The Crystal Structure of Bornite Cu5FeS4: Ordered Fe and Split Cu
Abstract
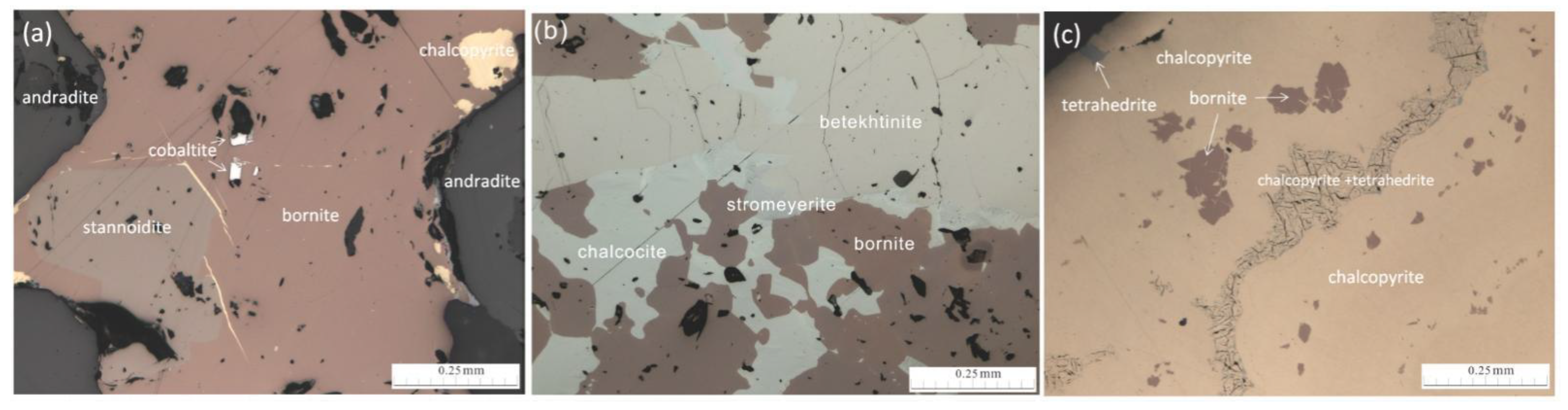
:1. Introduction
2. Samples and Experimental Methods
3. Results and Discussion
4. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morimoto, N.; Kullerud, G. Polymorphism in bornite. Am. Mineral. 1961, 46, 1270–1282. [Google Scholar]
- Morimoto, N. Structure of two polymorphic forms of Cu5FeS4. Acta Crystallogr. 1964, 17, 351–360. [Google Scholar] [CrossRef]
- Frueh, A.J. Disorder in the mineral bornite, Cu5FeS4. Am. Mineral. J. Earth Planet. Mater. 1950, 35, 185–192. [Google Scholar]
- Kanazawa, Y.; Koto, K.; Morimoto, N. Bornite (Cu5FeS4): Stability and crystal structure of the intermediate form. Can. Mineral. 1978, 16, 397–404. [Google Scholar]
- Kratz, T.; Fuess, H. Simultane strukturbestimmung von kupferkies und bornite an einem kristall. Zeitschrift für Kristallographie 1989, 186, 167–169. [Google Scholar]
- Tunnell, G.; Adams, C.E. On the symmetry and crystal structure of bornite. Am. Mineral. 1949, 34, 824–829. [Google Scholar]
- Koto, K.; Morimoto, N. Superstructure investigation of bornite, Cu5FeS4, by the modified partial Patterson function. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 2268–2273. [Google Scholar] [CrossRef]
- Ding, Y.; Veblen, D.R.; Prewitt, C.T. High-resolution transmission electron microscopy (HRTEM) study of the 4a and 6a superstructure of bornite Cu5FeS4. Am. Miner. 2005, 90, 1256–1264. [Google Scholar] [CrossRef]
- Martinelli, A.; Lepore, G.O.; Bernardini, F.; Giaccherini, A.; Di Benedetto, F. The puzzling structure of Cu5FeS4 (bornite) at low temperature. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2018, 74, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHEXLT-integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refienment with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Pierce, L.; Buseck, P.R. Superstructuring in the bornite-digenite series: A high-resolution electron microscopy study. Am. Mineral. 1978, 63, 1–16. [Google Scholar]
- Stevens, J.G.; Khasanov, A.M.; Miller, J.W.; Pollak, H.; Li, Z. Mössbauer Mineral Handbook; Mössbauer Effect Data Center: Dalian, China, 2005; p. 636. ISSN 0272-2755. [Google Scholar]
- Haidinger, W. Zweite Klasse: Geogenide. XIII. Ordnung. Kiese. V. Kupperkies. Bornite. In Handbuch der Bestimmenden Mineralogie; Bei Braumüller and Seidel: Wien, Austria, 1845; pp. 559–562. [Google Scholar]
- Harrington, B.J. On the formula of bornite. Am. J. Sci. 1903, s4-16, 151–154. [Google Scholar] [CrossRef]
- Allen, E.T. The composition of natural bornite. Am. J. Sci. 1916, s4-41, 409–413. [Google Scholar] [CrossRef]
- Borgheresi, M.; Di Benedetto, F.; Caneschi, A.; Pratesi, G.; Romanelli, M.; Sorace, L. An EPR and SQUID magnetometry study of bornite. Phys. Chem. Miner. 2007, 34, 609–619. [Google Scholar] [CrossRef]
- Przewoźnik, J.; Żukrowski, J.; Gondek, L.; Gąska, K.; Lemański, A.; Kapusta, C.; Piestrzyński, A. Structural, magnetic, and Mössbauer effect studies of bornite. Nukleonika 2013, 58, 43–46. [Google Scholar]
- Kullerud, G. The The Cu9S5-Cu5FeS4 join. Carnegie Inst. Wash. Year B 1960, 59, 114–116. [Google Scholar]


| Deposits | Element wt% | apfu. (Total Atoms = 10) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | Fe | Cu | Ag | Total | S | Fe | Cu | Ag | ||
| Saishitang | average | 25.38 | 11.64 | 61.48 | 0.59 | 99.09 | 4.01 | 1.06 | 4.90 | 0.03 |
| (N = 5) | stdev | 0.54 | 0.10 | 0.43 | 0.41 | 0.39 | 0.06 | 0.01 | 0.06 | 0.02 |
| Yushui | average | 25.21 | 11.17 | 62.46 | 0.34 | 99.47 | 3.98 | 1.04 | 4.97 | 0.02 |
| (N = 5) | stdev | 0.32 | 0.25 | 0.62 | 0.08 | 0.39 | 0.03 | 0.01 | 0.07 | 0.01 |
| Bofang | average | 25.82 | 11.91 | 62.59 | 0.03 | 100.34 | 4.02 | 1.06 | 4.92 | 0.00 |
| (N = 5) | stdev | 0.22 | 0.26 | 0.44 | 0.02 | 0.53 | 0.03 | 0.02 | 0.03 | 0.00 |
| Crystal Data | |||
| Sample source | Saishitang | Yushui | Bofang |
| Empirical formula | Cu5FeS4 | Cu5FeS4 | Cu5FeS4 |
| Formula weight | 501.79 | 501.71 | 501.99 |
| Crystal size/mm3 | 0.09 × 0.08 × 0.07 | 0.04 × 0.035 × 0.03 | 0.03 × 0.02 × 0.01 |
| Crystal system | orthorhombic | orthorhombic | orthorhombic |
| Space group | Pbca | Pbca | Pbca |
| Temperature °K | 293 | 293 | 293 |
| a Å | 10.97016(18) | 10.95863(16) | 10.9639(7) |
| b Å | 21.8803(4) | 21.8673(4) | 21.8774(12) |
| c Å | 10.9637(2) | 10.9569(2) | 10.9593(6) |
| V Å3 | 2631.61(8) | 2625.67(8) | 2628.7(3) |
| Z | 16 | 16 | 16 |
| ρcalc g/cm3 | 5.066 | 5.077 | 5.074 |
| μ/mm−1 | 19.163 | 45.839 | 19.194 |
| Data collection | |||
| Instrument | Rigaku Synergy | Rigaku Synergy | Rigaku Synergy |
| Radiation (λ Å) | Mo Kα (0.71073) | Cu Kα (1.54184) | Mo Kα (0.71073) |
| 2θ range ° | 5.26 to 56.53 | 8.086 to 154.668 | 5.262 to 56.552 |
| Absorption correction | ABSPACK | ABSPACK | ABSPACK |
| Reflections collected | 67,413 | 11,787 | 19,144 |
| Independent reflections | 3273 | 2732 | 3272 |
| Reflections with I > 2σ(I) | 2645 | 2258 | 1768 |
| Rint | 0.0345 | 0.0203 | 0.0564 |
| Rsigma | 0.0135 | 0.0271 | 0.0426 |
| Indices range of h | –14 ≤ h ≤ 14 | –7 ≤ h ≤ 13 | –14 ≤ h ≤ 14 |
| Indices range of k | –29 ≤ k ≤ 29 | –27 ≤ k ≤ 27 | –28 ≤ k ≤ 29 |
| Indices range of l | –14 ≤ l ≤ 14 | –13 ≤ l ≤ 13 | –11 ≤ l ≤ 14 |
| Refinement | |||
| No. of parameters, restraints | 283, 0 | 283, 0 | 282, 0 |
| R1, wR2 [I > 2σ(I)] | 0.0259, 0.0748 | 0.0299, 0.0749 | 0.0483, 0.1060 |
| R1, wR2 (all data) | 0.0338, 0.0792 | 0.0396, 0.0796 | 0.1067, 0.1284 |
| Goodness-of-fit | 1.023 | 1.041 | 1.013 |
| Δρmax, Δρmin (e− Å−3) | 1.15, –0.71 | 0.54, –0.60 | 2.02, –0.73 |
| Site | Atom/Occup. | x | y | z | Ueq | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S1.00 | 0.00400(6) | −0.00082(2) | 0.25260(5) | 0.0153(2) | 0.0153(3) | 0.0152(3) | 0.0156(3) | −0.0018(2) | 0.0007(2) | 0.0008(2) |
| S2 | S1.00 | 0.00255(5) | 0.25026(2) | 0.25351(6) | 0.0157(2) | 0.0160(3) | 0.0161(3) | 0.0150(3) | 0.0012(2) | −0.0015(2) | 0.0019(2) |
| S3 | S1.00 | 0.24441(5) | 0.12678(3) | 0.25587(6) | 0.0149(2) | 0.0155(3) | 0.0155(3) | 0.0138(3) | −0.0006(2) | 0.0011(2) | 0.0019(2) |
| S4 | S1.00 | 0.25185(5) | 0.00161(3) | 0.50844(5) | 0.0152(2) | 0.0154(3) | 0.0145(3) | 0.0156(3) | −0.0014(2) | −0.0002(2) | −0.0016(2) |
| S5 | S1.00 | 0.00092(6) | 0.12814(3) | 0.49451(6) | 0.0155(2) | 0.0144(3) | 0.0165(3) | 0.0157(3) | 0.0008(2) | 0.0020(2) | −0.0015(2) |
| S6 | S1.00 | −0.00676(6) | 0.12238(3) | −0.00617(6) | 0.0152(2) | 0.0144(3) | 0.0155(3) | 0.0156(3) | −0.0022(2) | −0.0014(2) | −0.0010(2) |
| S7 | S1.00 | 0.25509(5) | 0.12286(3) | 0.74808(6) | 0.0158(2) | 0.0160(4) | 0.0163(3) | 0.0150(3) | −0.0018(2) | 0.0024(2) | −0.0001(2) |
| S8 | S1.00 | 0.24376(5) | 0.24817(3) | 0.49624(5) | 0.0151(2) | 0.0156(3) | 0.0143(3) | 0.0154(3) | −0.0017(2) | −0.0003(2) | −0.0013(2) |
| M1a | Cu0.665(36) | 0.12769(51) | 0.05825(18) | 0.37157(36) | 0.0258(5) | 0.0293(12) | 0.0216(7) | 0.0265(6) | −0.0034(8) | −0.0032(7) | −0.0037(6) |
| M1b | Cu0.335(36) | 0.14591(164) | 0.05270(60) | 0.36457(95) | 0.0337(14) | 0.0280(29) | 0.0402(26) | 0.0330(21) | 0.0188(18) | −0.0127(16) | −0.0129(19) |
| M2a | Cu0.555(25) | 0.10702(27) | 0.06453(17) | 0.62799(37) | 0.0315(5) | 0.0278(8) | 0.0346(7) | 0.0320(8) | 0.0063(6) | −0.0044(10) | −0.0121(11) |
| M2b | Cu0.445(25) | 0.09920(56) | 0.05726(37) | 0.61058(90) | 0.0441(9) | 0.0474(18) | 0.0426(15) | 0.0424(18) | 0.0217(12) | 0.0258(12) | 0.0256(12) |
| M3a | Cu0.441(27) | 0.39174(39) | 0.06319(30) | 0.36114(74) | 0.0364(9) | 0.0355(14) | 0.0404(12) | 0.0333(16) | −0.0142(10) | −0.0108(13) | 0.0212(15) |
| M3b | Cu0.559(27) | 0.39210(31) | 0.05648(23) | 0.37724(50) | 0.0336(6) | 0.0340(11) | 0.0418(10) | 0.0251(9) | −0.0126(7) | 0.0126(10) | −0.0147(12) |
| M4 | Fe1.00 | 0.37625(3) | 0.06010(2) | 0.63211(3) | 0.0129(1) | 0.0129(2) | 0.0127(2) | 0.0131(2) | 0.0006(1) | 0.0001(1) | 0.0000(1) |
| M5 | Fe1.00 | 0.11933(3) | 0.18967(2) | 0.37289(3) | 0.0131(1) | 0.0132(2) | 0.0131(2) | 0.0132(2) | 0.0001(1) | −0.0002(1) | 0.0004(1) |
| M6a | Cu0.433(24) | 0.13093(47) | 0.19273(36) | 0.64528(38) | 0.0391(8) | 0.0459(12) | 0.0291(14) | 0.0421(15) | 0.0122(11) | 0.0295(15) | 0.0116(9) |
| M6b | Cu0.567(24) | 0.12080(31) | 0.18330(24) | 0.64253(23) | 0.0334(6) | 0.0361(7) | 0.0336(11) | 0.0304(8) | −0.0116(9) | −0.0116(11) | 0.0106(6) |
| M7a | Cu0.435(25) | 0.38411(47) | 0.18556(29) | 0.35932(36) | 0.0330(8) | 0.0373(12) | 0.0323(13) | 0.0295(12) | 0.0149(14) | −0.0123(14) | −0.0110(8) |
| M7b | Cu0.565(25) | 0.37318(43) | 0.19490(33) | 0.35581(35) | 0.0367(6) | 0.0297(8) | 0.0396(14) | 0.0407(13) | −0.0153(9) | 0.0193(10) | −0.0170(8) |
| M8a | Cu0.669(22) | 0.37928(29) | 0.19057(22) | 0.61967(30) | 0.0239(4) | 0.0256(5) | 0.0156(11) | 0.0304(6) | −0.0027(5) | −0.0003(6) | −0.0004(5) |
| M8b | Cu0.331(22) | 0.38490(69) | 0.20084(59) | 0.61229(74) | 0.0335(15) | 0.0382(18) | 0.0195(28) | 0.0427(19) | −0.0080(15) | −0.0235(17) | 0.0048(13) |
| M9a | Cu0.305(30) | 0.13393(59) | 0.06582(29) | 0.87871(62) | 0.0273(8) | 0.0224(12) | 0.0297(12) | 0.0298(13) | −0.0019(17) | 0.0011(11) | −0.0049(16) |
| M9b | Cu0.695(30) | 0.15070(68) | 0.07260(30) | 0.89771(75) | 0.0434(9) | 0.0386(13) | 0.0456(12) | 0.0459(15) | 0.0266(10) | 0.0220(11) | 0.0219(9) |
| M10a | Cu0.243(18) | 0.36553(68) | 0.06889(33) | 0.11586(59) | 0.0301(10) | 0.0298(17) | 0.0273(16) | 0.0333(14) | −0.0015(14) | 0.0013(15) | −0.0072(13) |
| M10b | Cu0.757(18) | 0.33485(52) | 0.08299(24) | 0.09033(45) | 0.0461(10) | 0.0511(13) | 0.0447(12) | 0.0425(11) | −0.0241(9) | 0.0268(10) | −0.0271(11) |
| M11a | Cu0.363(27) | 0.13149(42) | 0.18117(21) | 0.11239(41) | 0.0275(8) | 0.0325(10) | 0.0297(11) | 0.0205(13) | 0.0023(11) | −0.0032(10) | −0.0015(14) |
| M11b | Cu0.637(27) | 0.14964(75) | 0.17138(41) | 0.09043(87) | 0.0520(12) | 0.0499(15) | 0.0548(16) | 0.0514(18) | 0.0321(14) | −0.0312(13) | −0.0344(12) |
| M12a | Cu0.221(26) | 0.36837(74) | 0.18403(35) | 0.88087(76) | 0.0287(10) | 0.0268(17) | 0.0307(15) | 0.0287(17) | −0.0003(16) | 0.0010(17) | −0.0014(18) |
| M12b | Cu0.779(26) | 0.34422(66) | 0.17387(29) | 0.90399(63) | 0.0442(11) | 0.0457(14) | 0.0427(12) | 0.0442(13) | −0.0230(10) | −0.0243(12) | 0.0238(11) |
| Fe-S tetrahedron | |||||||||
| M4(Fe)- | Distance | Angle | M5(Fe)- | Distance | Angle | ||||
| S1 | 2.2701(7) | S2 | 2.2609(6) | ||||||
| S7 | 2.2954(7) | 110.77(3) | S5 | 2.2975(7) | 111.05(3) | ||||
| S4 | 2.3105(7) | 111.52(3) | 108.32(3) | S8 | 2.3087(7) | 110.43(3) | 108.61(3) | ||
| S6 | 2.3261(7) | 111.16(3) | 107.33(2) | 107.58(3) | S3 | 2.3286(7) | 111.19(3) | 107.85(3) | 107.58(2) |
| Average | 2.3005 | 109.45 | Average | 2.2989 | 109.45 | ||||
| Cu-S pyramidal triangles (including M1b 2b 7b 8b 9b 10b 11b 12b) | |||||||||
| M1b- | Distance | Angle | M10b- | Distance | Angle | ||||
| S4 | 2.2557(117) | S4 | 2.2665(15) | ||||||
| S3 | 2.2837(91) | 118.22(64) | S5 | 2.2716(16) | 121.98(6) | ||||
| S1 | 2.3025(89) | 117.98(45) | 113.74(34) | S3 | 2.2796(16) | 118.42(7) | 119.49(6) | ||
| Average | 2.2806 | 116.65 | Average | 2.2726 | 119.96 | ||||
| Cu-S distorted tetrahedra (including M1a 2a 3a 3b 6a 6b 7b 8a 9a 10a 11a 12a) | |||||||||
| M1a- | Distance | Angle | M10a- | Distance | Angle | ||||
| S1 | 2.2831(41) | S5 | 2.3133(47) | ||||||
| S3 | 2.3445(46) | 112.18(17) | S4 | 2.3291(49) | 117.49(24) | ||||
| S4 | 2.3755(49) | 113.98(17) | 111.24(22) | S3 | 2.3929(57) | 113.29(26) | 111.59(26) | ||
| S5 | 2.4677(47) | 109.14(22) | 101.95(15) | 107.54(15) | S1 | 2.5911(105) | 104.16(30) | 102.46(28) | 106.27(26) |
| Average | 2.3677 | 109.34 | Average | 2.4066 | 109.21 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Z.; Shen, C.; Lu, A.; Gu, X.; Liu, Z. The Crystal Structure of Bornite Cu5FeS4: Ordered Fe and Split Cu. Crystals 2021, 11, 1495. https://doi.org/10.3390/cryst11121495
Shu Z, Shen C, Lu A, Gu X, Liu Z. The Crystal Structure of Bornite Cu5FeS4: Ordered Fe and Split Cu. Crystals. 2021; 11(12):1495. https://doi.org/10.3390/cryst11121495
Chicago/Turabian StyleShu, Zhengxiang, Can Shen, Anhuai Lu, Xiangping Gu, and Zhongfa Liu. 2021. "The Crystal Structure of Bornite Cu5FeS4: Ordered Fe and Split Cu" Crystals 11, no. 12: 1495. https://doi.org/10.3390/cryst11121495







