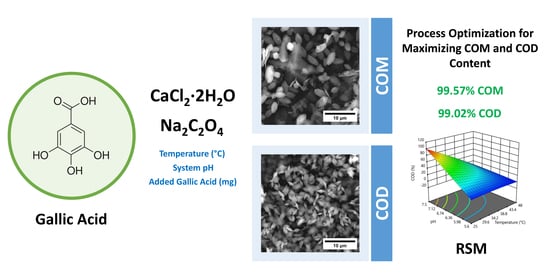
Calcium Oxalate and Gallic Acid: Structural Characterization and Process Optimization toward Obtaining High Contents of Calcium Oxalate Monohydrate and Dihydrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Precipitation Experiments
2.3. Sample Characterization of Calcium Oxalate Hydrate
2.4. Response Surface Methodology and Process Optimization in Calcium Oxalate Formation
3. Results and Discussion
3.1. The Structural Analysis
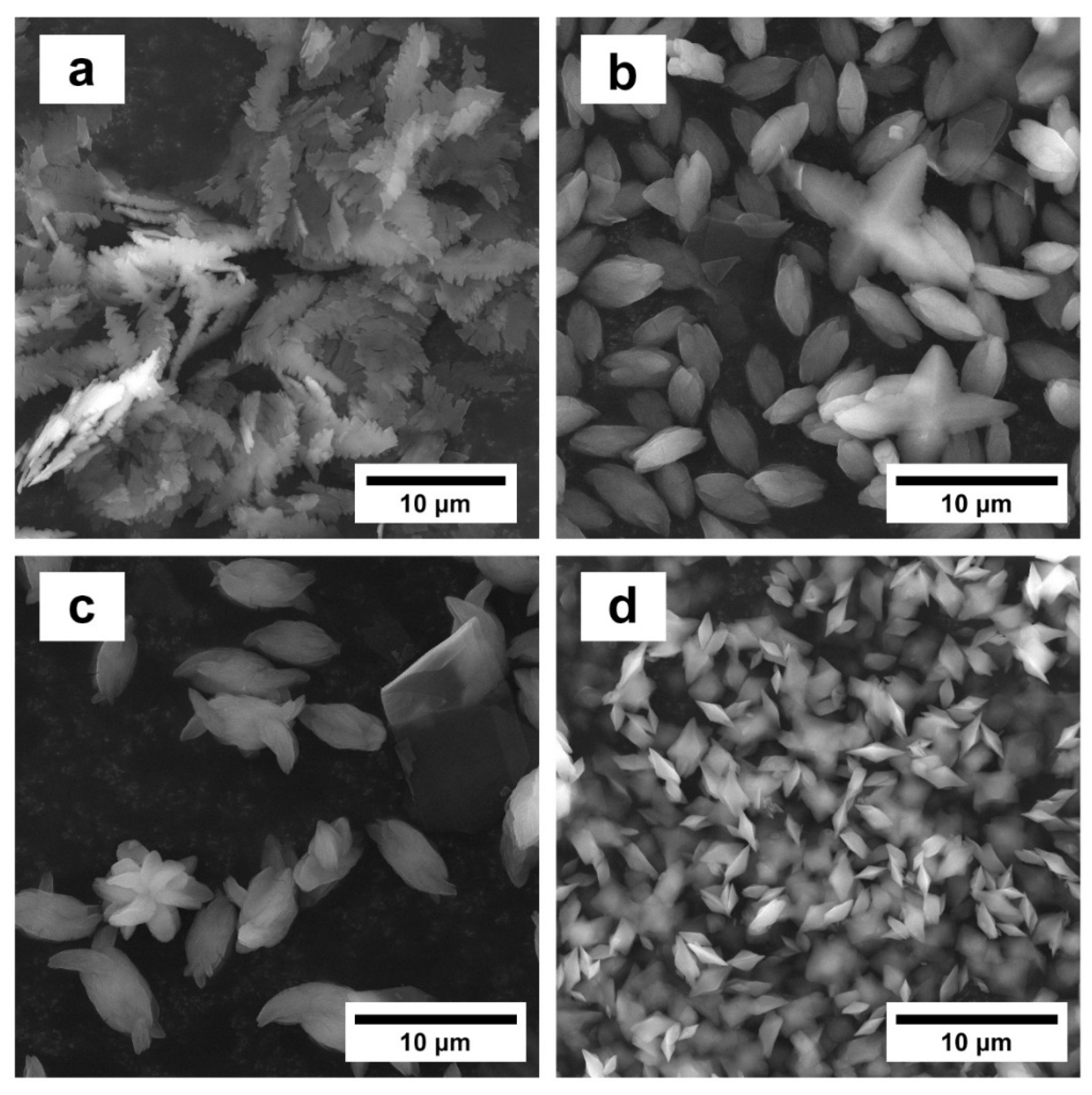
3.2. Morphology of Calcium Oxalate Crystals
3.3. The Influence of the Process Parameters on the Obtained Calcium Oxalate Hydrate Phases
3.4. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ivanovski, O.; Drüeke, T.B. A new era in the treatment of calcium oxalate stones? Kidney Int. 2013, 83, 998–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, M.; Hoppe, B. History, epidemiology and regional diversities of urolithiasis. Pediatr. Nephrol. 2010, 25, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.M.; Duan, L.; Tieke, B. Effects of carboxylic acids on the crystal growth of calcium oxalate nanoparticles in lecithin−water liposome systems. Langmuir 2003, 19, 8980–8985. [Google Scholar] [CrossRef]
- Tiselius, H.G.; Hallin, A.; Lindbäck, B. Crystallisation properties in stone forming and normal subjects' urine diluted using a standardised procedure to match the composition of urine in the distal part of the distal tubule and the middle part of the collecting duct. Urol. Res. 2001, 29, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.M. Effects of temperature on growth and aggregation of calcium oxalate in presence of various carboxylic acids in silica gel systems. Mater. Sci. Eng. C 2006, 26, 679–682. [Google Scholar] [CrossRef]
- De Bellis, R.; Piacentini, M.P.; Meli, M.A.; Mattioli, M.; Menotta, M.; Mari, M.; Valentini, L.; Palomba, L.; Desideri, D.; Chiarantini, L. In vitro effects on calcium oxalate crystallization kinetics and crystal morphology of an aqueous extract from Ceterach officinarum: Analysis of a potential antilithiatic mechanism. PLoS ONE 2019, 14, e0218734. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Wen, X.L.; Ding, Y.M.; Ouyang, J.M. Adsorption differences of acidic, neutral and alkaline amino acids onto nano/micron COM and COD crystals. Adv. Mater. Technol. 2017, 175–180. [Google Scholar] [CrossRef]
- Qiu, S.R.; Wierzbicki, A.; Orme, C.A.; Cody, A.M.; Hoyer, J.R.; Nancollas, G.H.; Zepeda, S.; De Yoreo, J.J. Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc. Natl. Acad. Sci. USA 2004, 101, 1811–1815. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.; Ackermann, D.; Finlayson, B. Calcium oxalate dihydrate (weddellite) precipitation. J. Cryst. Growth 1989, 98, 285–292. [Google Scholar] [CrossRef]
- Ou, Y.; Xue, J.F.; Tan, C.Y.; Gui, B.S.; Sun, X.Y.; Ouyang, J.M. Inhibition of urinary macromolecule heparin on aggregation of nano-COM and nano-COD crystals. Molecules 2015, 20, 1626–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.M.; Zheng, H.; Deng, S.P. Simultaneous formation of calcium oxalate (mono-, di-, and trihydrate) induced by potassium tartrate in gelatinous system. J. Cryst. Growth 2006, 293, 118–123. [Google Scholar] [CrossRef]
- Basavaraj, D.R.; Biyani, C.S.; Browning, A.J.; Cartledge, J.J. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser. 2007, 5, 126–136. [Google Scholar] [CrossRef]
- Bouatia, M.; Benzeid, H.; Idrissi, M.O.B.; Benramdane, L.; Draoui, M. In vitro effect of acetylsalicylic acid on calcium oxalate crystallization: An approach to antilithiasis. Int. J. Pharm. Pharmaceut. Sci. 2015, 7, 329–331. [Google Scholar]
- Spivacow, F.R.; Negri, A.L.; Polonsky, A.; Del Valle, E.E. Long-term treatment of renal lithiasis with potassium citrate. Urology 2010, 76, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Kvsrg, P.; Sujatha, D.; Bharathi, K. Herbal drugs in urolithiasis—A review. Pharmacog. Rev. 2007, 1, 175–178. [Google Scholar]
- Choubey, A.; Parasar, A.; Choubey, A.; Iyer, D.; Pawar, R.S.; Patil, U.K. Potential of medicinal plants in kidney, gall and urinary stones. Int. J. Drug. Dev. Res. 2010, 2, 431–447. [Google Scholar]
- Tiwari, A.H.; Soni, V.B.; Londhe, V.; Bhandarkar, A.; Bandawane, D.; Nipate, S. An overview on potent indigenous herbs for urinary tract infirmity: Urolithiasis. Asian J. Pharm. Clin. Res. 2012, 5, 7–12. [Google Scholar]
- Chitme, H.R.; Alok, S.; Jain, S.K.; Sabharwal, M. Herbal treatment for urinary stones. Int. J Pharm. Sci. Res. 2010, 1, 24–31. [Google Scholar] [CrossRef]
- Chu, Y.F.; Sun, J.I.E.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M.; Andrighetti-Fröhner, C.R.; Leal, P.C.; Nunes, R.J.; Yunes, R.A.; Trybala, E.; Bergström, T.; Barardi, C.R.M.; Simões, C.M.O. Evaluation of anti-HSV-2 activity of gallic acid and pentyl gallate. Biol. Pharm. Bull. 2008, 31, 903–907. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, W.; Shi, P.; Li, M.; Sun, J.; Gong, J. A New Perspective of Gallic Acid on Calcium Oxalate Nucleation. Cryst. Growth Des. 2020, 20, 3173–3181. [Google Scholar] [CrossRef]
- Šter, A.; Šafranko, S.; Bilić, K.; Marković, B.; Kralj, D. The effect of hydrodynamic and thermodynamic factors and the addition of citric acid on the precipitation of calcium oxalate dihydrate. Urolithiasis 2018, 46, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Stanković, A.; Šafranko, S.; Jurišić, K.; Balić, I.; Bijelić, J.; Jokić, S.; Medvidović-Kosanović, M. Investigation of system complexity and addition of vitamin C on calcium oxalate precipitation. Chem. Pap. 2020, 74, 3279–3291. [Google Scholar] [CrossRef]
- Stanković, A.; Šafranko, S.; Kontrec, J.; Njegić Džakula, B.; Lyons, D.M.; Marković, B.; Kralj, D. Calcium oxalate precipitation in model systems mimicking the conditions of hyperoxaluria. Cryst. Res. Technol. 2019, 54, 1800210. [Google Scholar] [CrossRef]
- Echigo, T.; Kimata, M.; Kyono, A.; Shimizu, M.; Hatta, T. Re-investigation of the crystal structure of whewellite [Ca(C2O4)· H2O] and the dehydration mechanism of caoxite [Ca(C2O4)·3H2O]. Mineral. Mag. 2005, 69, 77–88. [Google Scholar] [CrossRef]
- Kaloustian, J.; El-Moselhy, T.F.; Portugal, H. Determination of calcium oxalate (mono-and dihydrate) in mixtures with magnesium ammonium phosphate or uric acid: The use of simultaneous thermal analysis in urinary calculi. Clin. Chim. Acta 2003, 334, 117–129. [Google Scholar] [CrossRef]
- Maurice-Estepa, L.; Levillain, P.; Lacour, B.; Daudon, M. Advantage of zero-crossing-point first-derivative spectrophotometry for the quantification of calcium oxalate crystalline phases by infrared spectrophotometry. Clin. Chim. Acta 2000, 298, 1–11. [Google Scholar] [CrossRef]
- Conti, C.; Casati, M.; Colombo, C.; Realini, M.; Brambilla, L.; Zerbi, G. Phase transformation of calcium oxalate dihydrate–monohydrate: Effects of relative humidity and new spectroscopic data. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 413–419. [Google Scholar] [CrossRef]
- Girija, E.K.; Latha, S.C.; Kalkura, S.N.; Subramanian, C.; Ramasamy, P. Crystallization and microhardness of calcium oxalate monohydrate. Mater. Chem. Phys. 1998, 52, 253–257. [Google Scholar] [CrossRef]
- Petit, I.; Belletti, G.D.; Debroise, T.; Llansola-Portoles, M.J.; Lucas, I.T.; Leroy, C.; Bonhomme, C.; Bonhomme-Coury, L.; Bazin, D.; Daudon, M.; et al. Vibrational signatures of calcium oxalate polyhydrates. ChemistrySelect 2018, 3, 8801–8812. [Google Scholar] [CrossRef]
- King, M.; McClure, W.F.; Andrews, L.C.; Holomery, M.A. Powder Diffraction File Alphabetic Index, Inorganic Phases/Organic Phases; International Center for Diffraction Data: Newtown Square, PA, USA, 1992. [Google Scholar]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Kuz’mina, M.A.; Leoni, M.; Frank-Kamenetskaya, O.V. Hydrated calcium oxalates: Crystal structures, thermal stability, and phase evolution. Cryst. Growth Des. 2018, 18, 5465–5478. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Zhou, H.; Sang, L.; Li, X. Modulation of calcium oxalate crystallization by commonly consumed green tea. CrystEngComm 2010, 12, 845–852. [Google Scholar] [CrossRef]
- East, C.P.; Wallace, A.D.; Al-Hamzah, A.; Doherty, W.O.; Fellows, C.M. Effect of poly (acrylic acid) molecular mass and end-group functionality on calcium oxalate crystal morphology and growth. J. Appl. Polym. Sci. 2010, 115, 2127–2135. [Google Scholar] [CrossRef]
- Stanković, A.; Kontrec, J.; Džakula, B.N.; Kovačević, D.; Marković, B.; Kralj, D. Preparation and characterization of calcium oxalate dihydrate seeds suitable for crystal growth kinetic analyses. J. Cryst. Growth 2018, 500, 91–97. [Google Scholar] [CrossRef]
- Ouyang, J.; Deng, S.; Li, X.; Tan, Y.; Bernd, T. Effects of temperature and sodium carboxylate additives on mineralization of calcium oxalate in silica gel systems. Sci. China Ser. B Chem. 2004, 47, 311–319. [Google Scholar] [CrossRef]
- Yuzawa, M.; Tozuka, K.; Tokue, A. Effect of citrate and pyrophosphate on the stability of calcium oxalate dihydrate. Urol. Res. 1998, 26, 83–88. [Google Scholar] [CrossRef]
- Carvalho, M. Urinary pH in calcium oxalate stone formers: Does it matter? J. Bras. Nefrol. 2018, 40, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Grases, F.; Costa-Bauza, A.; Prieto, R.M. Renal lithiasis and nutrition. Nutr. J. 2006, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Manissorn, J.; Fong-Ngern, K.; Peerapen, P.; Thongboonkerd, V. Systematic evaluation for effects of urine pH on calcium oxalate crystallization, crystal-cell adhesion and internalization into renal tubular cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Su, Y.L.; Cheng, S.H. Sensitive and selective determination of gallic acid in green tea samples based on an electrochemical platform of poly (melamine) film. Anal. Chim. Acta 2015, 901, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Carloni, J.D.; Demarchi, B.; Sparks, D.; Reid, D.G.; Kunitake, M.E.; Tang, C.C.; Duer, M.J.; Freeman, C.L.; Pokroy, B.; et al. Tuning hardness in calcite by incorporation of amino acids. Nat. Mater. 2016, 15, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Independent Variable | Symbol | Level | ||
|---|---|---|---|---|
| Low (−1) | Center (0) | High (+1) | ||
| Temperature (°C) | X1 | 25 | 36.5 | 48 |
| System pH | X2 | 5.6 | 6.55 | 7.5 |
| Added Gallic Acid (mg) | X3 | 100 | 550 | 1000 |
| Operating Parameters | Response | |||||
|---|---|---|---|---|---|---|
| Run | Temperature | System pH | Added Gallic Acid | COM | COD | COT |
| °C | mg | % | ||||
| 1 | 36.5 | 5.60 | 1000 | 93.68 | 0.02 | 6.30 |
| 2 | 48.0 | 6.55 | 100 | 94.99 | 5.00 | 0.01 |
| 3 | 48.0 | 5.60 | 550 | 93.95 | 1.28 | 4.77 |
| 4 | 25.0 | 6.55 | 1000 | 6.53 | 90.51 | 2.96 |
| 5 | 25.0 | 5.60 | 550 | 95.66 | 2.94 | 1.40 |
| 6 | 48.0 | 7.50 | 550 | 91.47 | 8.53 | 0.01 |
| 7 | 36.5 | 5.60 | 100 | 96.73 | 3.03 | 0.24 |
| 8 | 25.0 | 6.55 | 100 | 90.28 | 6.30 | 3.42 |
| 9 | 36.5 | 6.55 | 550 | 71.25 | 15.61 | 13.15 |
| 10 | 36.5 | 6.55 | 550 | 74.08 | 11.75 | 14.17 |
| 11 | 36.5 | 6.55 | 550 | 77.32 | 12.41 | 10.27 |
| 12 | 36.5 | 6.55 | 550 | 75.36 | 12.97 | 11.67 |
| 13 | 48.0 | 6.55 | 1000 | 50.13 | 0.45 | 49.42 |
| 14 | 36.5 | 7.50 | 1000 | 8.00 | 91.61 | 0.39 |
| 15 | 25.0 | 7.50 | 550 | 0.34 | 99.32 | 0.34 |
| 16 | 36.5 | 7.50 | 100 | 85.97 | 14.02 | 0.02 |
| 17 | 36.5 | 6.55 | 550 | 76.08 | 12.55 | 11.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šafranko, S.; Goman, S.; Goman, D.; Jokić, S.; Marion, I.D.; Mlinarić, N.M.; Selmani, A.; Medvidović-Kosanović, M.; Stanković, A. Calcium Oxalate and Gallic Acid: Structural Characterization and Process Optimization toward Obtaining High Contents of Calcium Oxalate Monohydrate and Dihydrate. Crystals 2021, 11, 954. https://doi.org/10.3390/cryst11080954
Šafranko S, Goman S, Goman D, Jokić S, Marion ID, Mlinarić NM, Selmani A, Medvidović-Kosanović M, Stanković A. Calcium Oxalate and Gallic Acid: Structural Characterization and Process Optimization toward Obtaining High Contents of Calcium Oxalate Monohydrate and Dihydrate. Crystals. 2021; 11(8):954. https://doi.org/10.3390/cryst11080954
Chicago/Turabian StyleŠafranko, Silvija, Sara Goman, Dominik Goman, Stela Jokić, Ida Delač Marion, Nives Matijaković Mlinarić, Atiđa Selmani, Martina Medvidović-Kosanović, and Anamarija Stanković. 2021. "Calcium Oxalate and Gallic Acid: Structural Characterization and Process Optimization toward Obtaining High Contents of Calcium Oxalate Monohydrate and Dihydrate" Crystals 11, no. 8: 954. https://doi.org/10.3390/cryst11080954
APA StyleŠafranko, S., Goman, S., Goman, D., Jokić, S., Marion, I. D., Mlinarić, N. M., Selmani, A., Medvidović-Kosanović, M., & Stanković, A. (2021). Calcium Oxalate and Gallic Acid: Structural Characterization and Process Optimization toward Obtaining High Contents of Calcium Oxalate Monohydrate and Dihydrate. Crystals, 11(8), 954. https://doi.org/10.3390/cryst11080954