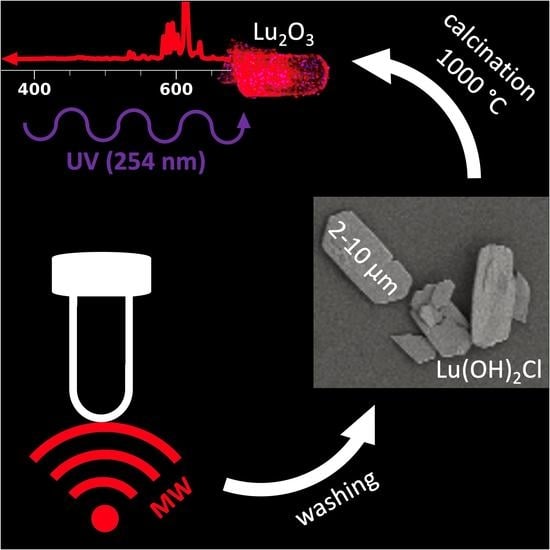
Heat-Induced Transformation of Luminescent, Size Tuneable, Anisotropic Eu:Lu(OH)2Cl Microparticles to Micro-Structurally Controlled Eu:Lu2O3 Microplatelets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Lu(OH)2Cl Microcrystals
2.2. Characterisation
3. Results and Discussion
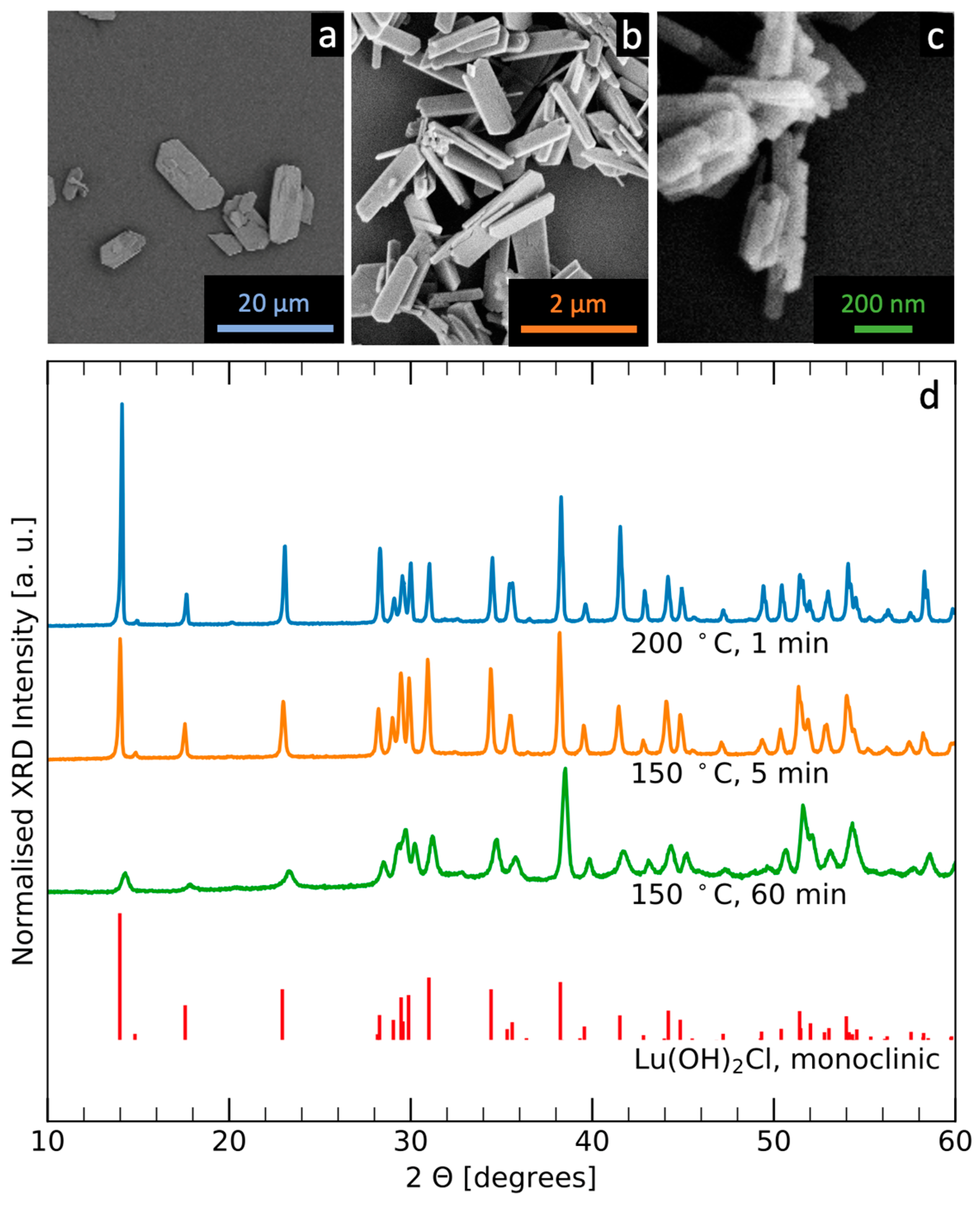
3.1. Synthesis of Size-Tuneable Eu:Lu(OH)2Cl Microparticles
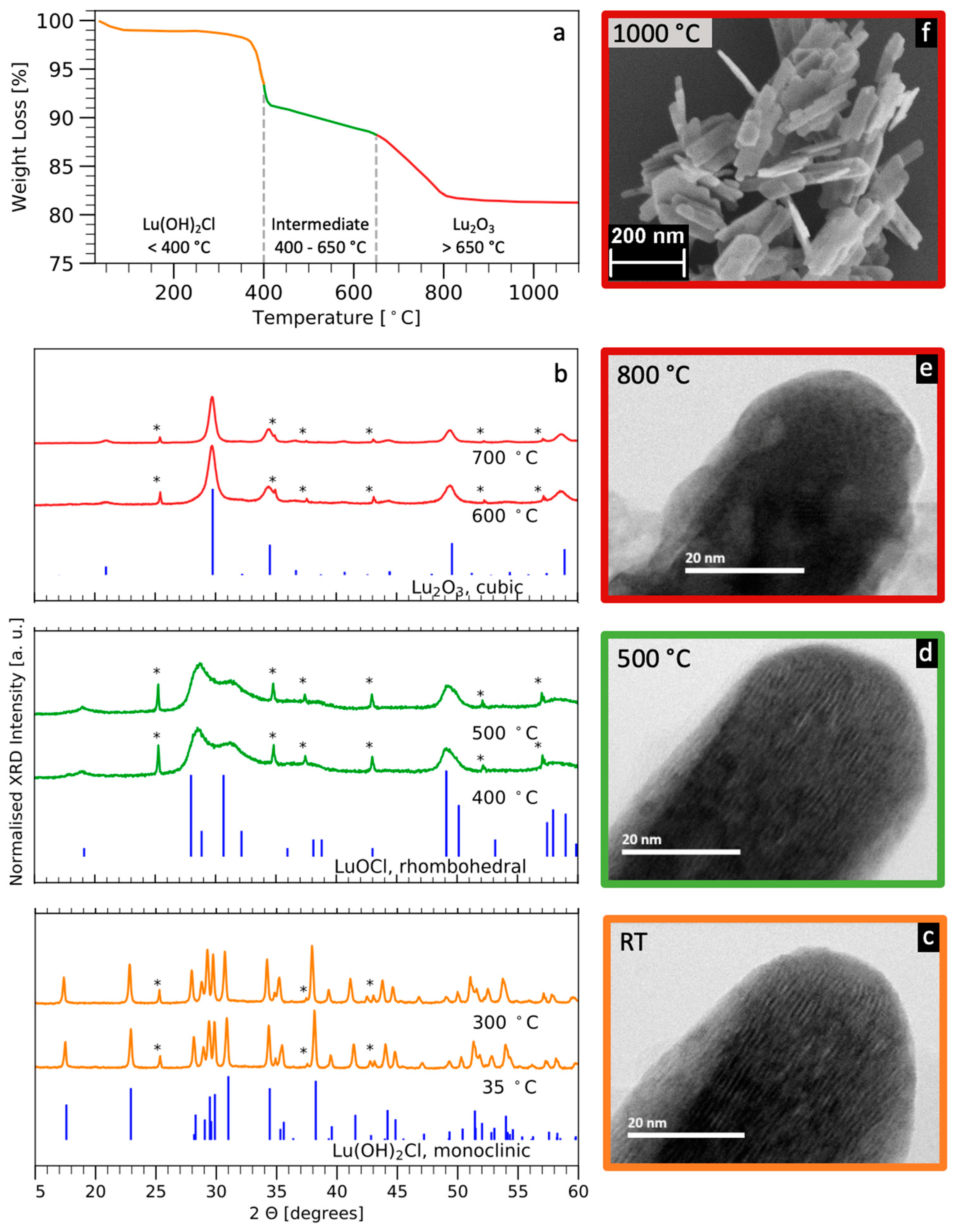
3.2. Thermal Evolution and Formation of Eu:Lu2O3 Platelets
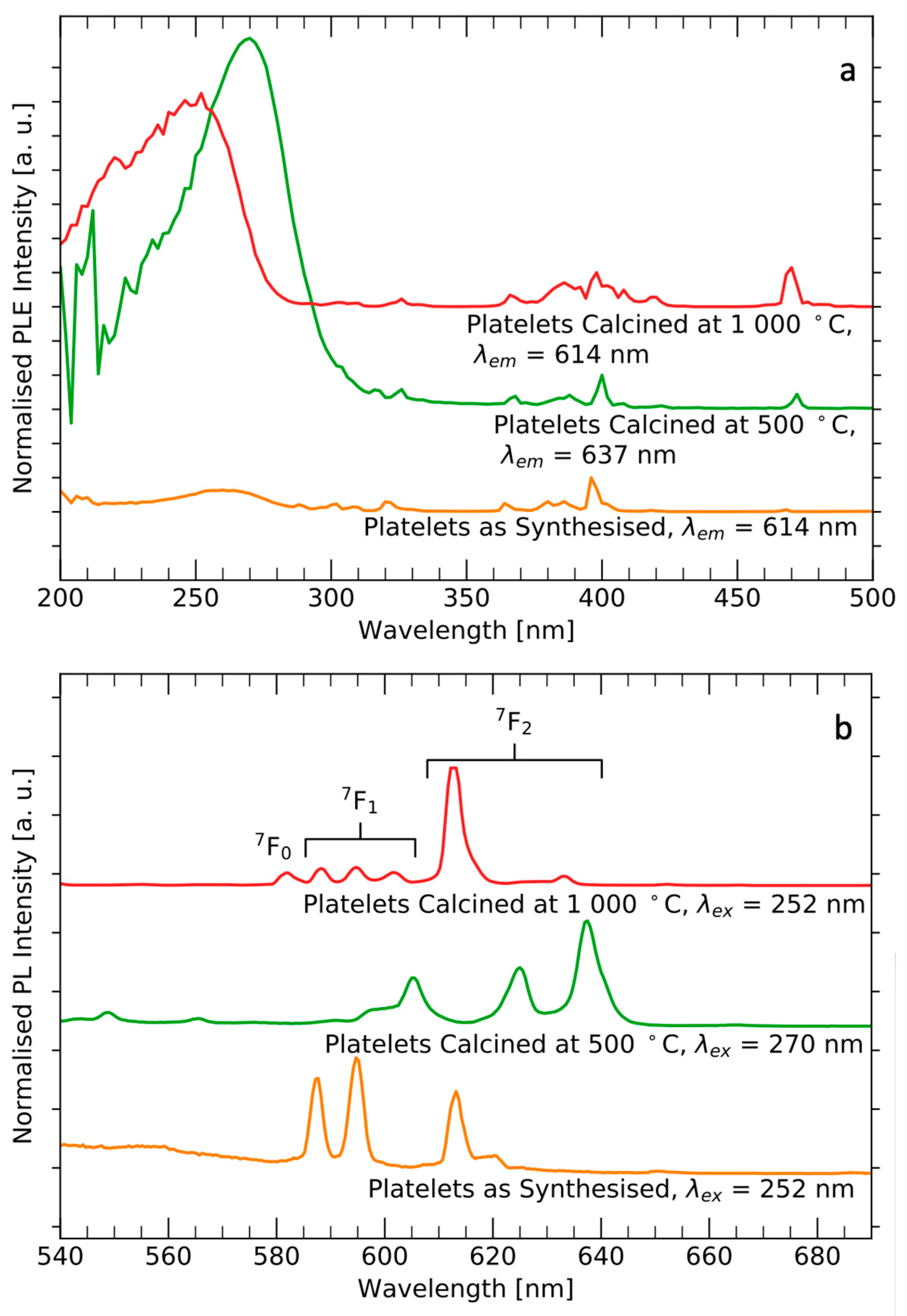
3.3. Luminescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seeley, Z.M.; Kuntz, J.D.; Cherepy, N.J.; Payne, S.A. Transparent Lu2O3:Eu ceramics by sinter and HIP optimization. Opt. Mater. 2011, 33, 1721–1726. [Google Scholar] [CrossRef]
- Satapathy, S.; Ahlawat, A.; Paliwal, A.; Singh, R.; Singh, M.K.; Gupta, P.K. Effect of calcination temperature on nanoparticle morphology and its consequence on optical properties of Nd:Y2O3 transparent ceramics. CrystEngComm. 2014, 16, 2723–2731. [Google Scholar] [CrossRef]
- Serrano, A.; Caballero-Calero, O.; García, M.Á.; Lazić, S.; Carmona, N.; Castro, G.R.; Martín-González, M.; Fernández, J.F. Cold sintering process of ZnO ceramics: Effect of the nanoparticle/microparticle ratio. J. Eur. Ceram. Soc. 2020, 40, 5535–5542. [Google Scholar] [CrossRef]
- Massera, J.; Gaussiran, M.; Gluchowski, P.; Lastusaari, M.; Hupa, L.; Petit, L. Processing and characterization of phosphate glasses containing CaAl2O4:Eu2+,Nd3+ and SrAl2O4:Eu2+,Dy3+ microparticles. J. Eur. Ceram. Soc. 2015, 35, 3863–3871. [Google Scholar] [CrossRef]
- Vanecek, V.; Kral, R.; Paterek, J.; Babin, V.; Jary, V.; Hybler, J.; Kodama, S.; Kurosawa, S.; Yokota, Y.; Yoshikawa, A.; et al. Modified vertical Bridgman method: Time and cost effective tool for preparation of Cs2HfCl6 single crystals. J. Cryst. Growth 2020, 533, 125479. [Google Scholar] [CrossRef]
- Guzik, M.; Pejchal, J.; Yoshikawa, A.; Ito, A.; Goto, T.; Siczek, M.; Lis, T.; Boulon, G. Structural investigations of Lu2O3 as single crystal and polycrystalline transparent ceramic. Cryst. Growth Des. 2014, 14, 3327–3334. [Google Scholar] [CrossRef]
- Erb, R.M.; Libanori, R.; Rothfuchs, N.; Studart, A.R. Composites Reinforced in Three Dimensions by Using Low Magnetic Fields. Science 2012, 335, 199–204. [Google Scholar] [CrossRef]
- Magrini, T.; Bouville, F.; Lauria, A.; Le Ferrand, H.; Niebel, T.P.; Studart, A.R. Transparent and tough bulk composites inspired by nacre. Nat. Commun. 2019, 10, 2794. [Google Scholar] [CrossRef] [Green Version]
- Magrini, T.; Moser, S.; Fellner, M.; Lauria, A.; Bouville, F.; Studart, A.R. Transparent Nacre-like Composites Toughened through Mineral Bridges. Adv. Funct. Mater. 2020, 30, 2002149. [Google Scholar] [CrossRef]
- Nydegger, M.; Deshmukh, R.; Tervoort, E.; Niederberger, M.; Caseri, W. Composites of Copper Nanowires in Polyethylene: Preparation and Processing to Materials with NIR Dichroism. ACS Omega 2019, 4, 11223–11228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernenko, K.A.; Gorokhova, E.I.; Eronko, S.B.; Sandulenko, A.V.; Venevtsev, I.D.; Wieczorek, H.; Rodnyi, P.A. Structural, Optical, and Luminescent Properties of ZnO:Ga and ZnO:In Ceramics. IEEE Trans. Nucl. Sci. 2018, 65, 2196–2202. [Google Scholar] [CrossRef]
- Shmurak, S.Z.; Kedrov, V.V.; Klassen, N.V.; Shakhrai, O.A. Spectroscopy of composite scintillators. Phys. Solid State 2012, 54, 2266–2276. [Google Scholar] [CrossRef]
- Kalyvas, N.; Liaparinos, P.; Michail, C.; David, S.; Fountos, G.; Wójtowicz, M.; Zych, E.; Kandarakis, I. Studying the luminescence efficiency of Lu2O3:Eu nanophosphor material for digital X-ray imaging applications. Appl. Phys. A 2012, 106, 131–136. [Google Scholar] [CrossRef]
- Zych, E.; Meijerink, A.; De, C.; Donegá, M. Quantum efficiency of europium emission from nanocrystalline powders of Lu2O3: Eu. J. Phys. Condens. Matter 2003, 15, 5145–5155. [Google Scholar] [CrossRef]
- Zych, E.; Trojan-Piegza, J. Low-Temperature Luminescence of Lu2O3: Eu Ceramics upon Excitation with Synchrotron Radiation in the Vicinity of Band Gap Energy. Chem. Mater. 2006, 18, 2194–2199. [Google Scholar] [CrossRef]
- Cao, Y.C. Synthesis of Square Gadolinium-Oxide Nanoplates. J. Am. Chem. Soc. 2004, 126, 7456–7457. [Google Scholar] [CrossRef]
- Mahajan, S.V.; Hart, J.; Hood, J.; Everheart, A.; Redigolo, M.L.; Koktysh, D.S.; Payzant, E.A.; Dickerson, J.H. Synthesis of RE(OH)2Cl and REOCl (RE=Eu, Tb) nanostructures. J. Rare Earths 2008, 26, 131–135. [Google Scholar] [CrossRef]
- Fang, Y.-P.; Xu, A.-W.; You, L.-P.; Song, R.-Q.; Yu, J.C.; Zhang, H.-X.; Li, Q.; Liu, H.-Q. Hydrothermal Synthesis of Rare Earth (Tb, Y) Hydroxide and Oxide Nanotubes. Adv. Funct. Mater. 2003, 13, 955–960. [Google Scholar] [CrossRef]
- Li, G.; Peng, C.; Zhang, C.; Xu, Z.; Shang, M.; Yang, D.; Kang, X.; Wang, W.; Li, C.; Cheng, Z.; et al. Eu3+ /Tb3+ -Doped La2O2CO 3/La2O3 Nano/Microcrystals with Multiform Morphologies: Facile Synthesis, Growth Mechanism, and Luminescence Properties. Inorg. Chem. 2010, 49, 10522–10535. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Zhu, Z.; Chen, L.; Hu, J.; Xu, M.; Cheng, C.; Ouyang, X.; Zhang, Z.; Ruan, J.; et al. Modified timing characteristic of a scintillation detection system with photonic crystal structures. Opt. Lett. 2017, 42, 987–990. [Google Scholar] [CrossRef]
- Knapitsch, A.; Auffray, E.; Fabjan, C.W.; Leclercq, J.L.; Lecoq, P.; Letartre, X.; Seassal, C. Photonic crystals: A novel approach to enhance the light output of scintillation based detectors. Nucl. Instrum. Methods Phys. Res. 2011, 628, 385–388. [Google Scholar] [CrossRef]
- Zehnder, R.A.; Clark, D.L.; Scott, B.L.; Donohoe, R.J.; Palmer, P.D.; Runde, W.H.; Hobart, D.E. Investigation of the Structural Properties of an Extended Series of Lanthanide Bis-hydroxychlorides Ln(OH)2Cl (Ln = Nd − Lu, except Pm and Sm). Inorg. Chem. 2010, 49, 4781–4790. [Google Scholar] [CrossRef]
- Olliges-Stadler, I.; Rossell, M.D.; Süess, M.J.; Ludi, B.; Bunk, O.; Pedersen, J.S.; Birkedal, H.; Niederberger, M. A comprehensive study of the crystallization mechanism involved in the nonaqueous formation of tungstite. Nanoscale 2013, 5, 8517. [Google Scholar] [CrossRef]
- Thoř, T.; Rubešová, K.; Jakeš, V.; Cajzl, J.; Nádherný, L.; Mikolášová, D.; Kučerková, R.; Nikl, M. Lanthanide-doped Lu2O3 phosphors and scintillators with green-to-red emission. J. Lumin. 2019, 215, 116647. [Google Scholar] [CrossRef]
- Brandt, G.; Diehl, R. Preparation, powder data and crystal structure of YbOCl. Mater. Res. Bull. 1974, 9, 411–419. [Google Scholar] [CrossRef]
- Pinna, N.; Garnweitner, G.; Beato, P.; Niederberger, M.; Antonietti, M. Synthesis of Yttria-Based Crystalline and Lamellar Nanostructures and their Formation Mechanism. Small 2004, 1, 112–121. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Lauria, A.; Chiodini, N.; Fasoli, M.; Mihóková, E.; Moretti, F.; Nale, A.; Nikl, M.; Vedda, A. Acetate–citrate gel combustion: A strategy for the synthesis of nanosized lutetium hafnate phosphor powders. J. Mater. Chem. 2011, 21, 8975. [Google Scholar] [CrossRef]
- Boyer, J.C.; Vetrone, F.; Capobianco, J.A.; Speghini, A.; Bettinelli, M. Variation of Fluorescence Lifetimes and Judd-Ofelt Parameters between Eu 3+ Doped Bulk and Nanocrystalline Cubic Lu2O3. J. Phys. Chem. B 2004, 108, 20137–20143. [Google Scholar] [CrossRef]
- Mahajan, S.V.; Redígolo, M.L.; Koktysh, D.S.; Dickerson, J.H. Stepwise assembly of europium sesquioxide nanocrystals and nanoneedles. In Proceedings of the Nanophotonic Materials III, San Diego, CA, USA, 13–17 August 2006; Gaburro, Z., Cabrini, S., Eds.; Volume 6321, p. 632102. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellner, M.; Soppelsa, A.; Lauria, A. Heat-Induced Transformation of Luminescent, Size Tuneable, Anisotropic Eu:Lu(OH)2Cl Microparticles to Micro-Structurally Controlled Eu:Lu2O3 Microplatelets. Crystals 2021, 11, 992. https://doi.org/10.3390/cryst11080992
Fellner M, Soppelsa A, Lauria A. Heat-Induced Transformation of Luminescent, Size Tuneable, Anisotropic Eu:Lu(OH)2Cl Microparticles to Micro-Structurally Controlled Eu:Lu2O3 Microplatelets. Crystals. 2021; 11(8):992. https://doi.org/10.3390/cryst11080992
Chicago/Turabian StyleFellner, Madeleine, Alberto Soppelsa, and Alessandro Lauria. 2021. "Heat-Induced Transformation of Luminescent, Size Tuneable, Anisotropic Eu:Lu(OH)2Cl Microparticles to Micro-Structurally Controlled Eu:Lu2O3 Microplatelets" Crystals 11, no. 8: 992. https://doi.org/10.3390/cryst11080992
APA StyleFellner, M., Soppelsa, A., & Lauria, A. (2021). Heat-Induced Transformation of Luminescent, Size Tuneable, Anisotropic Eu:Lu(OH)2Cl Microparticles to Micro-Structurally Controlled Eu:Lu2O3 Microplatelets. Crystals, 11(8), 992. https://doi.org/10.3390/cryst11080992