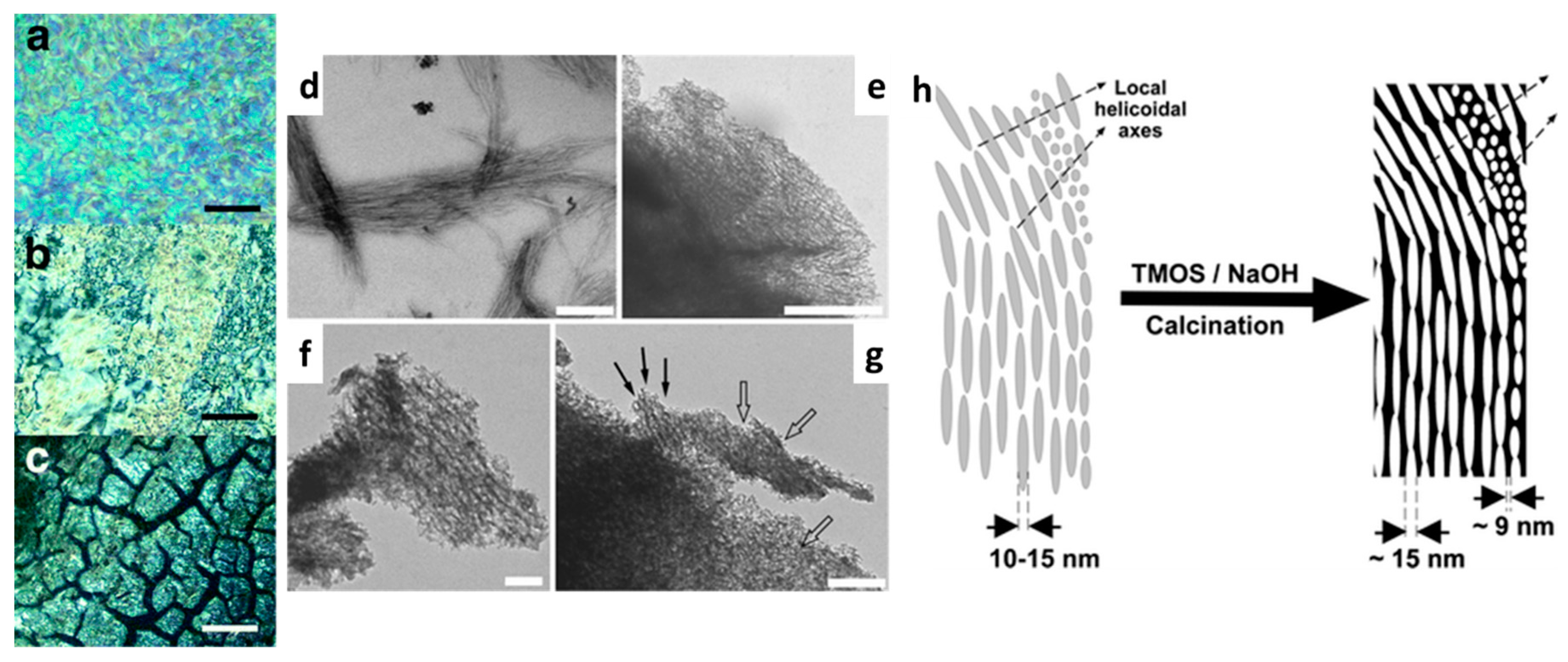
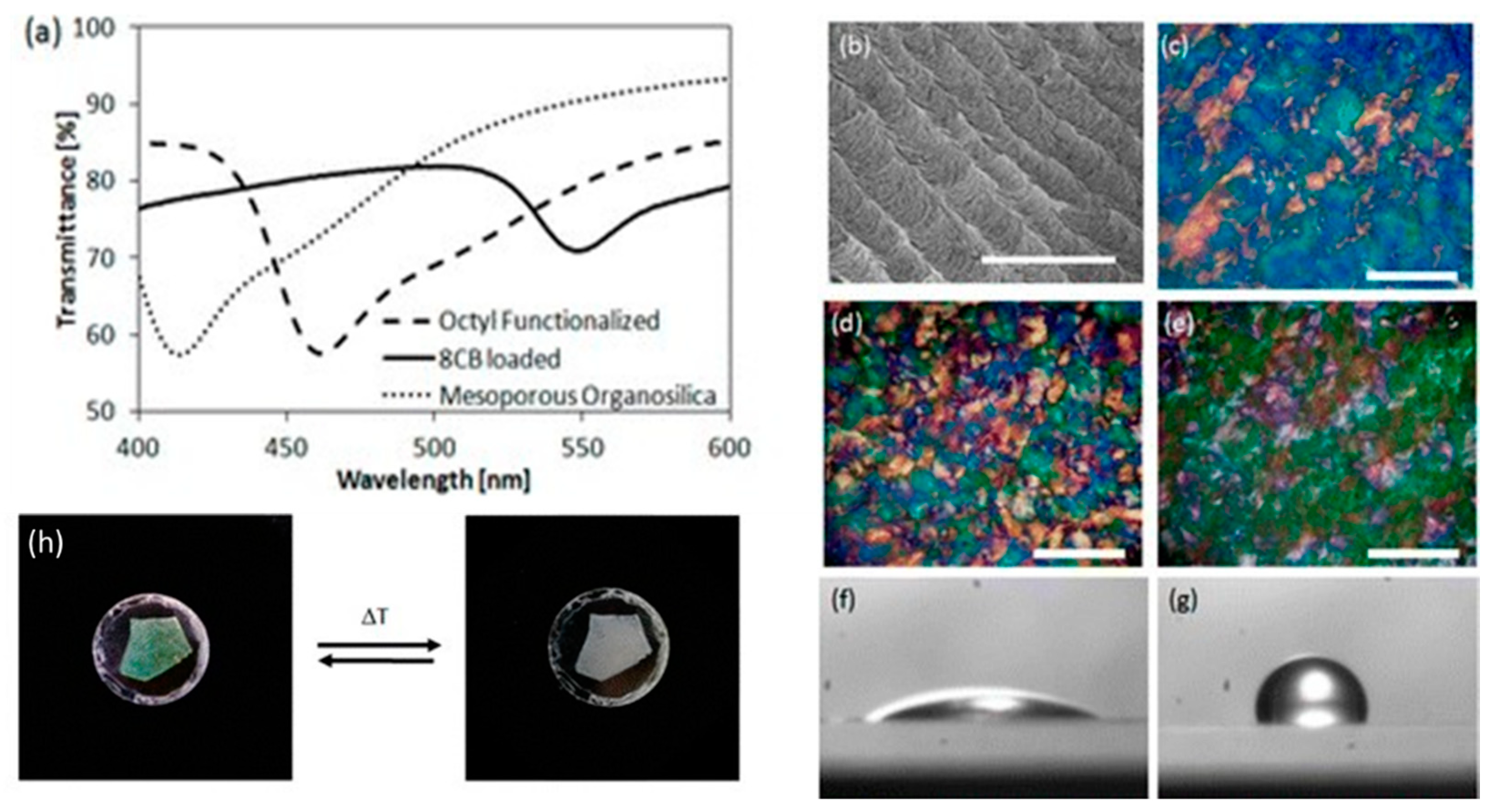
2.5. Cellulose Templating and Chiral Photonic Structures
2.6. Hydrogel Templates
3. Templating Thermotropic Liquid Crystal Phases
3.1. Chiral Nematic and Blue Phases
3.2. Smectic A Phase and Nanoporous Membranes
4. Conclusions
Funding
Conflicts of Interest
References
- Rumplecker, A.; Kleitz, F.; Salabas, E.L.; Schuth, F. Hard templating pathways for the synthesis of nanostructured porous Co3O4. Chem. Mater. 2007, 19, 485. [Google Scholar] [CrossRef]
- Li, W.; Lu, A.H.; Weidenthaler, C.; Schuth, F. Hard templating pathway to create mesoporous magnesium oxide. Chem. Mater. 2004, 16, 5676–5681. [Google Scholar] [CrossRef]
- Zhao, W.; Lang, M.; Li, Y.; Li, L.; Shi, J. Fabrication of uniform hollow mesoporous silica spheres and ellipsoids of tunable size through a facile hard-templating route. J. Mater. Chem. 2009, 19, 2778. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leanowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Ryoo, R. Birth of a class of nanomaterial. Nature 2019, 575, 40–41. [Google Scholar] [CrossRef] [Green Version]
- Attard, G.S.; Glyde, J.C.; Goltner, C.G. Liquid crystalline phases as templates for the synthesis of mesoporous silica. Nature 1995, 378, 366–368. [Google Scholar] [CrossRef]
- Seddon, J.M.; Raimondi, M.E. Liquid crystal templating of mesoporous materials. Mol. Cryst. Liq. Cryst. 2000, 347, 221–229. [Google Scholar] [CrossRef]
- Sakya, P.; Seddon, J.M.; Templer, R.H. Lyotropic phase-behavior of n-octyl-1-o-beta-d-glucopyranoside and its thio derivative n-octyl-1-s-beta-d-glucopyranoside. J. Phys. II 1994, 4, 1311–1331. [Google Scholar]
- Sakya, P.; Seddon, J.M.; Templer, R.H.; Mirkin, R.J.; Tiddy, G.J.T. Micellar cubic phases and their structural relationships: The nonionic surfactant system C12EO12/water. Langmuir 1997, 13, 3706–3714. [Google Scholar] [CrossRef]
- Rancon, Y.; Charvolin, J. Epitaxial relationships during phase transformations in a lyotropic liquid crystal. J. Phys. Chem. 1988, 92, 2646–2651. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C. Recent advances in the synthesis, characterization and applications of mesoporous molecular sieves. Curr. Opin. Solid State Mat. Sci. 1996, 1, 76–87. [Google Scholar] [CrossRef]
- Sayari, A.; Liu, P. Non-silica periodic mesostructured materials; recent progress. Microporous Mater. 1997, 12, 149–177. [Google Scholar] [CrossRef]
- Coma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar]
- Goltner, C.G.; Antonietti, M. Mesoporous materials by templating of liquid crystalline phases. Adv. Mater. 1997, 9, 431–436. [Google Scholar] [CrossRef]
- Mann, S.; Burkett, S.L.; Davis, S.A.; Fowler, C.E.; Mendelson, N.H.; Sims, S.D.; Walsh, D.; Whilton, N.T. Sol-gel synthesis of organized matter. Chem. Mater 1997, 9, 2300–2310. [Google Scholar] [CrossRef]
- Schuth, F. Superstructure of mesoporous silica. Curr. Opin. Colloid Interface Sci. 1998, 3, 174–180. [Google Scholar] [CrossRef]
- Brinker, C.J. Oriented inorganic films. Curr. Opin. Colloid Interface Sci. 1998, 3, 166–173. [Google Scholar] [CrossRef]
- Maschmeyer, T. Derivatised mesoporous solids. Curr. Opin. Solid State Mater. Sci. 1998, 3, 71–78. [Google Scholar] [CrossRef]
- Raimondi, M.E.; Seddon, J.M. Liquid crystal templating of porous materials. Liq. Cryst. 1999, 26, 305–339. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, L.; Liu, B.; He, J. Templated growth of crystalline mesoporous materials: From soft/hard templates to colloidal templates. Front. Chem. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Siril, P.F.; Remita, S. Swollen liquid crystals (SLCs): A versatile template for the synthesis of nano structured materials. RSC Adv. 2017, 7, 5733–5750. [Google Scholar] [CrossRef] [Green Version]
- Gin, D.L.; Gu, W.; Pindzola, B.A.; Zhou, W.-J. Polymerized lyotropic liquid crystal assemblies for materials applications. Acc. Chem. Res. 2001, 34, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, D.; Jiao, X. Lyotropic liquid crystal directed synthesis of nanostructured materials. Sci. Technol. Adv. Mater. 2009, 10, 023001. [Google Scholar] [CrossRef]
- Van der Asdonk, P.; Kouwer, P.H.J. Liquid crystal templating as an approach to spatially and temporally organise soft matter. Chem. Soc. Rev. 2017, 46, 5935–5949. [Google Scholar] [CrossRef] [Green Version]
- Alothman, Z.A. Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and applications of supramolecular templated mesoporous materials. Angew. Chem. Int. Ed. 1999, 38, 56. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Zhao, T.; Elzatahry, A.; Li, X.; Zhao, D. Single-micelle-directed synthesis of mesoporous materials. Nat. Rev. Mater. 2019, 4, 775–791. [Google Scholar] [CrossRef]
- Choi, M.; Heo, W.; Kleitz, F.; Ryoo, R. Facile synthesis of high quality mesoporous SBA-15 with enhanced control of the porous network connectivity and wall thickness. Chem. Commun. 2003, 1340–1341. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martinez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attard, G.S.; Bartlett, P.N.; Coleman, N.R.B.; Elliott, J.M.; Owen, J.R.; Wang, J.H. Mesoporous platinum films from lyotropic liquid crystalline phases. Science 1997, 278, 838–840. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.H.; Gin, D.L. Catalystic Pd nanoparticles synthesized using a lyotropic liquid crystal polymer template. Chem. Mater. 2000, 12, 22. [Google Scholar] [CrossRef]
- Huang, L.; Wang, H.; Wang, Z.; Mitra, A.P.; Zhao, D.; Yan, Y. Cuprite nanowires by electrodeposition from lyotropic reverse hexagonal liquid crystalline phase. Chem. Mater. 2002, 14, 876. [Google Scholar] [CrossRef]
- Schuth, F. Non-siliceous mesostructured and mesoporous materials. Chem. Mater. 2001, 13, 3184–3195. [Google Scholar] [CrossRef]
- Antonietti, M.; Caruso, R.A.; Hentze, H.-P.; Goltner, C.G. Hydrophilic gels with new superstructures and their hybrids by nanocasting technologies. Macromol. Symp. 2000, 152, 163–172. [Google Scholar] [CrossRef]
- Parisi, O.I.; Scrivano, L.; Candamano, S.; Ruffo, M.; Vattimo, A.F.; Spanedda, M.V.; Puoci, F. Molecularly imprinted microrods via mesophase polymerization. Molecules 2018, 23, 63. [Google Scholar] [CrossRef] [Green Version]
- Forney, B.S.; Baguenard, C.; Guymon, C.A. Effect of controlling polymer nanostructure using photopolymerzation within lyotropic liquid crystalline templates. Chem. Mater. 2013, 25, 2950–2960. [Google Scholar] [CrossRef]
- Zhao, B.; Moore, J.S. Fast pH- and ionic strength-responsive hydrogels in microchannels. Langmuir 2001, 17, 4758–4763. [Google Scholar] [CrossRef]
- Jung, M.; German, A.L.; Fischer, H.R. Polymerisation in lyotropic liquid-crystalline phases of ioctodecyldimethylammonium bromide. Colloid Polym. Sci. 2001, 279, 105–113. [Google Scholar] [CrossRef]
- Lester, C.L.; Colson, C.D.; Guymon, C.A. Photopolymerization kinetics and structure development of templated lyotropic liquid crystalline systems. Macromolecules 2001, 34, 4430. [Google Scholar] [CrossRef]
- Hentze, H.-P.; Kaler, E.W. Polymerization of and within self-organized media. Curr. Opin. Colloid Interface Sci. 2003, 8, 164–178. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, Y.; Lu, J.; Zhu, L.; He, W.; Qian, Y. Simultaneous in situ formation of ZnS nanowires in a liquid crystal template by γ-irradiation. Chem. Mater. 2001, 13, 1213. [Google Scholar] [CrossRef]
- Li, Y.; Wan, J.; Gu, Z. The formation of cadmium sulphide nanowires in different liquid crystal systems. Mater. Sci. Eng. 2000, A286, 106–109. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, L.; Cheng, H.; Ma, J. Preparation of ZnS nanorods by a liquid crystal template. J. Colloid Interface Sci. 2002, 246, 413. [Google Scholar] [CrossRef] [PubMed]
- Kijima, T.; Ikeda, T.; Yada, M.; Machida, M. Synthesis of AgBr and SnO2 microwires induced by mixed surfactant nematic liquid crystalline phases. Langmuir 2002, 18, 6453. [Google Scholar] [CrossRef]
- Kijima, T.; Yoshimura, T.; Uota, M.; Ikeda, T.; Fujikawa, D.; Mouri, S.; Uoyama, S. Noble-metal nanotubes (Pt, Pd, Ag) from lyotropic mixed-surfactant liquid crystal templates. Angew. Chem. Int. Ed. Engl. 2004, 43, 228. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhan, J.; Sui, Z.; Zhao, J.; Sun, Z. Controllable morphology formation of gold nano and micro plates in amphiphilic block copolymer based liquid crystalline phase. Chem. Lett. 2004, 33, 720. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhan, J.; Chai, Y.; Yang, C.; Xu, L.; Zhuang, W.; Jing, B. Synthesis of gold nano and microplates in hexagonal liquid crystals. J. Phys. Chem. B 2005, 109, 3189. [Google Scholar] [CrossRef]
- Hulvat, J.F.; Stupp, S.I. Liquid crystal templating of conducting polymers. Angew. Chem. Int. Ed. 2003, 42, 778–781. [Google Scholar] [CrossRef]
- Gurunathan, K.; Vadivel Murugan, A.; Marimuthu, R.; Mulik, U.P.; Amalanerkar, D.P. Electrochemically synthesised conducting polymeric materials for applications towards technology in electronics, optoelectronics and energy storage devices. Mater. Chem. Phys. 1999, 61, 173–191. [Google Scholar] [CrossRef]
- Greiner, A. Design and synthesis of polymers for light-emitting diodes. Polym. Adv. Technol. 1998, 9, 371. [Google Scholar] [CrossRef]
- Durardin, E.; Blaseby, M.; Mann, S. Synthesis of mesoporous silica by sol-gel mineralisation of cellulose nanorod nematic suspension. J. Mater. Chem. 2003, 13, 696–699. [Google Scholar] [CrossRef]
- Che, S.; Liu, Z.; Ohsuna, T.; Sakamoto, K.; Terasaki, O.; Tatsumi, T. Synthesis and characterization of chiral mesoporous silica. Nature 2004, 429, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Inoue, Y.; Che, S. Supramolecular chiral transcription and recognition by mesoporous silica prepared by chiral imprinting of a helical micelle. Angew. Chem. Int. Ed. 2009, 48, 3069–3072. [Google Scholar] [CrossRef] [PubMed]
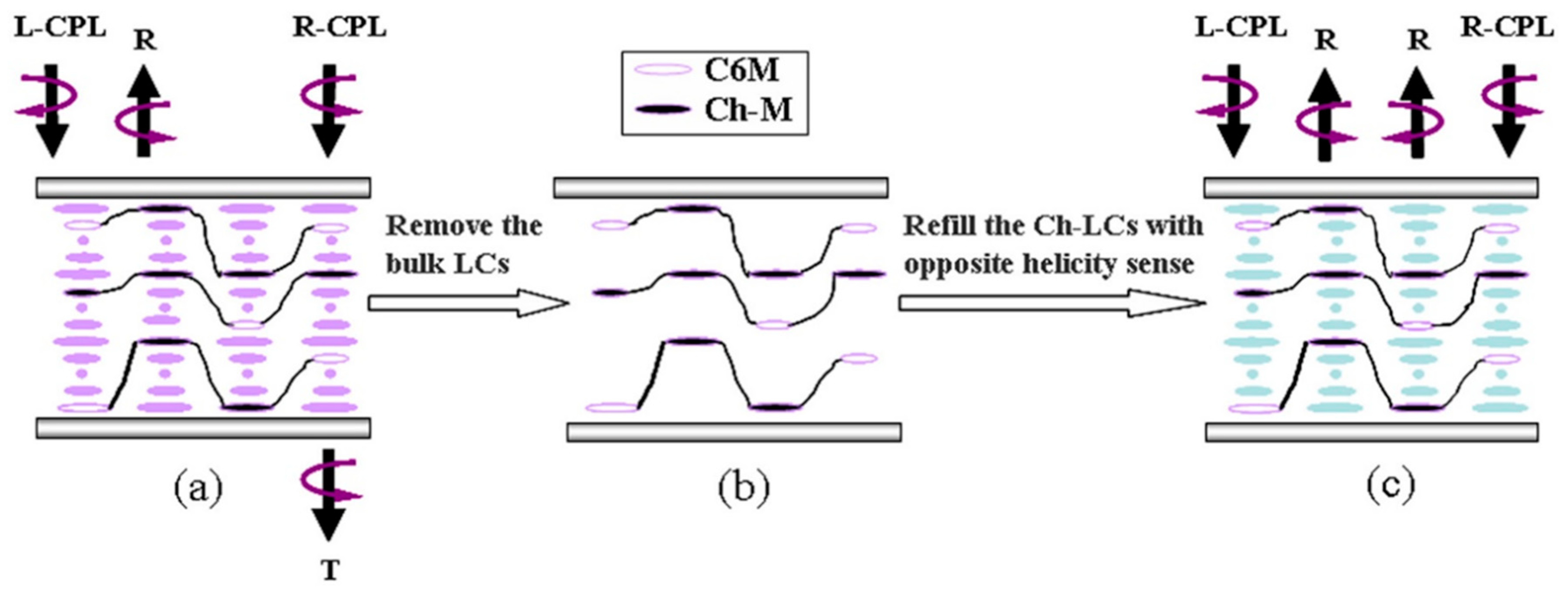
- Shopsowitz, K.E.; Qi, H.; Hamad, W.Y.; MacLachlan, M.J. Free standing mesoporous silica films with tunable chiral nematic structures. Nature 2010, 468, 422–425. [Google Scholar] [CrossRef]
- Giese, M.; De Witt, J.C.; Shopsowitz, K.E.; Manning, A.P.; Dong, R.Y.; Michal, C.A. Thermal switching of the reflection in chiral nematic mesoporous organosilica films infiltrated with liquid crystals. ACS Appl. Mater. Interfaces 2013, 5, 6854–6859. [Google Scholar] [CrossRef]
- Giese, M.; Krappitz, T.; Dong, R.Y.; Michal, C.A.; Hamad, W.Y.; Patrick, B.O. Tuning the photonic properties of chiral nematic mesoporous organosilica with hydrogen-bonded liquid-crystalline assemblies. J. Mater. Chem. C 2015, 3, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Tardy, B.L.; Mattos, B.D.; Greca, L.G.; Kamarainen, T.; Klockars, K.W.; Rojas, O.J. Tessellation of chiral nematic cellulose nanocrystal films by microtemplating. Adv. Funct. Mater. 2019, 29, 1808518. [Google Scholar] [CrossRef]
- Cartwright, J.H.; Checa, A.G. The dynamics of nacre self-assembly. J. R. Soc. Interface 2007, 4, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.S.; Vreeland, W.N.; DePaoli Lacerda, D.S.; Locascio, L.E.; Gaitan, M.; Raghavan, S.R. Liposome-templated supramolecular assembly of responsive alginate nanogels. Langmuir 2008, 24, 4092–4096. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Braun, P.V. Tunable inverse opal hydrogel pH sensors. Adv. Mater. 2003, 15, 563–566. [Google Scholar] [CrossRef]
- Lester, C.L.; Smith, S.M.; Colson, C.D.; Guymon, C.A. Physical properties of hydrogels synthesized from lyotropic liquid crystalline templates. Chem. Mater. 2003, 15, 3376–3384. [Google Scholar] [CrossRef]
- Texter, J. Templating hydrogels. Colloid Polym. Sci. 2009, 287, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Pruzinsky, S.A.; Braun, P.V. Glucose-sensitive inverse opal hydrogels: Analysis of optical diffraction response. Langmuir 2004, 20, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, Z.; Hill, A.J.; She, F.H.; Thornton, A.W.; Hoang, M.; Kong, L.X. Structure retention in cross-linked poly(ethylene glycol) diacrylate hydrogel templated from a hexagonal lyotropic liquid crystal by controlling the surface tension. Soft Matter 2012, 8, 2087–2094. [Google Scholar] [CrossRef]
- De Pierro, M.A.; Carpenter, K.G.; Guymon, C.A. Influence of polymerization conditions on nanostructure and properties of polyacrylamide hydrogels templated from lyotropic liquid crystals. Chem. Mater. 2006, 18, 5609–5617. [Google Scholar] [CrossRef]
- McLaughlin, J.R.; Abbott, N.L.; Guymon, C.A. Responsive superabsorbent hydrogels via photopolymerisation in lyotropic liquid crystal templates. Polymer 2018, 142, 119–126. [Google Scholar] [CrossRef]
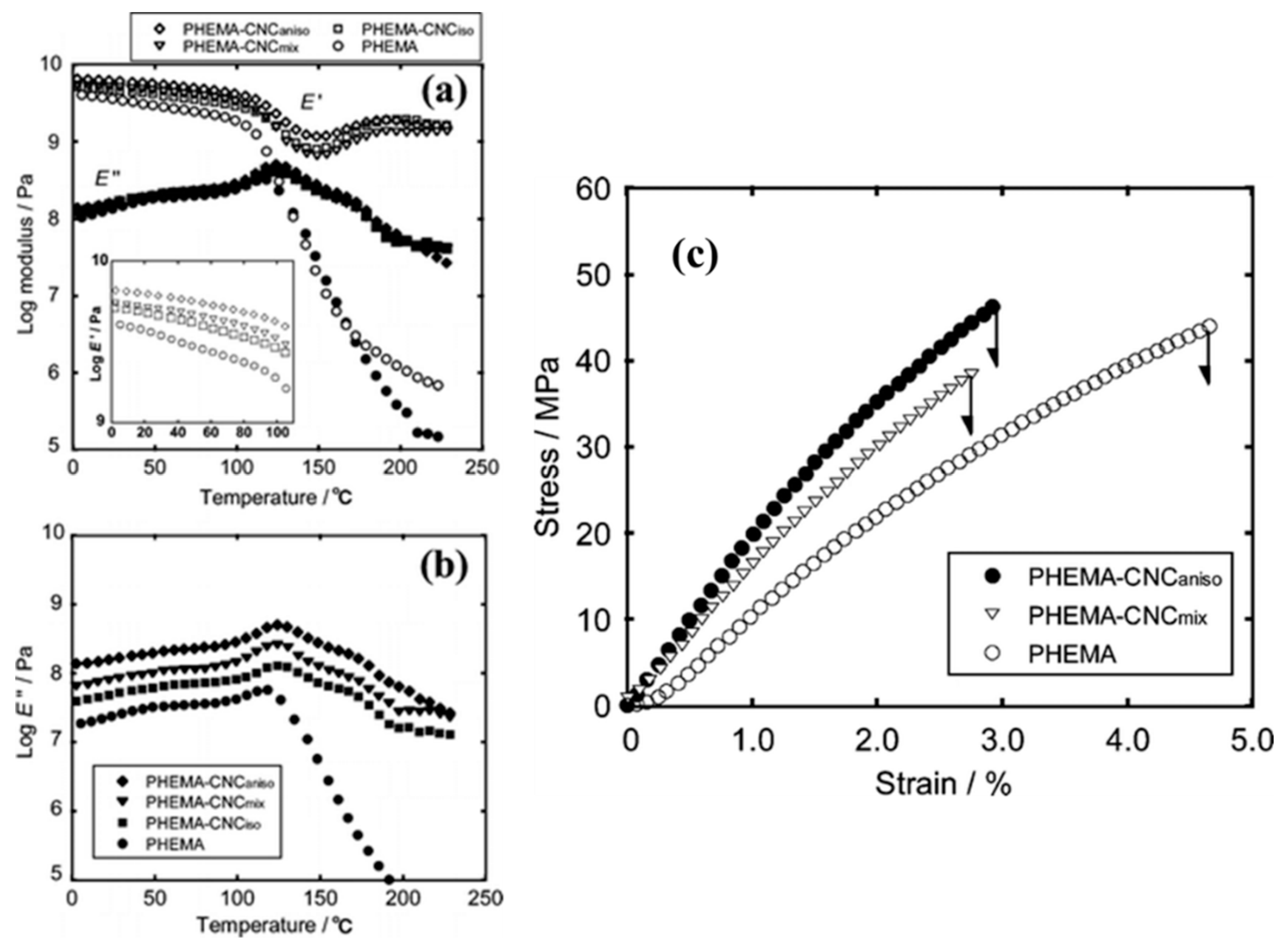
- Tatsumi, M.; Teramoto, Y.; Nishio, Y. Polymer composites reinforced by locking in a liquid crystalline assembly of cellulose nanocrystallites. Biomacromolecules 2012, 13, 1584–1591. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef]
- Boott, C.E.; Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Cellulose nanocrystal elastomers with reversible visible color. Angew. Chem. Int. Ed. 2020, 59, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Cao, H.; Wei, J.; Zhang, D.; Liu, F.; Pan, G.; Zhao, D.; He, W.; Yang, H. Polymer stabilized liquid crystal films reflecting both right and left circularly polarized light. Appl. Phys. Lett. 2008, 93, 201901. [Google Scholar] [CrossRef]
- Bouligand, Y. Liquid crystalline order in biological materials. In Liquid Crystalline Order in Polymers; Blumstein, A., Ed.; Academic Press: New York, NY, USA, 1978; pp. 261–297. [Google Scholar]
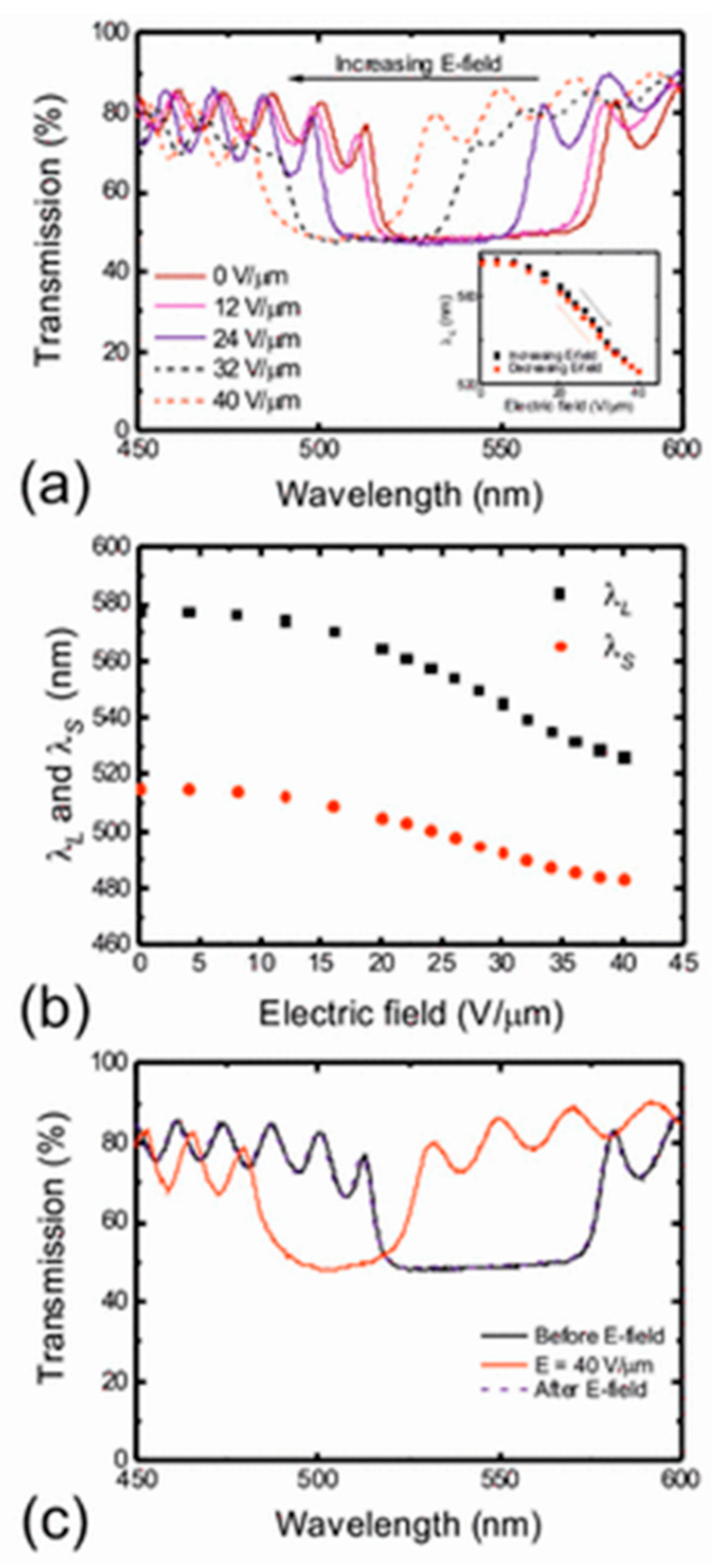
- Wood, S.M.; Fells, J.A.J.; Elston, S.J.; Morris, S.M. Wavelength tuning of the photonic band gap of an achiral nematic liquid crystal filled into a chiral polymer scaffold. Macromolecules 2016, 49, 8643–8652. [Google Scholar] [CrossRef] [Green Version]
- Batir, O.; Bat, E.; Bukusoglu, E. Strain enhanced sensitivity of polymeric sensors templated from cholesteric liquid crystals. Soft Matter 2020. [Google Scholar] [CrossRef] [PubMed]
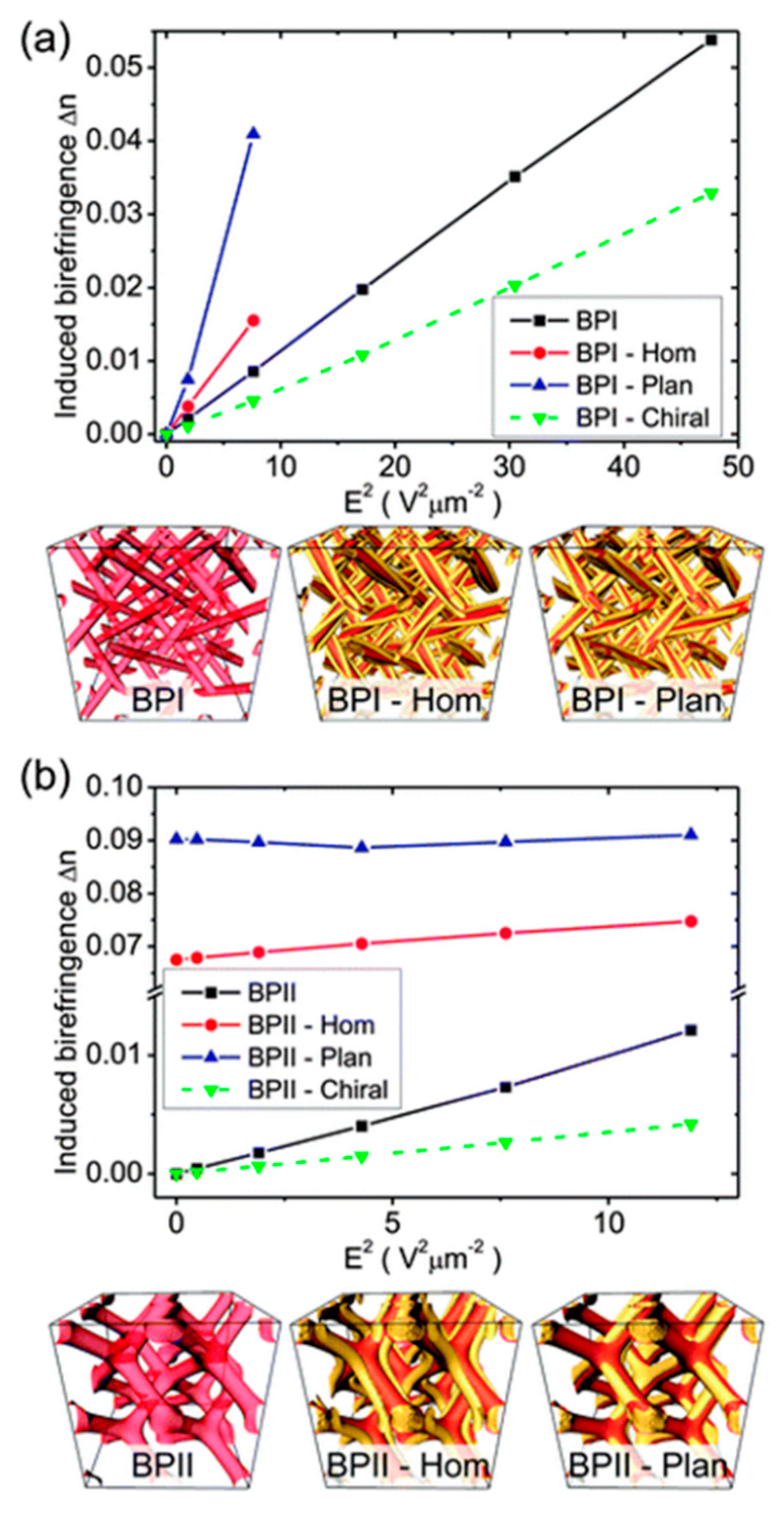
- Castles, F.; Day, F.V.; Morris, S.M.; Ko, D.-H.; Gardiner, D.J.; Qasim, M.M.; Nosheen, S.; Hands, P.J.W.; Choi, S.S.; Friend, R.H.; et al. Blue phase templated fabrication of three dimensional nanostructures for photonic applications. Nat. Mater. 2012, 11, 599–603. [Google Scholar] [CrossRef]
- Kikuchi, H.; Izena, S.; Higuchi, H.; Okumura, Y.; Higashiguchi, K. Interaction between topological defects of liquid crystals and polymers. Soft Matter 2015, 11, 4572–4575. [Google Scholar] [CrossRef]
- Castles, F.; Morris, S.M.; Hung, J.M.C.; Qasim, M.M.; Wright, A.D.; Nosheen, S.; Choi, S.; Outram, B.I.; Elston, S.J.; Burgess, C.; et al. Stretchable liquid crystal blue phase gels. Nat. Mater. 2014, 13, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Ravnik, M.; Fukuda, J. Templated blue phases. Soft Matter 2015, 11, 8417. [Google Scholar] [CrossRef] [Green Version]
- Jau, H.-C.; Lin, Y.-T.; Li, C.-C.; Chen, C.-W.; Lin, T.-H. Optically rewritable dynamic phase grating based on blue phase templated azobenzene liquid crystal. Optics Express 2019, 27, 10580–10585. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, H.; Ko, Y.H.; Chang, Y.J.; Oh, N.-K.; Zin, W.-C.; Kim, K. Synthesis of a nanoporous polymer with hexagonal channels from supramolecular discotic liquid crystals. Angew. Chem. Int. Ed. 2001, 40, 2669–2671. [Google Scholar] [CrossRef]
- Bögels, G.M.; Lugger, J.A.M.; Goor, O.J.G.M.; Sijbesma, R.P. Size-selective binding of sodium and potassium ions in nanoporous thin films of polymerized liquid crystals. Adv. Funct. Mater. 2016, 26, 8023–8030. [Google Scholar] [CrossRef]
- Gracia, I.; Romero, P.; Serrano, J.L.; Barberá, J.; Omenat, A. Templated nanoporous membranes based on hierarchically self-assembled materials. J. Mater. Chem. C 2017, 5, 2033–2042. [Google Scholar] [CrossRef]
- Feng, X.; Tousley, M.E.; Cowan, M.G.; Wiesenauer, B.R.; Nejati, S.; Choo, Y.; Noble, R.D.; Elimelech, M.; Gin, D.L.; Osuji, C.O. Scalable fabrication of polymer membranes with vertically aligned 1 Nm pores by magnetic field directed self-assembly. ACS Nano 2014, 8, 11977–11986. [Google Scholar] [CrossRef]
- Lugger, J.; Mulder, D.J.; Sijbesma, R.; Schenning, A. Nanoporous polymers based on liquid crystals. Materials 2018, 11, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-C.; Huang, W.-Y. Insertion of poly(acrylamide) disc-columnar liquid crystals as a functional template in organic photovoltaics. J. Appl. Polym. Sci. 2012, 126, E70–E77. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Kidd, T.J.; Noble, R.D.; Gin, D.L. Supported lyotropic liquid crystal polymer membranes: Promising materials for molecular size selective aqueous nanofiltration. Adv. Mater. 2005, 17, 1850–1853. [Google Scholar] [CrossRef]
- Ishida, Y.; Amano, S.; Saigo, K. Template polymerization of columnar architectures based on the salts of a carboxylic acid and 2-amino alcohols: Application to the molecular recognition of 2-amino alcohols. Chem. Commun. 2003, 2338–2339. [Google Scholar] [CrossRef]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Chem. Rev. 2009, 109, 6275–6540. [CrossRef]
- Beginn, U.; Zipp, G.; Mouller, A.; Walther, P.; Möller, M. Membranes containing oriented supramolecular transport channels. Adv. Mater. 2000, 12, 513–516. [Google Scholar] [CrossRef]
- Karausta, A.; Bukisoglu, E. Liquid crystal templated synthesis of mesporous membranes with predetermined pore alignment. ACS Appl. Mater. Interfaces 2018, 10, 33484–33492. [Google Scholar] [CrossRef]
- Kishikawa, K.; Hirai, A.; Kohmoto, S. Fixation of multi-layered structures of liquid crystalline 2:1 complexes of benzoic acid derivatives and dipyridyl compounds and the effect of nanopillars on removal of the dipyridyl molecules from the polymers. Chem. Mater. 2008, 20, 1931–1935. [Google Scholar] [CrossRef]
- Kamarudin, M.A.; Khan, A.A.; Williams, C.; Rughoobur, G.; Said, S.M.; Nosheen, S.; Flewitt, A.J.; Qasim, M.M.; Wilkinson, T.D. Self-assembled liquid crystalline nanotemplates and their incorporation in dye-sensitised solar cells. Electrochim. Acta 2016, 222, 657–667. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, M. Liquid Crystals Templating. Crystals 2020, 10, 648. https://doi.org/10.3390/cryst10080648
Nagaraj M. Liquid Crystals Templating. Crystals. 2020; 10(8):648. https://doi.org/10.3390/cryst10080648
Chicago/Turabian StyleNagaraj, Mamatha. 2020. "Liquid Crystals Templating" Crystals 10, no. 8: 648. https://doi.org/10.3390/cryst10080648
APA StyleNagaraj, M. (2020). Liquid Crystals Templating. Crystals, 10(8), 648. https://doi.org/10.3390/cryst10080648



