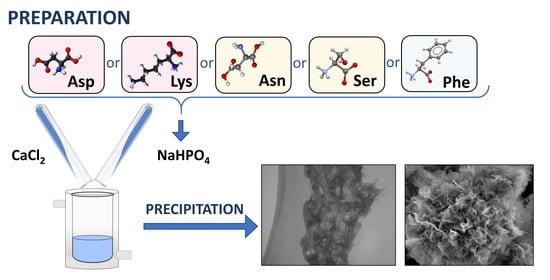
Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Precipitation Systems
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Powder X-ray Diffraction (PXRD)
2.4. Scanning Electron Microscopy (SEM)
2.5. Transmission Electron Microscopy (TEM)
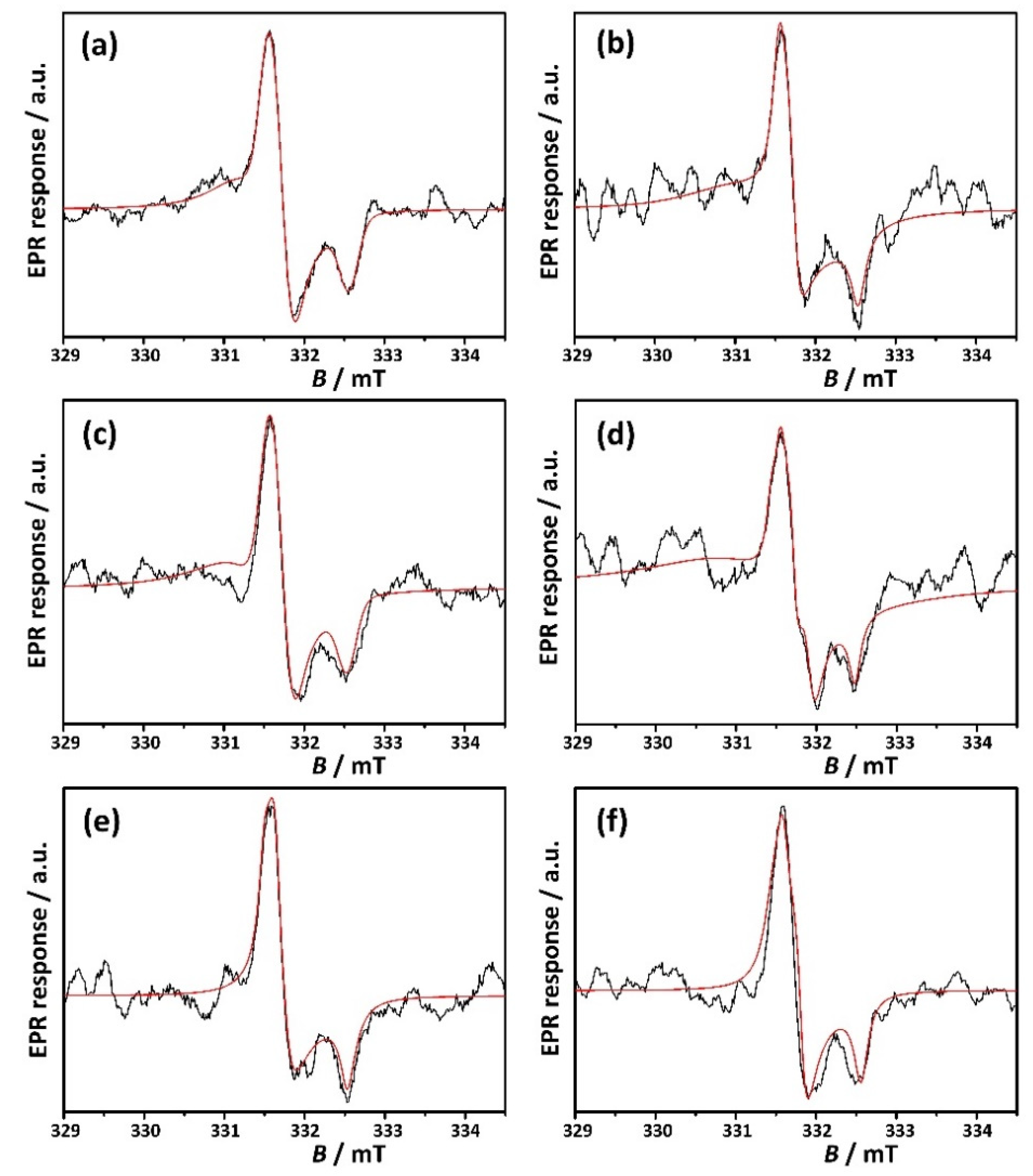
2.6. Electron Paramagnetic Resonance Spectroscopy (EPR)
3. Results
3.1. Influence of Amino Acids on the Rate of CaP Transformation
- −
- In the initial precipitation stage (stage I), slight or negligible pH changes are observed, which correspond to the formation of ACP;
- −
- A subsequent rapid decrease in pH (stage II) is associated with secondary precipitation of crystalline phase upon the formation of ACP;
- −
- A final slight pH change (stage III) corresponds to the solution-mediated crystal growth and phase transformation of the crystalline phase formed in stage II.
3.2. Influence of Amino Acids on the Properties of Formed CaPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nudelman, F.; Sommerdijk, N.A.J.M. Biomineralization as an Inspiration for Materials Chemistry. Angew. Chem. Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem. A Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and Peptide-Directed Syntheses of Inorganic Materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. THE MATERIAL BONE: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S. The Strategic Role of Adsorption Phenomena in Biomineralization. Cryst. Res. Technol. 2013, 48, 864–876. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989; ISBN 978-0-19-504977-0. [Google Scholar]
- Crichton, R. Biomineralization. In Biological Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–544. ISBN 978-0-12-811741-5. [Google Scholar]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford chemistry masters; Oxford University Press: New York, NY, USA, 2001; ISBN 978-0-19-850882-3. [Google Scholar]
- De, M.; Gosh, P.S.; Rotello, V.M. Applications of Nanoparticles in Biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.J. The Interaction of Proteins with Solid Surfaces. Curr. Opin. Struct. Biol. 2004, 14, 110–115. [Google Scholar] [CrossRef]
- Hudson, S.P.; Cooney, J.; Magner, E. Proteins in Mesoporous Silicates. Angew. Chem. 2008, 47, 8582–8594. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem.-Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Fomin, A.S.; Sinel’nikov, A.A.; Kolyagin, Y.G.; Barinov, S.M. Effect of an Amino Acid on the Formation of Calcium Phosphate Particles on Chitosan Macromolecules. Inorg. Mater. 2015, 51, 1017–1024. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates. Application in Nature, Biology and Medicine; Pan Stanford Publishing: Singapore, 2012. [Google Scholar]
- Sikirić, M.D.; Füredi-Milhofer, H. The Influence of Surface Active Molecules on the Crystallization of Biominerals in Solution. Adv. Colloid Interface Sci. 2006, 128–130, 135–158. [Google Scholar] [CrossRef]
- Bleek, K.; Taubert, A. New Developments in Polymer-Controlled, Bioinspired Calcium Phosphate Mineralization from Aqueous Solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef]
- Gonzalez-McQuire, R.; Chane-Ching, J.-Y.; Vignaud, E.; Lebugle, A.; Mann, S. Synthesis and Characterization of Amino Acid-Functionalized Hydroxyapatite Nanorods. J. Mater. Chem. 2004, 14, 2277. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The Role of Amino Acids in Hydroxyapatite Mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solonenko, A.P.; Belskaya, L.V.; Golovanova, O.A. Features of Calcium Phosphate Crystallization in the Presence of Amino Acids. Chem. Sustain. Dev. 2010, 1, 69–76. [Google Scholar]
- Koutsopoulos, S.; Dalas, E. Inhibition of Hydroxyapatite Formation in Aqueous Solutions by Amino Acids with Hydrophobic Side Groups. Langmuir 2000, 16, 6739–6744. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. Hydroxyapatite Crystallization in the Presence of Serine, Tyrosine and Hydroxyproline Amino Acids with Polar Side Groups. J. Cryst. Growth 2000, 216, 443–449. [Google Scholar] [CrossRef]
- Spanos, N.; Klepetsanis, P.G.; Koutsoukos, P.G. Model Studies on the Interaction of Amino Acids with Biominerals: The Effect of L-Serine at the Hydroxyapatite–Water Interface. J. Colloid Interface Sci. 2001, 236, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S.; Dalas, E. Hydroxyapatite Crystallization in the Presence of Amino Acids with Uncharged Polar Side Groups: Glycine, Cysteine, Cystine, and Glutamine. Langmuir 2001, 17, 1074–1079. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. The Effect of Acidic Amino Acids on Hydroxyapatite Crystallization. J. Cryst. Growth 2000, 217, 410–415. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. The Crystallization of Hydroxyapatite in the Presence of Lysine. J. Colloid Interface Sci. 2000, 231, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.T.; Yao, G.; Cerruti, M. The Importance of Amino Acid Interactions in the Crystallization of Hydroxyapatite. J. R. Soc. Interface 2012, 10, 20120906. [Google Scholar] [CrossRef]
- Yang, X.; Xie, B.; Wang, L.; Qin, Y.; Henneman, Z.J.; Nancollas, G.H. Influence of Magnesium Ions and Amino Acids on the Nucleation and Growth of Hydroxyapatite. CrystEngComm 2011, 13, 1153–1158. [Google Scholar] [CrossRef]
- Tsai, T.W.T.; Chen, W.-Y.; Tseng, Y.-H.; Chan, J.C.C. Phase Transformation of Calcium Phosphates in the Presence of Glutamic Acid. Can. J. Chem. 2011, 89, 885–891. [Google Scholar] [CrossRef]
- Ikawa, N.; Kimura, T.; Oumi, Y.; Sano, T. Amino Acid Containing Amorphous Calcium Phosphates and the Rapid Transformation into Apatite. J. Mater. Chem. 2009, 19, 4906. [Google Scholar] [CrossRef]
- Shimbayashi, S.; Tanizawa, Y. Formation of Hydroxyapatite in the Presence of Phosphorylated Polyvinylalcohol as a Simplified Compound for Mineralization Regulator Phosphoproteins. Chem. Pharm. Bull. 1990, 38, 1810. [Google Scholar] [CrossRef] [Green Version]
- Jack, K.S.; Vizcarra, T.G.; Trau, M. Characterization and Surface Properties of Amino-Acid-Modified Carbonate-Containing Hydroxyapatite Particles. Langmuir 2007, 23, 12233–12242. [Google Scholar] [CrossRef]
- Palazzo, B.; Walsh, D.; Iafisco, M.; Foresti, E.; Bertinetti, L.; Martra, G.; Bianchi, C.L.; Cappelletti, G.; Roveri, N. Amino Acid Synergetic Effect on Structure, Morphology and Surface Properties of Biomimetic Apatite Nanocrystals. Acta Biomater. 2009, 5, 1241–1252. [Google Scholar] [CrossRef]
- Bar-Yosef Ofir, P.; Govrin-Lippman, R.; Garti, N.; Füredi-Milhofer, H. The Influence of Polyelectrolytes on the Formation and Phase Transformation of Amorphous Calcium Phosphate. Cryst. Growth Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Chandra Bose, A.; Rameshbabu, N. X-Ray Peak Broadening Studies of Nanocrystalline Hydroxyapatite by Williamson–Hall Analysis. Phys. B Condens. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Majer, M.; Roguljić, M.; Knežević, Ž.; Starodumov, A.; Ferenček, D.; Brigljević, V.; Mihaljević, B. Dose Mapping of the Panoramic 60Co Gamma Irradiation Facility at the Ruđer Bošković Institute–Geant4 Simulation and Measurements. Appl. Radiat. Isot. 2019, 154, 108824. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Brečević, L.; Füredi-Milhofer, H. Precipitation of Calcium Phosphates from Electrolyte Solutions: II. The Formation and Transformation of the Precipitates. Calcif. Tissue Res. 1972, 10, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Despotović, R.; Filipović-Vinceković, N.; Füredi-Milhofer, H. Precipitation of Calcium Phosphates from Electrolyte Solutions. Calcif. Tissue Res. 1975, 18, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Eans, E.D.; Gillessen, I.H.; Posner, A.S. Intermediate States in the Precipitation of Hydroxyapatite. Nature 1965, 208, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Brečević, L.; Hlady, V.; Füredi-Milhofer, H. Influence of Gelatin on the Precipitation of Amorphous Calcium Phosphate. Colloids Surf. 1987, 28, 301–313. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.K.; Gilbert, P.U.P.A. Transient Amorphous Calcium Phosphate in Forming Enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous Calcium Phosphate Is a Major Component of the Forming Fin Bones of Zebrafish: Indications for an Amorphous Precursor Phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef] [Green Version]
- Selmani, A.; Coha, I.; Magdić, K.; Čolović, B.; Jokanović, V.; Šegota, S.; Gajović, S.; Gajović, A.; Jurašin, D.; Dutour Sikirić, M. Multiscale Study of the Influence of Cationic Surfactants on Amorphous Calcium Phosphate Precipitation. CrystEngComm 2015, 17, 8529–8548. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Wang, C.-G.; Liao, J.-W.; Gou, B.-D.; Huang, J.; Tang, R.-K.; Tao, J.-H.; Zhang, T.-L.; Wang, K. Crystallization at Multiple Sites inside Particles of Amorphous Calcium Phosphate. Cryst. Growth Des. 2009, 9, 2620–2626. [Google Scholar] [CrossRef]
- Li, S.; Wang, L. Phosphorylated Osteopontin Peptides Inhibit Crystallization by Resisting the Aggregation of Calcium Phosphate Nanoparticles. CrystEngComm 2012, 14, 8037. [Google Scholar] [CrossRef]
- Čadež, V.; Erceg, I.; Selmani, A.; Domazet Jurašin, D.; Šegota, S.; Lyons, D.; Kralj, D.; Sikirić, M. Amorphous Calcium Phosphate Formation and Aggregation Process Revealed by Light Scattering Techniques. Crystals 2018, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Christoffersen, J.; Christoffersen, M.R.; Kibalczyc, W.; Andersen, F.A. A Contribution to the Understanding of the Formation of Calcium Phosphates. J. Cryst. Growth 1989, 94, 767–777. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovic, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-Association Complexes Unite Classical and Non-Classical Theories for the Biomimetic Nucleation of Calcium Phosphate. Nat. Commun. 2013, 4, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepić, R.; Jurković, L.; Klementyeva, K.; Ukrainczyk, M.; Gredičak, M.; Smith, D.M.; Kralj, D.; Smith, A.-S. Adsorption of Aspartate Derivatives to Calcite Surfaces in Aqueous Environment. Cryst. Growth Des. 2020, 20, 2853–2859. [Google Scholar] [CrossRef]
- Štajner, L.; Kontrec, J.; Njegić Džakula, B.; Maltar-Strmečki, N.; Plodinec, M.; Lyons, D.M.; Kralj, D. The Effect of Different Amino Acids on Spontaneous Precipitation of Calcium Carbonate Polymorphs. J. Cryst. Growth 2018, 486, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Mihelj Josipović, T.; Kovačević, M.; Mateša, S.; Kostešić, M.; Matijaković, N.; Radatović, B.; Lyons, D.M.; Kralj, D.; Dutour Sikirić, M. The Influence of Different Classes of Amino Acids on Calcium Phosphates Seeded Growth. Materials 2020, 13, 4798. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Amorphous Calcium (Ortho)Phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Buljan Meić, I.; Kontrec, J.; Domazet Jurašin, D.; Selmani, A.; Njegić Džakula, B.; Maltar-Strmečki, N.; Lyons, D.M.; Plodinec, M.; Čeh, M.; Gajović, A.; et al. How Similar Are Amorphous Calcium Carbonate and Calcium Phosphate? A Comparative Study of Amorphous Phase Formation Conditions. CrystEngComm 2018, 20, 35–50. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Mochales, C.; Wilson, R.M.; Dowker, S.E.P.; Ginebra, M.-P. Dry Mechanosynthesis of Nanocrystalline Calcium Deficient Hydroxyapatite: Structural Characterisation. J. Alloy. Compd. 2011, 509, 7389–7394. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous Calcium Phosphates: Synthesis, Properties and Uses in Biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, S.-C.; Chen, S.-Y.; Lee, H.-Y.; Bow, J.-S. Structural Characterization of Nano-Sized Calcium Deficient Apatite Powders. Biomaterials 2004, 25, 189–196. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Hamada, Y.; Taira, M.; Takahashi, J. Crystallinity and Solubility Characteristics of Hydroxyapatite Adsorbed Amino Acid. Biomaterials 2002, 23, 2241–2247. [Google Scholar] [CrossRef]
- Schramm, D.U.; Rossi, A.M. Electron Spin Resonance (ESR) Studies of CO2− Radicals in Irradiated A and B-Type Carbonate-Containing Apatites. Appl. Radiat. Isot. 2000, 52, 1085–1091. [Google Scholar] [CrossRef]
- Cevc, P.; Schara, M.; Ravnik, Č.; Ravnik, C. Electron Paramagnetic Resonance Study of Irradiated Tooth Enamel. Radiat. Res. 1972, 51, 581. [Google Scholar] [CrossRef]
- Callens, F.J.; Verbeeck, R.M.H.; Matthys, P.F.A.; Martens, L.C.; Boesman, E.R. The Contribution of CO3 3− and CO2− to the ESR Spectrum near G=2 of Powdered Human Tooth Enamel. Calcif. Tissue Int. 1987, 41, 124–129. [Google Scholar] [CrossRef]
- Callens, F.J.; Verbeeck, R.M.H.; Naessens, D.E.; Matthys, P.F.A.; Boesman, E.R. The Effect of Carbonate Content and Drying Temperature on the ESR-Spectrum near G=2 of Carbonated Calciumapatites Synthesized from Aqueous Media. Calcif. Tissue Int. 1991, 48, 249–259. [Google Scholar] [CrossRef]
- Callens, F.J.; Verbeeck, R.M.H.; Matthys, P.F.A.; Martens, L.C.; Boesman, E.R.; Driessens, F.C.M. The ESR Spectrum Near G=2 of Carbonated Calciumapatites Synthesized at High Temperature. Bull. Soc. Chim. Belg. 2010, 95, 589–596. [Google Scholar] [CrossRef]
- Ikeya, M. New Applications of Electron Spin Resonance: Dating, Dosimetry and Microscopy; WORLD SCIENTIFIC: Singapore, 1993; ISBN 978-981-02-1199-8. [Google Scholar]
- Sadlo, J.; Strzelczak, G.; Lewandowska-Szumiel, M.; Sterniczuk, M.; Pajchel, L.; Michalik, J. Carbon-Centered Radicals in γ-Irradiated Bone Substituting Biomaterials Based on Hydroxyapatite. J. Mater. Sci. Mater. Med. 2012, 23, 2061–2068. [Google Scholar] [CrossRef]
- Strzelczak, G.; Sadło, J.; Danilczuk, M.; Stachowicz, W.; Callens, F.; Vanhaelewyn, G.; Goovaerts, E.; Michalik, J. Multifrequency Electron Paramagnetic Resonance Study on Deproteinized Human Bone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1206–1209. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Mamin, G.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Combination of EPR Measurements and DFT Calculations To Study Nitrate Impurities in the Carbonated Nanohydroxyapatite. J. Phys. Chem. A 2014, 118, 1519–1526. [Google Scholar] [CrossRef]
- Eiden-Aßmann, S.; Viertelhaus, M.; Heiß, A.; Hoetzer, K.A.; Felsche, J. The Influence of Amino Acids on the Biomineralization of Hydroxyapatite in Gelatin. J. Inorg. Biochem. 2002, 91, 481–486. [Google Scholar] [CrossRef]




| (ti ± SD)/min | |||||
|---|---|---|---|---|---|
| c(AA)/mmol dm−3 | Asp | Lys | Asn | Ser | Phe |
| 0 (CS) | 18.2 ± 0.5 | ||||
| 1.0 | 21.4 ± 1.5 | 17.4 ± 1.1 | 21.5 ± 0.7 | 20.3 ± 1.0 | 18.8 ± 1.1 |
| 2.5 | 16.4 ± 0.5 | 19.7 ± 0.3 | 22.2 ± 0.2 | 18.8 ± 1.6 | 20.8 ± 0.5 |
| 5.0 | 23.1 ± 0.2 | 19.3 ± 0.1 | 16.7 ± 0.9 | 113.7 ± 6.3 | 15.9 ± 0.1 |
| D002/nm | |
|---|---|
| CS | 17.40 |
| Asp | 14.10 |
| Lys | 17.50 |
| Asn | 17.50 |
| Ser | 16.10 |
| Phe | 19.60 |
| R-Value | |
|---|---|
| CS | 0.5505 |
| Asp | 0.8535 |
| Lys | 0.5821 |
| Asn | 0.5714 |
| Ser | 0.6487 |
| Phe | 0.5477 |
| Sample | CS | Asp | Lys | Asn | Ser | Phe |
|---|---|---|---|---|---|---|
| Species | ||||||
| orth. gx=2.0022 gy=1.9973 gz=2.0034 | 53.46% | 44.63% | 35.11% | 13.82% | 70.64% | 75.51% |
| orth. gx=2.0034 gy=1.9973 gz=2.0017 | -- | 28.50% | 9.79% | - | 21.40% | -- |
| iso. go=2.0008 | -- | -- | -- | 2.48% | -- | 11.35% |
| axial gx=2.0039 gy=2.0039 gz=2.0014 | -- | -- | 55.10% | 45.52% | -- | -- |
| axial gx=2.0044 gy=2.0044 gz=2.0020 | 46.54% | 26.87% | -- | 38.18% | 7.96% | 13.14% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erceg, I.; Maltar-Strmečki, N.; Jurašin, D.D.; Strasser, V.; Ćurlin, M.; Lyons, D.M.; Radatović, B.; Mlinarić, N.M.; Kralj, D.; Sikirić, M.D. Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates. Crystals 2021, 11, 792. https://doi.org/10.3390/cryst11070792
Erceg I, Maltar-Strmečki N, Jurašin DD, Strasser V, Ćurlin M, Lyons DM, Radatović B, Mlinarić NM, Kralj D, Sikirić MD. Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates. Crystals. 2021; 11(7):792. https://doi.org/10.3390/cryst11070792
Chicago/Turabian StyleErceg, Ina, Nadica Maltar-Strmečki, Darija Domazet Jurašin, Vida Strasser, Marija Ćurlin, Daniel Mark Lyons, Borna Radatović, Nives Matijaković Mlinarić, Damir Kralj, and Maja Dutour Sikirić. 2021. "Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates" Crystals 11, no. 7: 792. https://doi.org/10.3390/cryst11070792
APA StyleErceg, I., Maltar-Strmečki, N., Jurašin, D. D., Strasser, V., Ćurlin, M., Lyons, D. M., Radatović, B., Mlinarić, N. M., Kralj, D., & Sikirić, M. D. (2021). Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates. Crystals, 11(7), 792. https://doi.org/10.3390/cryst11070792