Synthesis and Characterization of Ammonium Potassium Tellurium Polyoxomolybdate: (NH4)2K2TeMo6O22·2H2O with One-Dimensional Anionic Polymeric Chain [TeMo6O22]4−
Abstract
1. Introduction
2. Materials and Methods
2.1. Properties Characterization
2.2. Synthesis
2.3. Crystal Structure Determination
3. Results and Discussion
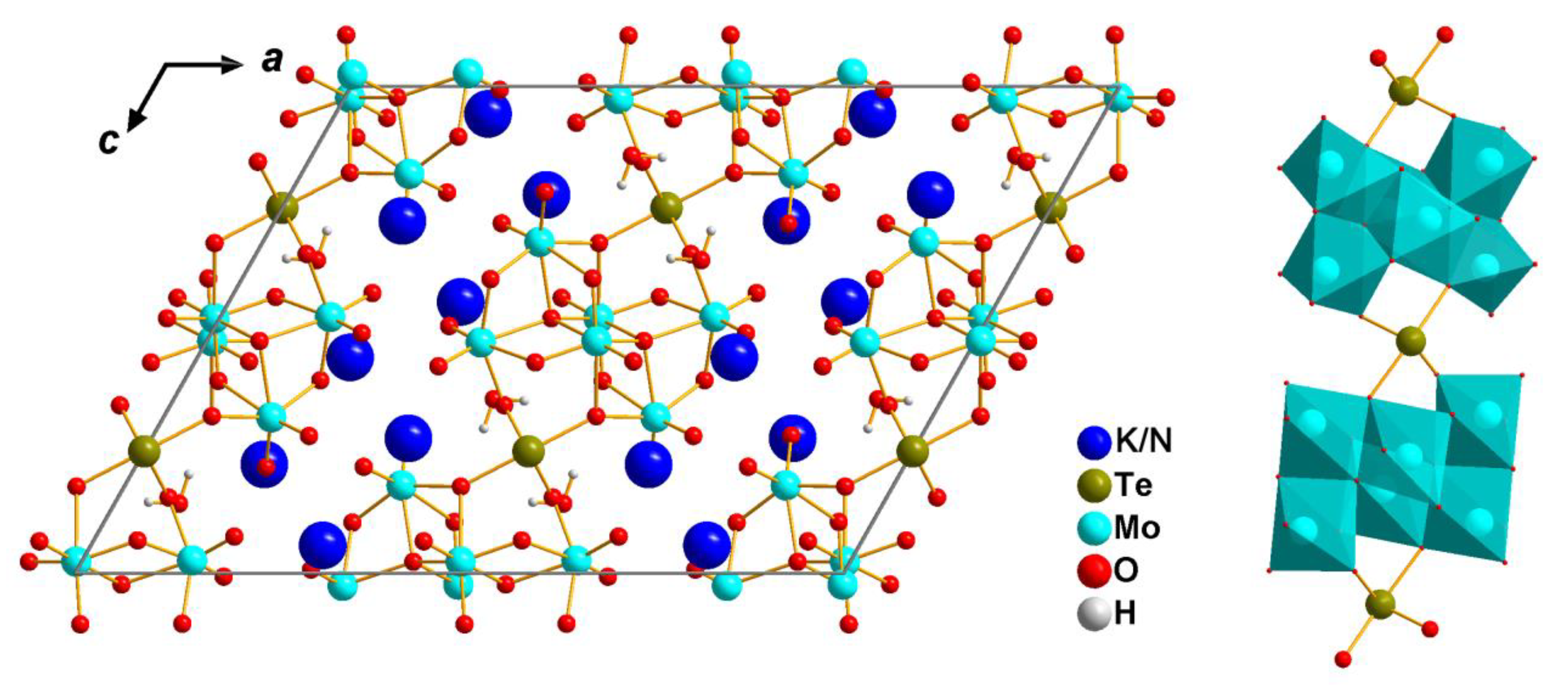
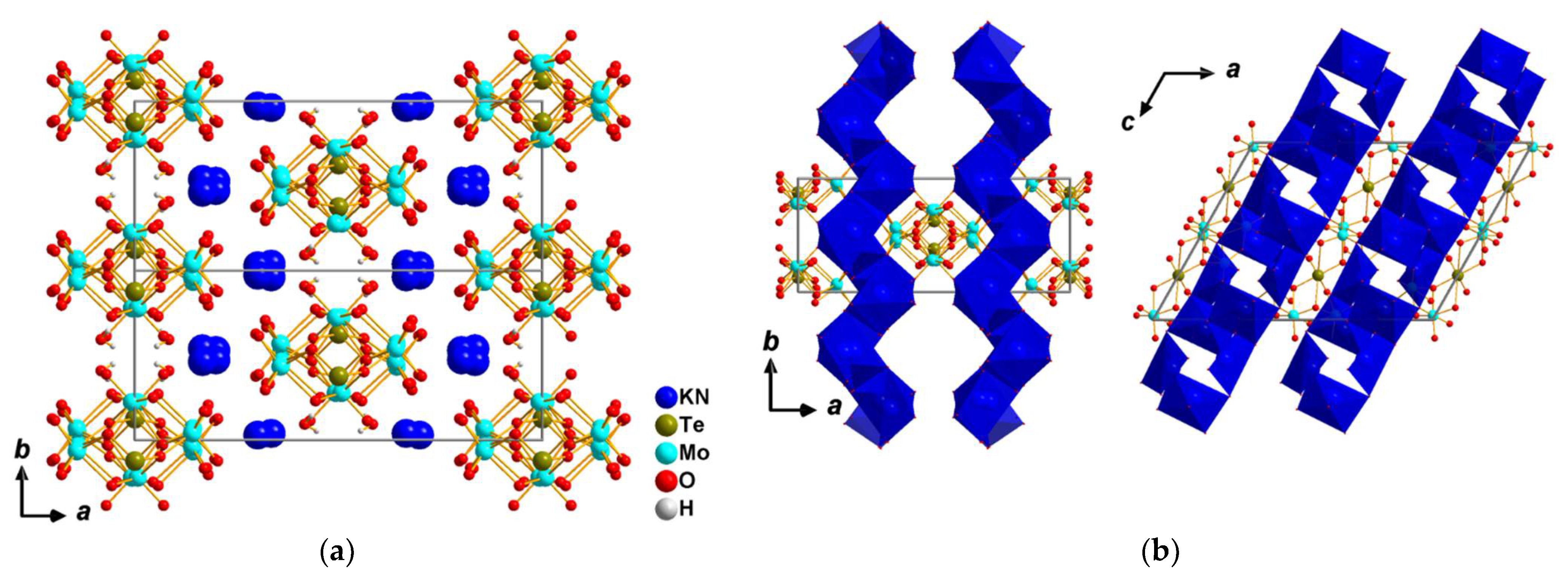
3.1. Crystal Structure
3.2. Dipole Moments and Local Distortion
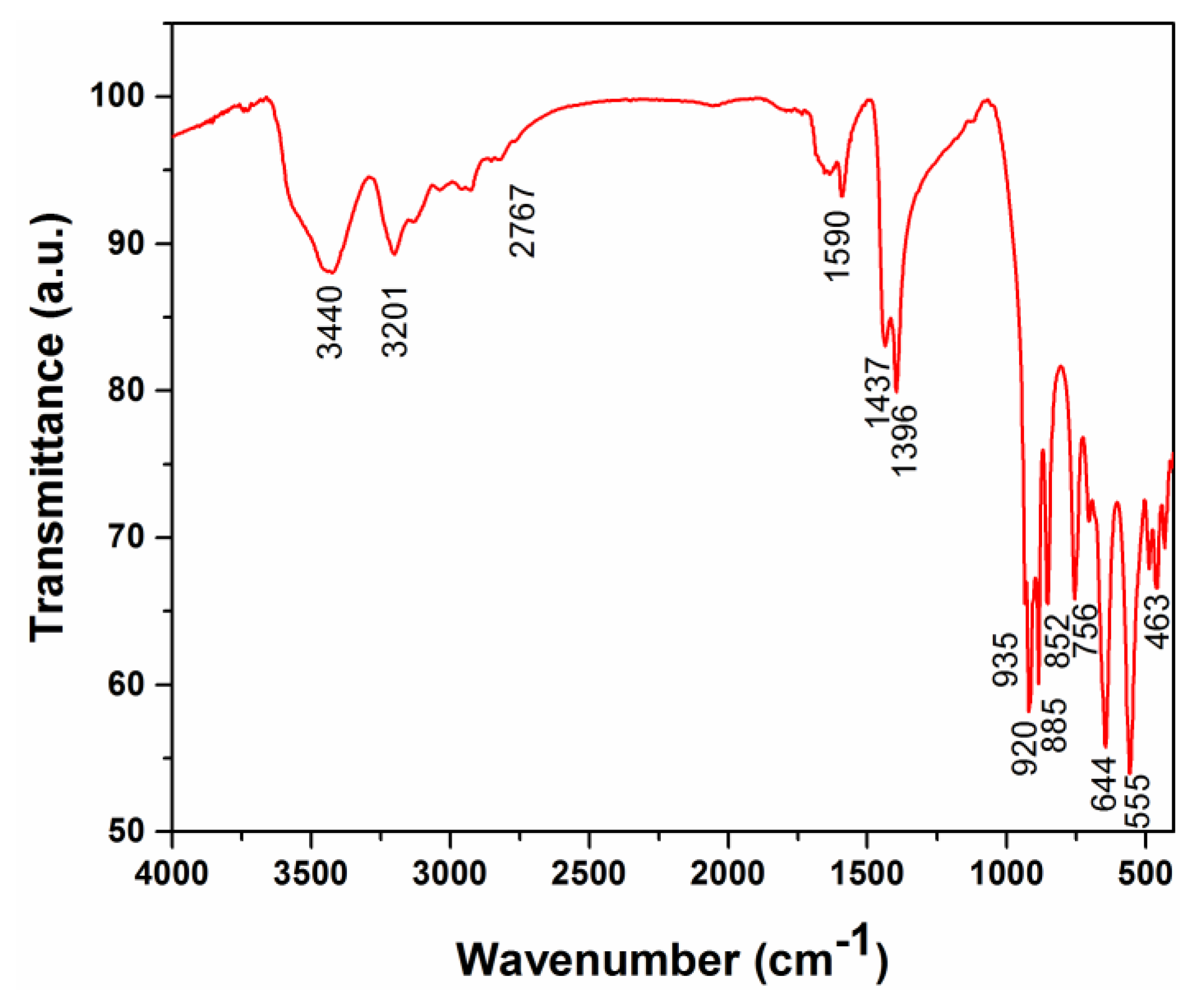
3.3. Thermal Stability
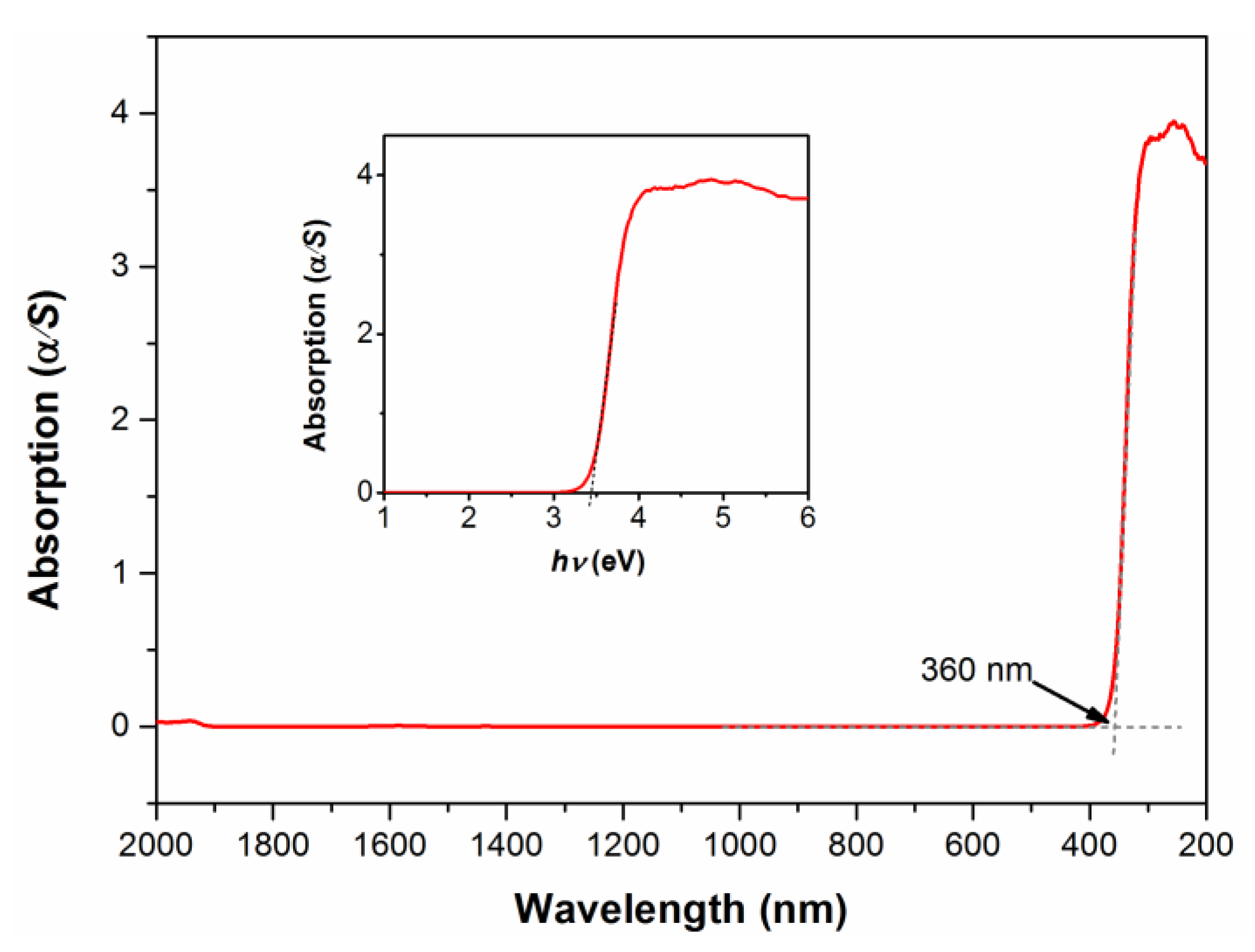
3.4. Optical Absorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodey, J.; Broussard, J.; Halasyamani, P.S. Synthesis, structure, and characterization of a new second-harmonic-generating tellurite: Na2TeW2O9. Chem. Mater. 2002, 14, 3174–3180. [Google Scholar] [CrossRef]
- Hubbard, D.J.; Johnston, A.R.; Casalongue, H.S.; Sarjeant, A.N.; Norquist, A.J. Synthetic Approaches for Noncentrosymmetric Molybdates. Inorg. Chem. 2008, 47, 8518–8525. [Google Scholar] [CrossRef] [PubMed]
- Kongmark, C.; Martis, V.; Pirovano, C.; Lofberg, A.; van Beek, W.; Sankar, G.; Rubbens, A.; Cristol, S.; Vannier, R.N.; Bordes-Richard, E. Synthesis of gamma-Bi2MoO6 catalyst studied by combined high-resolution powder diffraction, XANES and Raman spectroscopy. Catal. Today 2010, 157, 257–262. [Google Scholar] [CrossRef]
- Obregon, S.; Hernandez-Uresti, D.B.; Vazquez, A.; Sanchez-Martinez, D. Electrophoretic deposition of PbMoO4 nanoparticles for photocatalytic degradation of tetracycline. Appl. Surf. Sci. 2018, 457, 501–507. [Google Scholar] [CrossRef]
- Nasri, R.; Larbi, T.; Amlouk, M.; Zid, M.F. Investigation of the physical properties of K2Co2(MoO4)(3) for photocatalytic application. J. Mater. Sci. Mater. Electron. 2018, 29, 18372–18379. [Google Scholar] [CrossRef]
- Yong, Y.; Yang, S.T.; Zhao, Z.G. A smart cluster paradigm based Mo-containing polyoxometalate as a new therapeutic strategy for tumor-specific photothermal therapy. Sci. Bull. 2018, 63, 877–878. [Google Scholar] [CrossRef]
- Dianat, S.; Bordbar, A.K.; Tangestaninejad, S.; Zarkesh-Esfahani, S.H.; Habibi, P.; Kajani, A.A. ctDNA interaction of Co-containing Keggin polyoxomolybdate and in vitro antitumor activity of free and its nano-encapsulated derivatives. J. Iran. Chem. Soc. 2016, 13, 1895–1904. [Google Scholar] [CrossRef]
- Croce, M.; Conti, S.; Maake, C.; Patzke, G.R. Nanocomposites of Polyoxometalates and Chitosan-Based Polymers as Tuneable Anticancer Agents. Eur. J. Inorg. Chem. 2019, 3–4, 348–356. [Google Scholar] [CrossRef]
- Chen, Z.; Loo, B.H.; Ma, Y.; Cao, Y.; Ibrahim, A.; Yao, J. Photochromism of Novel Molybdate/Alkylamine Composite Thin Films. ChemPhysChem 2004, 5, 1020–1026. [Google Scholar] [CrossRef]
- Lu, J.K.; Zhang, X.; Ma, P.T.; Singh, V.; Zhang, C.; Niu, J.Y.; Wang, J.P. Photochromic behavior of a new polyoxomolybdate/alkylamine composite in solid state. J. Mater. Sci. 2018, 53, 3078–3086. [Google Scholar] [CrossRef]
- Chi, E.O.; Ok, K.M.; Porter, Y.; Halasyamani, P.S. Na2Te3Mo3O16: A new molybdenum tellurite with second-harmonic generating and pyroelectric properties. Chem. Mater. 2006, 18, 2070–2074. [Google Scholar] [CrossRef]
- Mao, J.G.; Jiang, H.L.; Kong, F. Structures and properties of functional metal selenites and tellurites. Inorg. Chem. 2008, 47, 8498–8510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, C.-L.; Hu, T.; Kong, F.; Mao, J.-G. Explorations of new second-order NLO materials in the AgI-MoVI/WVI-TeIV-O systems. Dalton Trans. 2009, 29, 5747–5754. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.D.; Kim, S.-H.; Halasyamani, P.S. Synthesis, Characterization, and Structure–Property Relationships in Two New Polar Oxides: Zn2(MoO4)(SeO3) and Zn2(MoO4)(TeO3). Inorg. Chem. 2011, 50, 5215–5222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Sun, Y.; Zhang, C.; Zhang, S.; Liu, Y.; Tao, X. MgTeMoO6: A neutral layered material showing strong second-harmonic generation. J. Mater. Chem. 2012, 22, 9921–9927. [Google Scholar] [CrossRef]
- Feng, Y.; Fan, H.; Zhong, Z.; Wang, H.; Qiu, D. Cd3(MoO4)(TeO3)2: A Polar 3D Compound Containing d10–d0 SCALP-Effect Cations. Inorg. Chem. 2016, 55, 11987–11992. [Google Scholar] [CrossRef]
- Evans, H.T. The Crystal Structures of Ammonium and Potassium Molybdotellurates. J. Am. Chem. Soc. 1948, 70, 1291–1292. [Google Scholar] [CrossRef]
- Balraj, V.; Vidyasagar, K. Low-Temperature Syntheses and Characterization of Novel Layered Tellurites, A2Mo3TeO12 (A = NH4, Cs), and “Zero-Dimensional” Tellurites, A4Mo6Te2O24·6H2O (A = Rb, K). Inorg. Chem. 1998, 37, 4764–4774. [Google Scholar] [CrossRef]
- Evans, H.T. Refined molecular structure of the heptamolybdate and hexamolybdotellurate ions. J. Am. Chem. Soc. 1968, 90, 3275–3276. [Google Scholar] [CrossRef]
- Blazevic, A.; Rompel, A. The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Saito, A. Systematics of some properties of hexamolybdotellurate(VI) salt hydrates with di- and trivalent metals, Mx[TeMo6O24]·nH2O. Inorg. Chim. Acta 1994, 217, 93–99. [Google Scholar] [CrossRef]
- Ratheesh, R.; Suresh, G.; Nayar, V.U. Infrared and Polarized Raman Spectra of M6[TeMo6O24] · 7H2O[M = K, NH4] and (NH4)6[TeMo6O24] · Te(OH)6 · 7H2O Single Crystals. J. Solid State Chem. 1995, 118, 341–356. [Google Scholar] [CrossRef]
- Cruywagen, J.J. Protonation, Oligomerization, and Condensation Reactions of Vanadate(V), Molybdate(vi), and Tungstate(vi). In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 49, pp. 127–182. [Google Scholar]
- Ma, H.-Y.; Wu, L.-Z.; Pang, H.-J.; Meng, X.; Peng, J. Hydrothermal synthesis of two Anderson POM-supported transition metal organic–inorganic compounds. J. Mol. Struct. 2010, 967, 15–19. [Google Scholar] [CrossRef]
- Zhang, P.; Singh, V.; Jia, J.; Zhang, D.; Ma, P.; Wang, J.; Niu, J. Organometallic functionalized non-classical polyoxometalates: Synthesis, characterization and electrochemical properties. Dalton Trans. 2018, 47, 9317–9323. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Lin, H.; Chang, Z.; Wang, X.; Liu, G. A series of Anderson-type polyoxometalate-based metal–organic complexes: Their pH-dependent electrochemical behaviour, and as electrocatalysts and photocatalysts. Dalton Trans. 2016, 45, 12465–12478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Lin, H.; Chang, Z.; Tian, A.; Liu, G.; Wang, X. Novel Anderson-type [TeMo6O24]6−-based metal–organic complexes tuned by different species and their coordination modes: Assembly, various architectures and properties. Dalton Trans. 2016, 45, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Rompel, A. Ten Good Reasons for the Use of the Tellurium-Centered Anderson–Evans Polyoxotungstate in Protein Crystallography. Acc. Chem. Res. 2017, 50, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Zhu, Z.; Sun, L.; Su, T.; Liao, W.; Deng, C.; Zhao, Y.; Ren, W.; Lü, H. Construction of biomimetic catalysis system coupling polyoxometalates with deep eutectic solvents for selective aerobic oxidation desulfurization. Appl. Catal. B Environ. 2019, 259, 118089. [Google Scholar] [CrossRef]
- Tewari, S.; Adnan, M.; Kumar, V.; Jangra, G.; Prakash, G.V.; Ramanan, A. Photoluminescence Properties of Two Closely Related Isostructural Series Based on Anderson-Evans Cluster Coordinated with Lanthanides [Ln(H2O)7{X(OH)6Mo6O18}]•yH2O, X = Al, Cr. Front. Chem. 2019, 6, 631. [Google Scholar] [CrossRef]
- Drewes, D.; Limanski, E.M.; Krebs, B. A series of novel lanthanide polyoxometalates: Condensation of building blocks dependent on the nature of rare earth cations. Dalton Trans. 2004, 14, 2087–2091. [Google Scholar] [CrossRef]
- Drewes, D.; Krebs, B. Synthesis and Structure of a Novel Type of Polyoxomolybdate Lanthanide Complex: [(Ln(H2O)6)2(TeMo6O24)] (Ln = Ho, Yb). Z. Anorg. Allg. Chem. 2005, 631, 2591–2594. [Google Scholar] [CrossRef]
- Yu, M.; Gao, B.; Liang, D. 1D Chain-Like Architecture in Anderson Heteropolymolybdate {[Eu(H2O)6]2(TeMo6O24)}·6H2O: Synthesis and Characterization. J. Cluster Sci. 2014, 25, 377–385. [Google Scholar] [CrossRef]
- Balraj, V.; Vidyasagar, K. Hydrothermal Synthesis and Characterization of Novel One-Dimensional Tellurites of Molybdenum(VI), A4Mo6TeO22·2H2O (A = NH4, Rb). Inorg. Chem. 1999, 38, 1394–1400. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Wendlandt, W.W.; Hecht, H.G. Reflectance Spectroscopy; Interscience: New York, NY, USA, 1966. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Pinlac, R.A.F.; Stern, C.L.; Poeppelmeier, K.R. New Layered Oxide-Fluoride Perovskites: KNaNbOF5 and KNaMO2F4 (M = Mo6+, W6+). Crystals 2011, 1, 3–14. [Google Scholar] [CrossRef]
- Zang, X.-S.; Tan, H.-Q.; Wu, Q.; Li, Y.; Li, Y.-G.; Wang, E.-B. The synthesis and characterization of a new butterfly-like polyoxometalate: K5Na2[Mo9V3O38]·9H2O. Inorg. Chem. Commun. 2010, 13, 471–474. [Google Scholar] [CrossRef]
- Khal’baeva, K.M.; Solodovnikov, S.F.; Khaikina, E.G.; Kadyrova, Y.M.; Solodovnikova, Z.A.; Basovich, O.M. Phase formation features in the systems M2MoO4–Fe2(MoO4)3 (M=Rb, Cs) and crystal structures of new double polymolybdates M3FeMo4O15. J. Solid State Chem. 2010, 183, 712–719. [Google Scholar] [CrossRef]
- Lü, M.; Jo, H.; Oh, S.-J.; Lee, S.; Choi, K.-Y.; Yu, Y.; Ok, K.M. Syntheses, Structures, and Characterization of Quaternary Tellurites, Li3MTe4O11 (M = Al, Ga, and Fe). Inorg. Chem. 2017, 56, 5873–5879. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.-L.; Ma, Y.-X.; Hu, C.-L.; Kong, F.; Mao, J.-G. A(VO2F)(SeO3) (A = Sr, Ba) and Ba(MOF2)(TeO4) (M = Mo, W): First examples of alkali-earth selenites/tellurites with a fluorinated d0-TM octahedron. Dalton Trans. 2018, 47, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Feger, C.R.; Schimek, G.L.; Kolis, J.W. Hydrothermal Synthesis and Characterization of M2Te3O8(M=Mn, Co, Ni, Cu, Zn): A Series of Compounds with the Spiroffite Structure. J. Solid State Chem. 1999, 143, 246–253. [Google Scholar] [CrossRef]
- Feger, C.R.; Kolis, J.W. Synthesis and Characterization of Two New Copper Tellurites, Ba2Cu4Te4O11Cl4 and BaCu2Te2O6Cl2, in Supercritical H2O. Inorg. Chem. 1998, 37, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal-Structure Database. Acta Crystallogr. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Brese, N.E.; O’keeffe, M. Bond-Valence Parameters for Solids. Acta Crystallogr. Sect. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Maggard, P.A.; Nault, T.S.; Stern, C.L.; Poeppelmeier, K.R. Alignment of acentric MoO3F33− anions in a polar material: (Ag3MoO3F3)(Ag3MoO4)Cl. J. Solid State Chem. 2003, 175, 27–33. [Google Scholar] [CrossRef]
- Ok, K.M.; Halasyamani, P.S. Mixed-Metal Tellurites: Synthesis, Structure, and Characterization of Na1.4Nb3Te4.9O18 and NaNb3Te4O16. Inorg. Chem. 2005, 44, 3919–3925. [Google Scholar] [CrossRef]
- Galy, J.; Meunier, G.; Andersson, S.; Åström, A. Stéréochimie des eléments comportant des paires non liées: Ge (II), As (III), Se (IV), Br (V), Sn (II), Sb (III), Te (IV), I (V), Xe (VI), Tl (I), Pb (II), et Bi (III) (oxydes, fluorures et oxyfluorures). J. Solid State Chem. 1975, 13, 142–159. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sivakumar, T.; Ok, K.M.; Halasyamani, P.S. Polar Hexagonal Tungsten Bronze-Type Oxides: KNbW2O9, RbNbW2O9, and KTaW2O9. Inorg. Chem. 2008, 47, 8511–8517. [Google Scholar] [CrossRef]
- Halasyamani, P.S. Asymmetric cation coordination in oxide materials: Influence of lone-pair cations on the intra-octahedral distortion in d(0) transition metals. Chem. Mater. 2004, 16, 3586–3592. [Google Scholar] [CrossRef]
- Gatehouse, B.M.; Leverett, P. Crystal structure of potassium tetramolybdate, K2Mo4O13, and its relationship to the structures of other univalent metal polymolybdates. J. Chem. Soc. A 1971, 2107–2112. [Google Scholar] [CrossRef]
- Forestier, P.; Goreaud, M. Structure cristalline de l’oxyde a valence mixte TeMo5O16 orthorombique. C. R. Hebd. Seances l’Acad. Sci. 1991, 312, 1141–1145. [Google Scholar]
- Ok, K.M.; Halasyamani, P.S. New d0 Transition Metal Iodates: Synthesis, Structure, and Characterization of BaTi(IO3)6, LaTiO(IO3)5, Ba2VO2(IO3)4·(IO3), K2MoO2(IO3)4, and BaMoO2(IO3)4·H2O. Inorg. Chem. 2005, 44, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-P.; Hu, C.-L.; Xu, X.; Sun, C.-F.; Zhang, J.-H.; Mao, J.-G. NaVO2(IO3)2(H2O): A Unique Layered Material Produces A Very Strong SHG Response. Chem. Mater. 2010, 22, 1545–1550. [Google Scholar] [CrossRef]
| Formula | (NH4)2K2TeMo6O22·2H2O |
| F.W. | 1197.49 |
| Temperature (K) | 296(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a (Å) | 21.172(2) |
| b (Å) | 7.660(0) |
| c (Å) | 15.419(1) |
| β (deg.) | 119.545(3) |
| V (Å3), Z | 2175.5(3), 4 |
| Calculated density (g/mm3) | 3.656 |
| F (000) | 2208 |
| Data collection range θ (deg.) | 2.211 to 27.610 |
| Limiting indices | −27 ≤ h ≤ 26 |
| −9 ≤ k ≤ 8 | |
| −20 ≤ l ≤ 20 | |
| Reflections collected/unique, Rint | 9472/2521, 0.0219 |
| Completeness to θ = 25.242 | 99.8% |
| GOF a on F2 | 1.053 |
| R1, wR2 (I > 2σ (I)] b | 0.0180, 0.0485 |
| R1, wR2 (all data) | 0.0189, 0.0489 |
| Largest diff. peak and hole (eA−3) | 0.621 and −0.693 |
| Atoms | Bond Lengths | Atoms | Bond Lengths |
|---|---|---|---|
| K(1)–O(10)#1 | 2.843(3) | Te(1)–O(5) | 1.878(2) |
| K(1)–O(4)#2 | 2.855(3) | Te(1)–O(3)#9 | 2.113(2) |
| K(1)–O(9)#3 | 2.868(3) | Te(1)–O(3)#3 | 2.113(2) |
| K(1)–O(12)#2 | 2.879(4) | Mo(1)–O(9) | 1.704(3) |
| K(1)–O(10) | 2.925(3) | Mo(1)–O(11) | 1.711(3) |
| K(1)–O(6)#4 | 2.958(3) | Mo(1)–O(3) | 1.944(2) |
| K(1)–O(7) | 3.029(3) | Mo(1)–O(6) | 1.956(2) |
| K(1)–O(7)#4 | 3.103(3) | Mo(1)–O(1)#9 | 2.189(2) |
| K(1)–O(11)#3 | 3.252(3) | Mo(1)–O(8) | 2.392(3) |
| K(1)–O(2)#4 | 3.300(3) | Mo(2)–O(4) | 1.719(3) |
| K(2)–O(12)#2 | 2.770(4) | Mo(2)–O(8) | 1.745(2) |
| K(2)–O(9)#5 | 2.829(4) | Mo(2)–O(2) | 1.814(2) |
| K(2)–O(7)#4 | 2.912(3) | Mo(2)–O(1) | 1.979(2) |
| K(2)–O(4)#6 | 2.922(3) | Mo(2)–O(3)#9 | 2.274(2) |
| K(2)–O(11)#7 | 2.968(3) | Mo(2)–O(1)#9 | 2.374(2) |
| K(2)–O(11) | 3.012(3) | Mo(3)–O(10) | 1.703(3) |
| K(2)–O(6) | 3.073(3) | Mo(3)–O(7) | 1.706(3) |
| K(2)–O(10) | 3.373(3) | Mo(3)–O(6) | 1.923(2) |
| K(2)–O(3)#7 | 3.401(3) | Mo(3)–O(5) | 2.008(2) |
| K(2)–O(8)#5 | 3.426(3) | Mo(3)–O(1)#9 | 2.183(2) |
| Te(1)–O(5)#8 | 1.878(2) | Mo(3)–O(2) | 2.234(2) |
| Units | a | b | c | Total |
|---|---|---|---|---|
| Te1–O | 0.000 | 9.610 | 0.000 | 9.610 |
| Mo1–O | 6.050 | −2.245 | 7.013 | 6.999 |
| Mo2–O | −1.693 | 8.539 | 2.490 | 9.281 |
| Mo3–O | 5.696 | −1.596 | −0.052 | 5.940 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, L.; Wang, Y. Synthesis and Characterization of Ammonium Potassium Tellurium Polyoxomolybdate: (NH4)2K2TeMo6O22·2H2O with One-Dimensional Anionic Polymeric Chain [TeMo6O22]4−. Crystals 2021, 11, 375. https://doi.org/10.3390/cryst11040375
Geng L, Wang Y. Synthesis and Characterization of Ammonium Potassium Tellurium Polyoxomolybdate: (NH4)2K2TeMo6O22·2H2O with One-Dimensional Anionic Polymeric Chain [TeMo6O22]4−. Crystals. 2021; 11(4):375. https://doi.org/10.3390/cryst11040375
Chicago/Turabian StyleGeng, Lei, and Yunjian Wang. 2021. "Synthesis and Characterization of Ammonium Potassium Tellurium Polyoxomolybdate: (NH4)2K2TeMo6O22·2H2O with One-Dimensional Anionic Polymeric Chain [TeMo6O22]4−" Crystals 11, no. 4: 375. https://doi.org/10.3390/cryst11040375
APA StyleGeng, L., & Wang, Y. (2021). Synthesis and Characterization of Ammonium Potassium Tellurium Polyoxomolybdate: (NH4)2K2TeMo6O22·2H2O with One-Dimensional Anionic Polymeric Chain [TeMo6O22]4−. Crystals, 11(4), 375. https://doi.org/10.3390/cryst11040375






