Biosynthesis of CeO2 Nanoparticles Using Egg White and Their Antibacterial and Antibiofilm Properties on Clinical Isolates
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis Method for the Preparation of CeO2 Nanoparticles
2.2. Characterizations
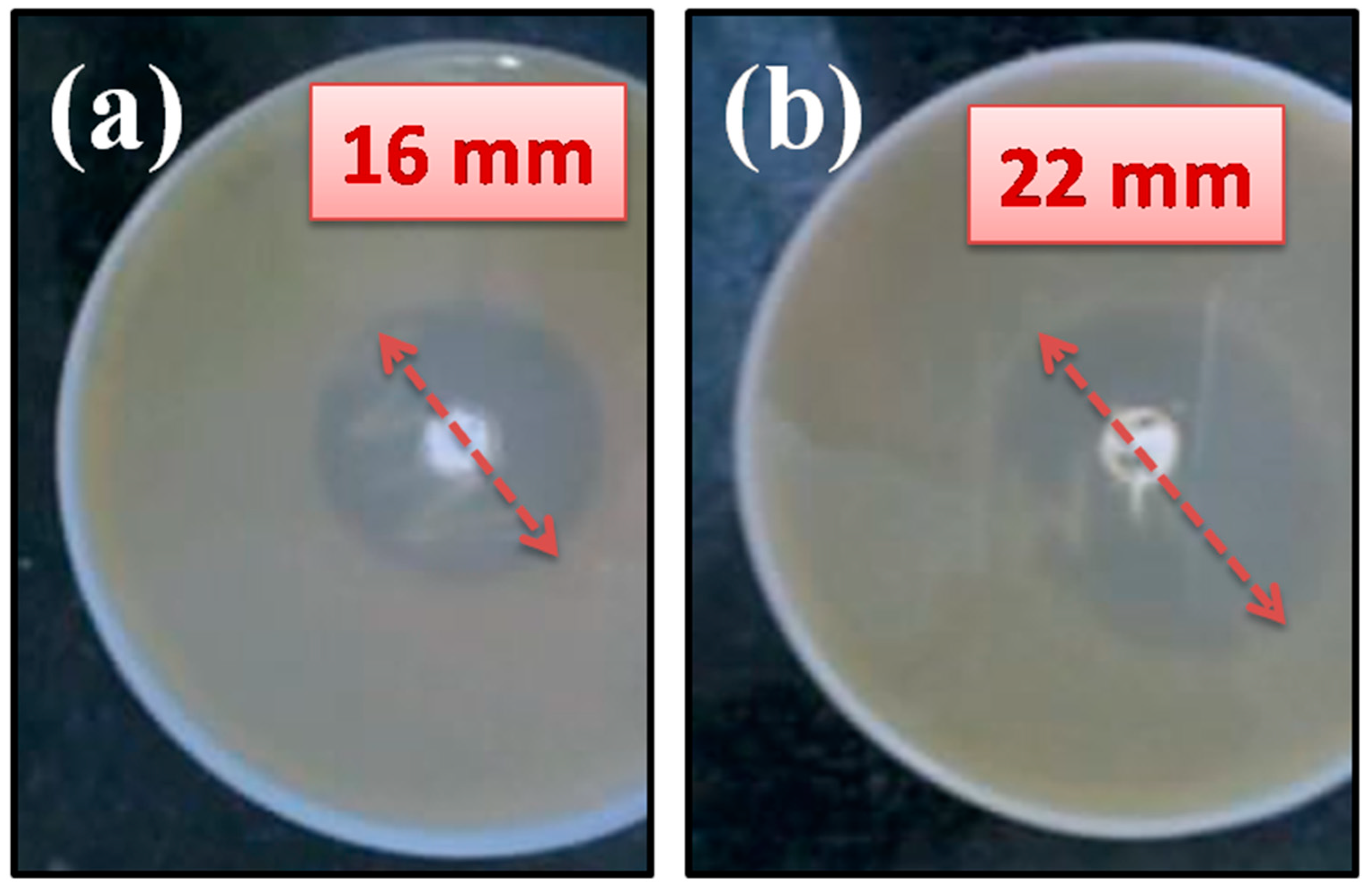
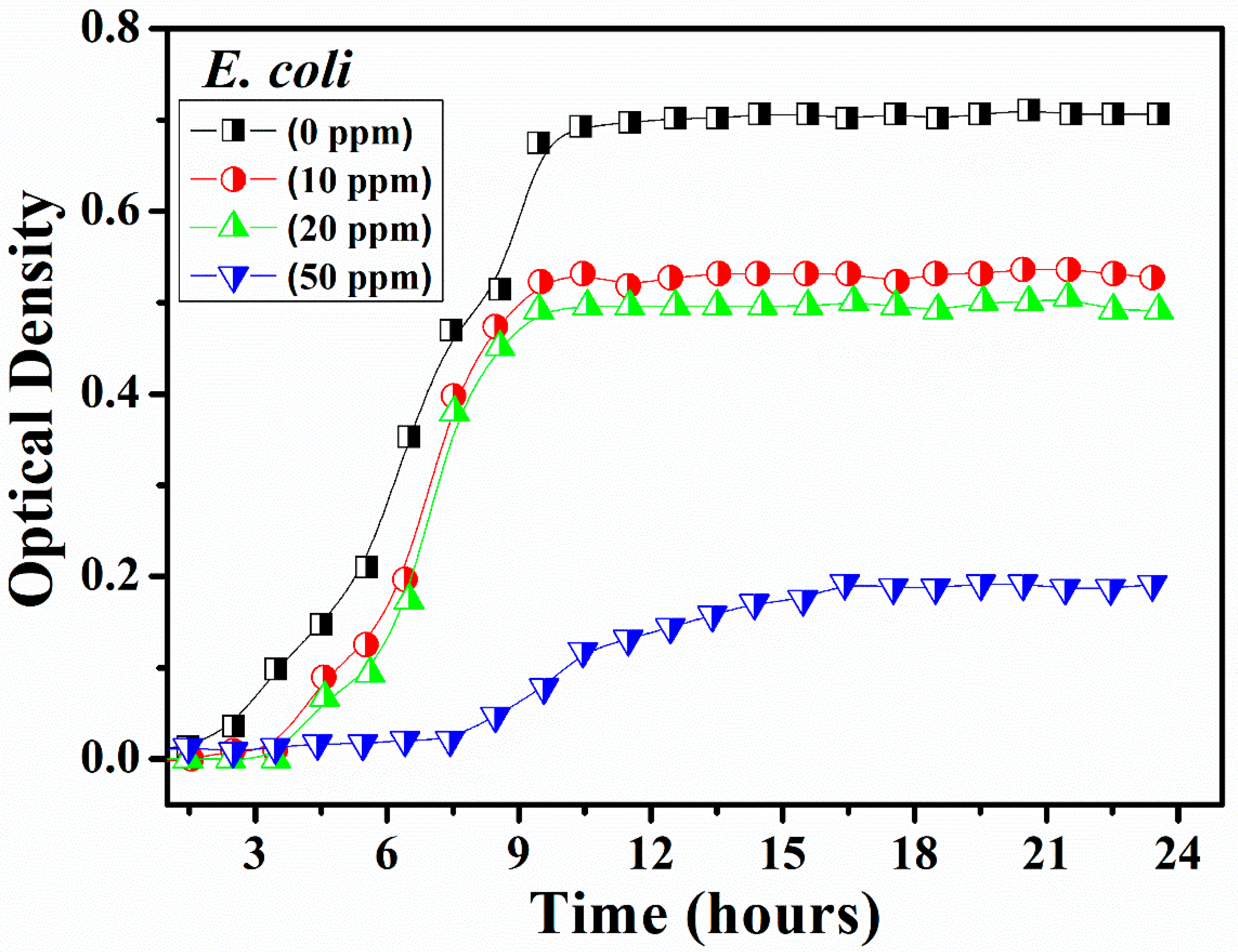
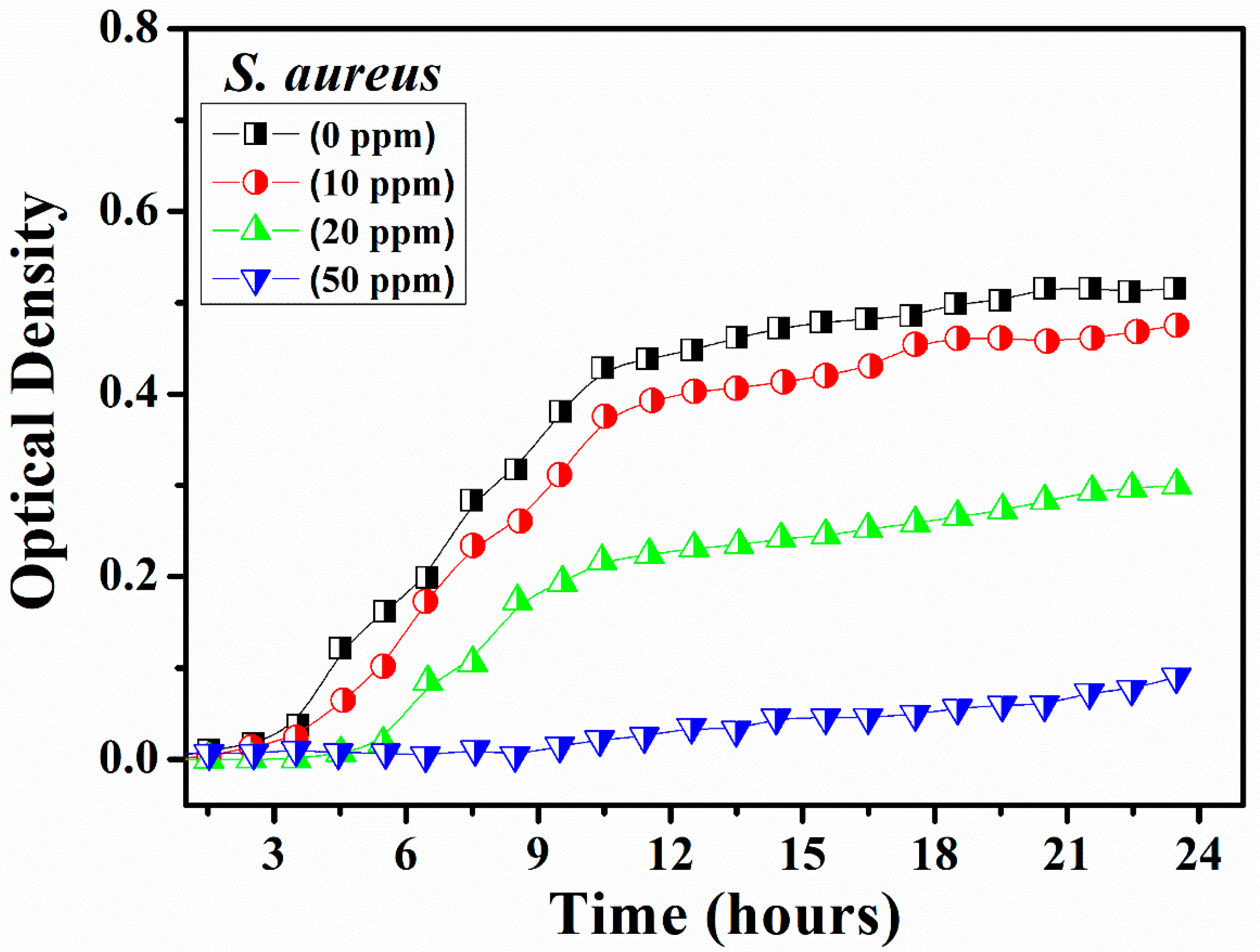
2.3. Antibacterial Assessment
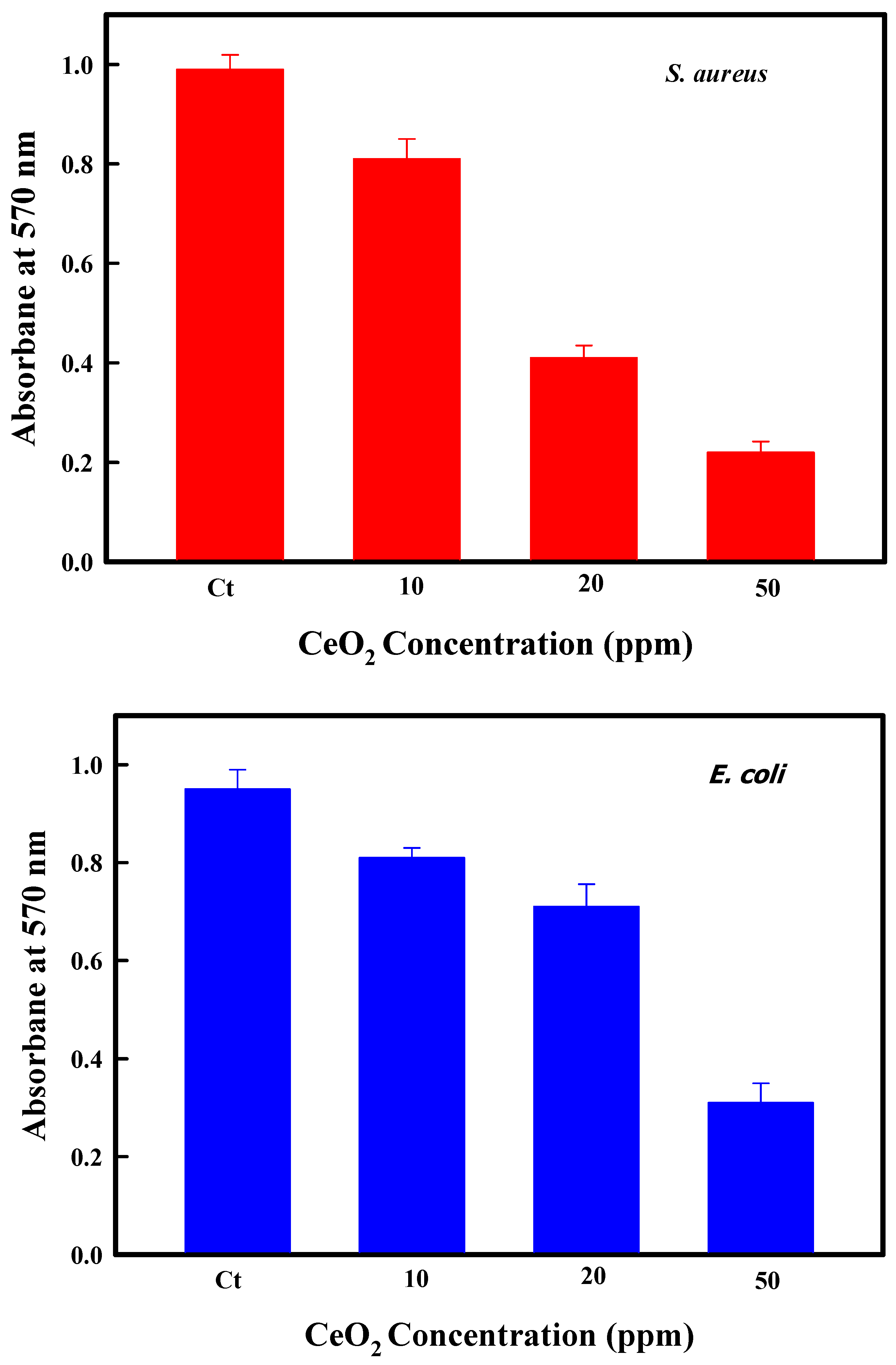
2.4. Antibiofilm Assessment
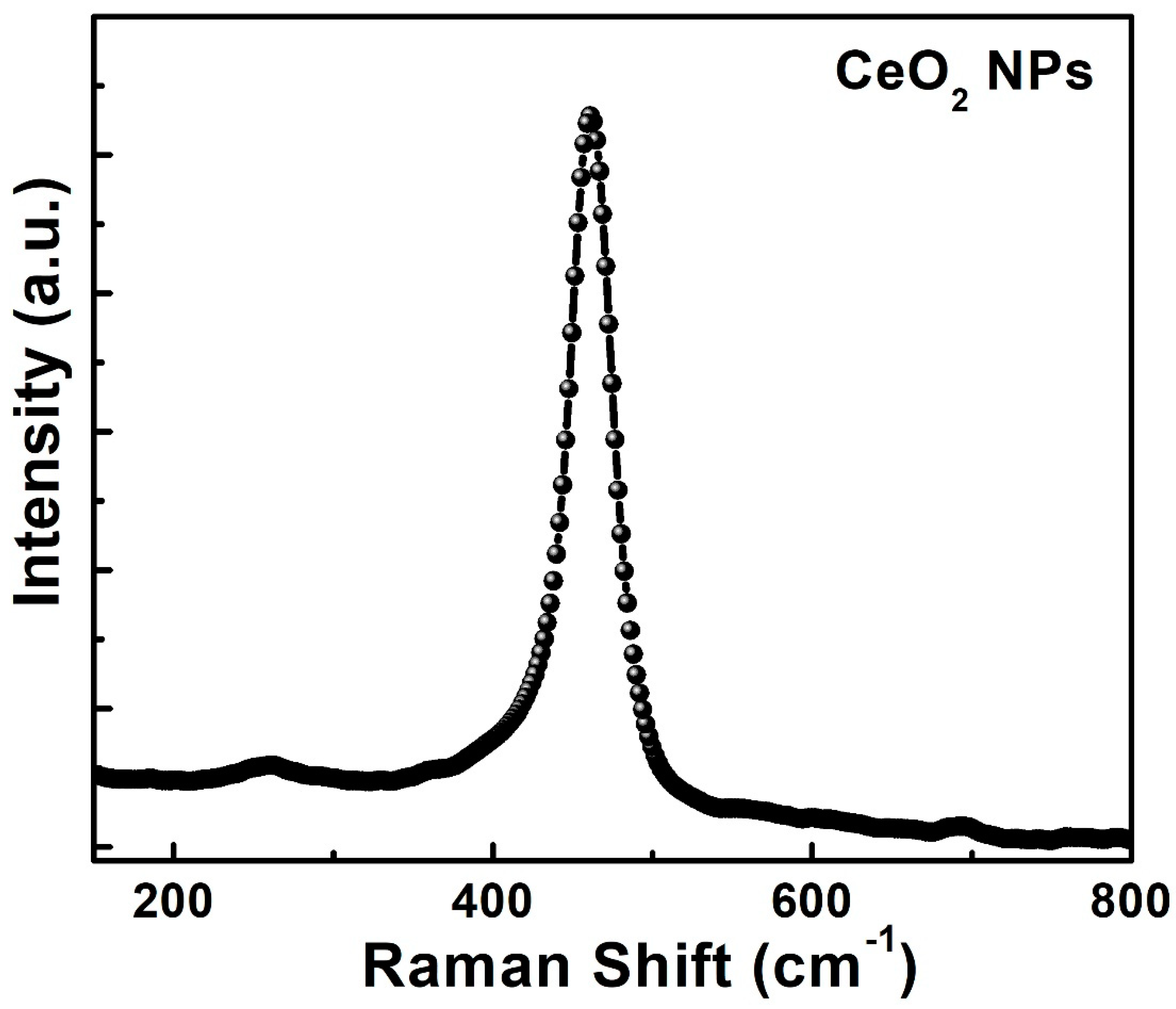
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alpaslan, E.; Geilich, B.M.; Yazici, H.; Webster, T.J. PH-Controlled Cerium Oxide Nanoparticle Inhibition of Both Gram-Positive and Gram-Negative Bacteria Growth. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Antibacterial natural products in medicinal chemistry—Exodus or revival? Angew. Chem.-Int. Ed. 2006, 45, 5072–5129. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Bera, P.; Gayen, A.; Hegde, M.S.; Lalla, N.P.; Spadaro, L.; Frusteri, F.; Arena, F. Promoting effect of CeO2 in combustion synthesized Pt/CeO2 catalyst for CO oxidation. J. Phys. Chem. B 2003, 107, 6122–6130. [Google Scholar] [CrossRef]
- Jacobs, G.; Williams, L.; Graham, U.; Sparks, D.; Davis, B.H. Low-Temperature Water-Gas Shift: In-Situ DRIFTS-Reaction Study of a Pt/CeO2 Catalyst for Fuel Cell Reformer Applications. J. Phys. Chem. B 2003, 107, 10398–10404. [Google Scholar] [CrossRef]
- Li, R.; Yabe, S.; Yamashita, M.; Momose, S.; Yoshida, S.; Yin, S.; Sato, T. Synthesis and UV-shielding properties of ZnO- and CaO-doped CeO2 via soft solution chemical process. Solid State Ion. 2002, 151, 235–241. [Google Scholar] [CrossRef]
- Sohlberg, K.; Pantelides, S.T.; Pennycook, S.J. Interactions of hydrogen with CeO2. J. Am. Chem. Soc. 2001, 123, 6609–6611. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, P.; Suzuki, T.; Anderson, H.U. Nanocrystalline undoped ceria oxygen sensor. Sens. Actuators B Chem. 2003, 95, 73–77. [Google Scholar] [CrossRef]
- Goubin, F.; Rocquefelte, X.; Whangbo, M.; Montardi, Y.; Brec, R.; Jobic, S. Experimental and Theoretical Characterization of the. Chem. Mater. 2004, 16, 662–669. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Caruso, R.A. Template synthesis and photocatalytic properties of porous metal oxide spheres formed by nanoparticle infiltration. Chem. Mater. 2004, 16, 2287–2292. [Google Scholar] [CrossRef]
- Soren, S.; Jena, S.R.; Samanta, L.; Parhi, P. Antioxidant Potential and Toxicity Study of the Cerium Oxide Nanoparticles Synthesized by Microwave-Mediated Synthesis. Appl. Biochem. Biotechnol. 2015, 177, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.M.; Asati, A.; Nath, S.; Kaittanis, C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 2008, 4, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Tarnuzzer, R.W.; Colon, J.; Patil, S.; Seal, S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005, 5, 2573–2577. [Google Scholar] [CrossRef] [PubMed]
- Alifanti, M.; Baps, B.; Blangenois, N.; Naud, J.; Grange, P.; Delmon, B. Characterization of CeO2-ZrO2 mixed oxides. Comparison of the citrate and sol-gel preparation methods. Chem. Mater. 2003, 15, 395–403. [Google Scholar] [CrossRef]
- Laberty-Robert, C.; Long, J.W.; Lucas, E.M.; Pettigrew, K.A.; Stroud, R.M.; Doescher, M.S.; Rolison, D.R. Sol-gel-derived ceria nanoarchitectures: Synthesis, characterization, and electrical properties. Chem. Mater. 2006, 18, 50–58. [Google Scholar] [CrossRef]
- Hirano, M.; Inagaki, M. Preparation of monodispersed cerium. J. Mater. Chem. 2000, 10, 473–477. [Google Scholar] [CrossRef]
- Hirano, M.; Fukuda, Y.; Iwata, H.; Hotta, Y.; Inagaki, M. Preparation and Spherical Agglomeration of Crystalline Cerium(IV) Oxide Nanoparticles by Thermal Hydrolysis. J. Am. Ceram. Soc. 2004, 83, 1287–1289. [Google Scholar] [CrossRef]
- Zhou, Y.; Rahaman, M.N. Effect of redox reaction on the sintering behavior of cerium oxide. Acta Mater. 1997, 45, 3635–3639. [Google Scholar] [CrossRef]
- Gopal, C.B.; Garcıa, M.; Lee, S.C.; Shi, Y.; Shavorskiy, A.; Monti, M.; Guan, Z.; Sinclair, R.; Bluhm, H.; Vojvodic, A.; et al. Equilibrium oxygen storage capacity of ultrathin CeO2-δ depends non-monotonically on large biaxial strain. Nat. Commun. 2017, 8, 15360. [Google Scholar] [CrossRef]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Flame-made ceria nanoparticles. J. Mater. Res. 2002, 17, 1356–1362. [Google Scholar] [CrossRef] [Green Version]
- Masui, T.; Fujiwara, K.; Machida, K.I.; Adachi, G.Y.; Sakata, T.; Mori, H. Characterization of Cerium(IV) Oxide Ultrafine Particles Prepared Using Reversed Micelles. Chem. Mater. 1997, 9, 2197–2204. [Google Scholar] [CrossRef]
- Sathyamurthy, S.; Leonard, K.J.; Dabestani, R.T.; Paranthaman, M.P. Reverse micellar synthesis of cerium oxide nanoparticles. Nanotechnology 2005, 16, 1960–1964. [Google Scholar] [CrossRef]
- Mokkelbost, T.; Kaus, I.; Grande, T.; Einarsrud, M.A. Combustion synthesis and characterization of nanocrystalline CeO2-based powders. Chem. Mater. 2004, 16, 5489–5494. [Google Scholar] [CrossRef]
- Liao, X.H.; Zhu, J.M.; Zhu, J.J.; Xu, J.Z.; Chen, H.Y. Preparation of monodispersed nanocrystalline CeO2 powders by microwave irradiation. Chem. Commun. 2001, 10, 937–938. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; He, H.; Zhang, Y.; Qin, X.; Wang, B. Oxygen vacancy clusters essential for the catalytic activity of CeO2 nanocubes for o-xylene oxidation. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Sarani, M. Biosynthesis, characterization and cytotoxic activity of CeO2 nanoparticles. Ceram. Int. 2018, 44, 12642–12647. [Google Scholar] [CrossRef]
- Nyoka, M.; Choonara, Y.E.; Kumar, P.; Kondiah, P.P.D.; Pillay, V. Synthesis of Cerium Oxide Nanoparticles Using Various Methods: Implications for Biomedical Applications. Nanomaterials 2020, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.; Ahmed, B.; Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Microwave accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus leaf extract and their antibacterial and antibiofilm activity on clinical isolates. PLoS ONE 2015, 10, e0131178. [Google Scholar] [CrossRef]
- Maensiri, S.; Masingboon, C.; Laokul, P.; Jareonboon, W.; Promarak, V.; Anderson, P.L.; Seraphin, S. Egg White Synthesis and Photoluminescence of Platelike Clusters of CeO2 Nanoparticles. Cryst. Growth Design 2007, 7, 950–955. [Google Scholar] [CrossRef]
- Kargar, H.; Ghazavi, H.; Darroudi, M. Size-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effects. Ceram. Int. 2015, 41, 4123–4128. [Google Scholar] [CrossRef]
- Casas-Cabanas, M.; Reynaud, M.; Rikarte, J.; Horbach, P.; Rodríguez-Carvajal, J. FAULTS: A program for refinement of structures with extended defects. J. Appl. Crystallogr. 2016, 49, 2259–2269. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Lee, C.G. Structural and magnetic properties of Ni doped CeO2 nanoparticles. J. Nanosci. Nanotechnol. 2010, 10, 7204–7207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, K.; Vij, A.; Hashim, M.; Chawla, A.K.; Kumar, S. Structural and optical properties of Cu doped CeO2 nanoparticles. AIP Conf. Proc. 2019, 2093, 2–6. [Google Scholar]
- Kumar, S.; Koo, B.H.; Sharma, S.K.; Knobel, M.; Lee, C.G. Influence of Co doping on structural, optical and magnetic studies of Co doped CeO2 nanoparticles. Nano 2010, 5, 349–355. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, S.; Dalela, B.; Kumar, S.; Alvi, P.A.; Dalela, S. Defects and oxygen vacancies tailored structural and optical properties in CeO2 nanoparticles doped with Sm3+ cation. J. Alloy. Compd. 2018, 752, 520–531. [Google Scholar] [CrossRef]
- Kumari, K.; Aljawfi, R.N.; Katharria, Y.S.; Dwivedi, S.; Chae, K.H.; Kumar, R.; Alshoaibi, A.; Alvi, P.A.; Dalela, S.; Kumar, S. Study the contribution of surface defects on the structural, electronic structural, magnetic, and photocatalyst properties of Fe: CeO2 nanoparticles. J. Electron Spectrosc. Relat. Phenom. 2019, 235, 29–39. [Google Scholar] [CrossRef]
- Kumari, K.; Aljawfi, R.N.; Chawla, A.K.; Kumar, R.; Alvi, P.A.; Alshoaibi, A.; Vij, A.; Ahmed, F.; Abu-samak, M.; Kumar, S. Engineering the optical properties of Cu doped CeO2 NCs for application in white LED. Ceram. Int. 2020, 46, 7482–7488. [Google Scholar] [CrossRef]
- Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Ukritnukun, S.; Nattestad, A.; Chen, J.; Phanichphant, S. Photocatalytic degradation of methyl orange by CeO2 and Fe-doped CeO2 films under visible light irradiation. Sci. Rep. 2014, 4, 5757. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Kumar, S.; Ahamed, M. Structural, electrical and optical properties of nanocrystalline silicon thin films deposited by pulsed laser ablation. Mater. Sci. Semicond. Process. 2015, 30, 169–173. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Saravanan, P.; Chen, S.-H.; Dong, C.-L.; Chen, C.L.; Chen, S.-Y.; Asokan, K.; Rajendra Kumar, R.T. Enhanced Room-Temperature Ferromagnetism on Co-Doped CeO 2 Nanoparticles: Mechanism and Electronic and Optical Properties. J. Phys. Chem. C 2014, 118, 27039–27047. [Google Scholar] [CrossRef]
- Hiramatsu, K. Vancomycin-resistant Staphylococcus aureus: A new model of antibiotic resistance. Lancet Infect. Dis. 2001, 1, 147–155. [Google Scholar] [CrossRef]
- Barnard, A.S. Nanohazards: Knowledge is our first defence. Nat. Mater. 2006, 5, 245–248. [Google Scholar] [CrossRef] [PubMed]
- De Faria, L.A.; Trasatti, S. The Point of Zero Charge of CeO2. J. Colloid Interface Sci. 1994, 167, 352–357. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Seaton, A.; MacNee, W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 2001, 109, 523–527. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ahmed, F.; Shaalan, N.M.; Saber, O. Biosynthesis of CeO2 Nanoparticles Using Egg White and Their Antibacterial and Antibiofilm Properties on Clinical Isolates. Crystals 2021, 11, 584. https://doi.org/10.3390/cryst11060584
Kumar S, Ahmed F, Shaalan NM, Saber O. Biosynthesis of CeO2 Nanoparticles Using Egg White and Their Antibacterial and Antibiofilm Properties on Clinical Isolates. Crystals. 2021; 11(6):584. https://doi.org/10.3390/cryst11060584
Chicago/Turabian StyleKumar, Shalendra, Faheem Ahmed, Nagih M. Shaalan, and Osama Saber. 2021. "Biosynthesis of CeO2 Nanoparticles Using Egg White and Their Antibacterial and Antibiofilm Properties on Clinical Isolates" Crystals 11, no. 6: 584. https://doi.org/10.3390/cryst11060584







