Defect-Related Etch Pits on Crystals and Their Utilization
Abstract
:1. Introduction
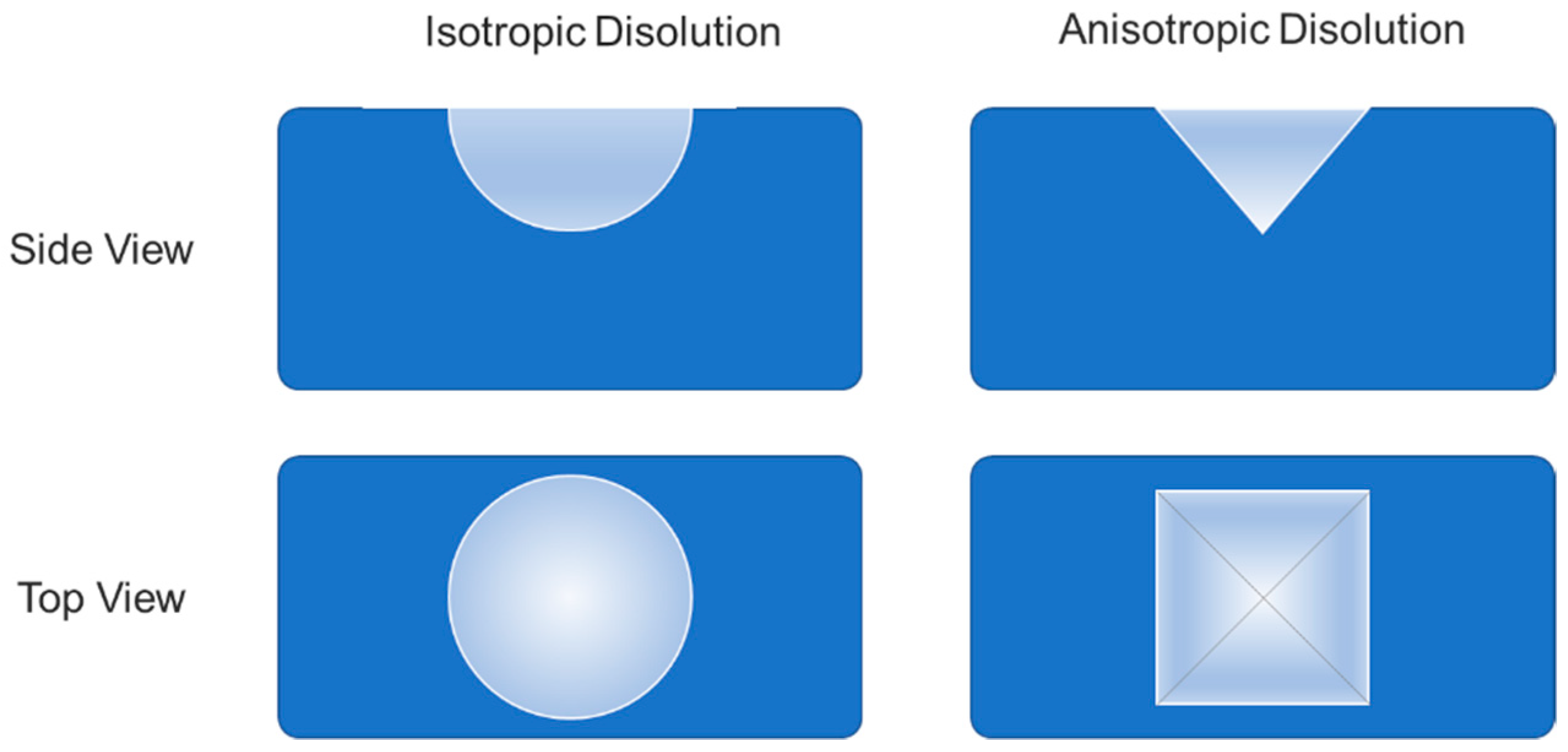
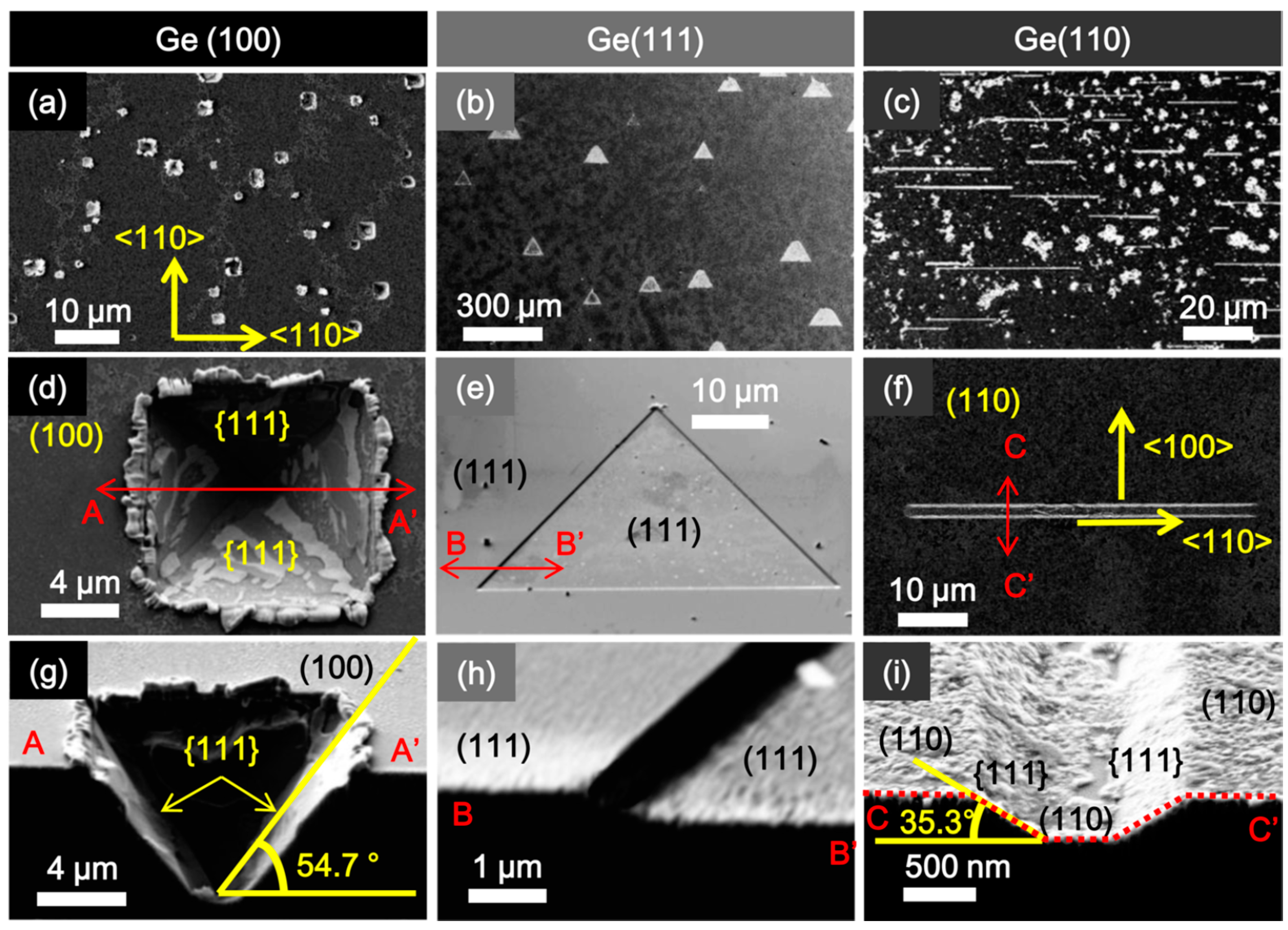
2. The Morphology of Etch Pits
3. The Origin of Etch Pits
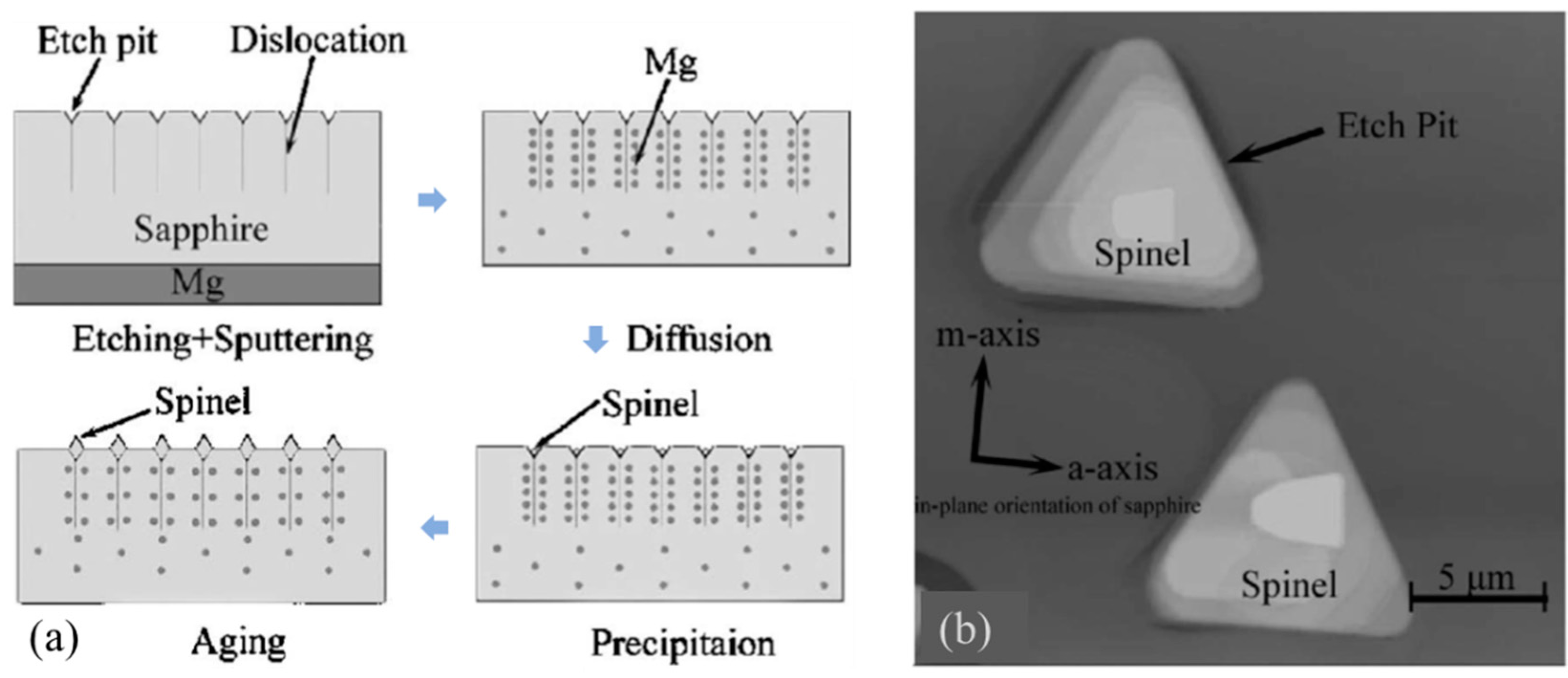
3.1. Relationship between Etch Pits and Dislocations
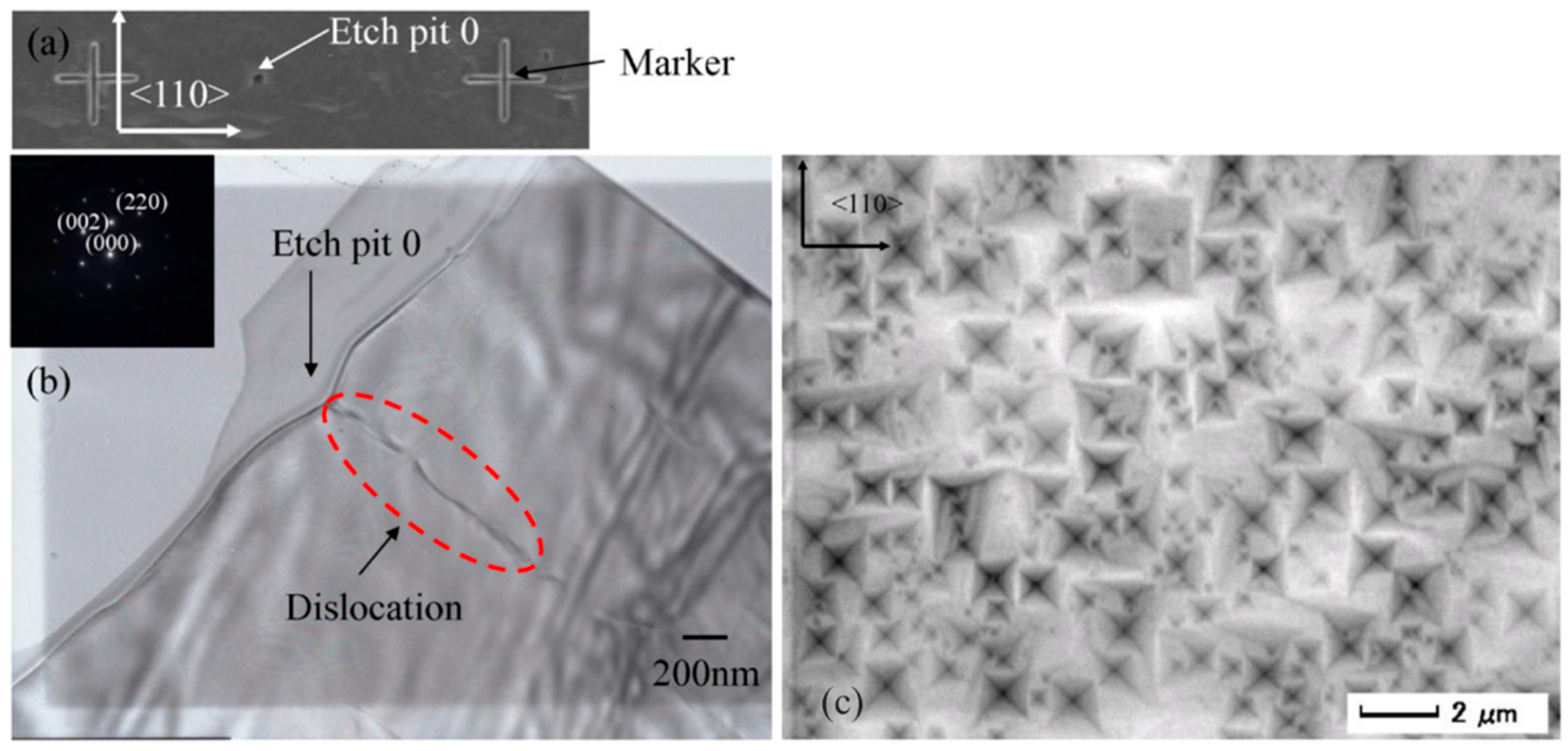
3.1.1. Identification of the Relationship between Dislocations and Etch Pits
3.1.2. Identification of Dislocation Types
3.2. Other Defects
4. Influencing Factors for the Formation of Etch Pits
4.1. The Etchant
4.1.1. Effect on the Morphology of Etch Pits
4.1.2. Effect on the Density of Etch Pits
4.1.3. Effect on the Etching Rate
4.2. The Etching Time
4.2.1. Effect on the Morphology of Etch Pits
4.2.2. Effect on the Density of Etch Pits
4.3. The Etching Temperature
4.4. The Matrix
4.5. Electrochemical Parameters
4.6. The Atmosphere
4.7. Other Factors
5. Applications of Etch Pit Technology
5.1. Investigation of Dislocations
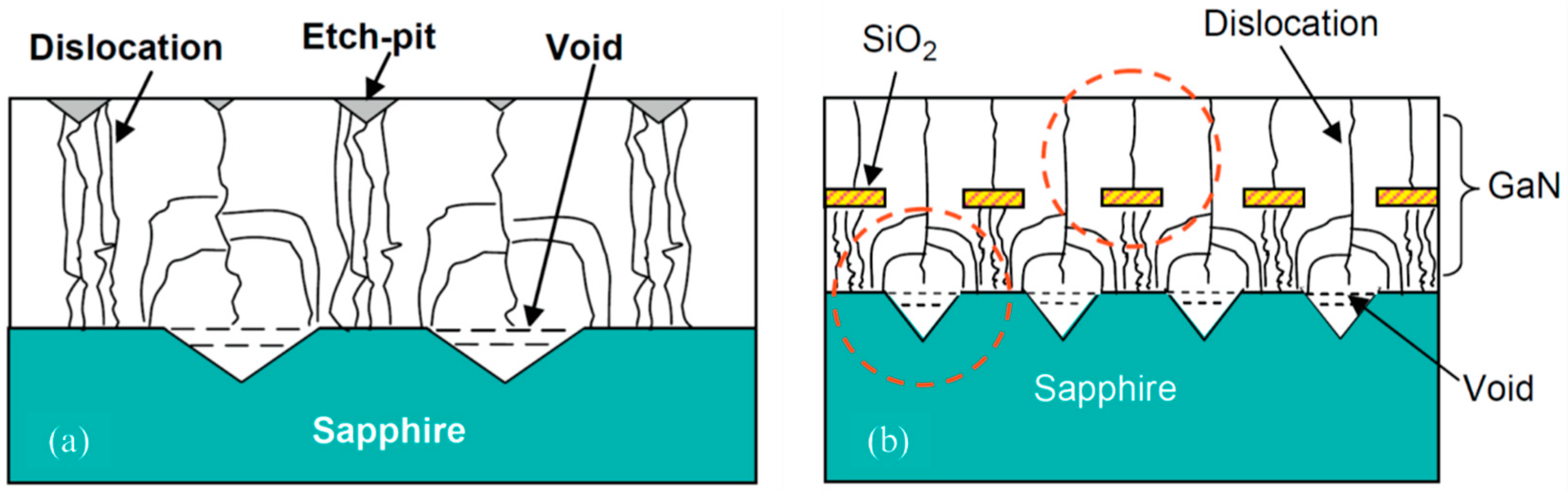
5.2. Reduction of Dislocations
5.3. Count of Precipitates
5.4. Polytype Identification
5.5. Polarity Detemination
5.6. Detection of Leakage Current
6. Trends Related to Etch Pit Technology
6.1. Characterization of Etch Pits
6.2. Simulation of Disolution
6.3. Etching on Macro Patterns
6.4. Self-Assemble of Particles at Etch Pits
6.5. Crystal Growth at Etch Pits
7. Summary
8. Outlook
- Atomic Characterization
- In-situ Characterization
- Dissolution Mechanism
- Numerical Simulation
- Etch Pit Cluster
- Micro- and Nanofabrication
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gautier, J.M.; Oelkers, E.H.; Schott, J. Are quartz dissolution rates proportional to B.E.T. surface areas? Geochim. Cosmochim. Acta 2001, 65, 1059–1070. [Google Scholar] [CrossRef]
- Pina, C.M.; Pimentel, C.; García-Merino, M. High resolution imaging of the dolomite (104) cleavage surface by atomic force microscopy. Surf. Sci. 2010, 604, 1877–1881. [Google Scholar] [CrossRef] [Green Version]
- Mercier, M.; Raimi, M.K.; Bonpunt, L. Observation by transmission electron microscopy of etch figures obtained on an organic molecular crystal. Microsc. Res. Tech. 1992, 21, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sugawara, S.; Sato, T.; Guo, J.Q.; Tsai, A.P. Unique shapes of micro-pits formed in an Al-Pd-Mn icosahedral quasicrystal by anodic etching. Mater. Trans. JIM 2000, 41, 1221–1225. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.Z.; Wu, M.J. Observation of etch pits in Fe-36wt%Ni Invar alloy. Int. J. Miner. Metall. Mater. 2014, 21, 682–686. [Google Scholar] [CrossRef]
- Bondokov, R.T.; Mueller, S.G.; Morgan, K.E.; Slack, G.A.; Schujman, S.; Wood, M.C.; Smart, J.A.; Schowalter, L.J. Large-area AlN substrates for electronic applications: An industrial perspective. J. Cryst. Growth 2008, 310, 4020–4026. [Google Scholar] [CrossRef]
- He, Z.; Zhao, B.; Zhu, S.; Chen, B.; Huang, W.; Lin, L.; Feng, B. Crystal growth and dislocation etch pits observation of chalcopyrite CdSiP2. J. Cryst. Growth 2018, 481, 29–34. [Google Scholar] [CrossRef]
- Jo, W.; Kim, S.J.; Kim, D.Y. Analysis of the etching behavior of ZnO ceramics. Acta Mater. 2005, 53, 4185–4188. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.F.; Wang, H.; Zhu, J.J.; Zhang, S.M.; Zhao, D.G.; Jiang, D.S.; Yang, H.; Jahn, U.; Ploog, K.H. Measurement of threading dislocation densities in GaN by wet chemical etching. Semicond. Sci. Technol. 2006, 21, 1229–1235. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Lee, R.G.; Nyakiti, L.; Armstrong, J.; Gu, Z.; Edgar, J.H.; Wen, J.G. Transmission electron microscopy study of defect-selective etched (010) ScN crystals. Mater. Lett. 2008, 62, 27–29. [Google Scholar] [CrossRef]
- Zeng, D.; Jie, W.; Wang, T.; Zhang, J.; Zha, G. Type and formation mechanism of thermal etch pit on annealed (111) CdZnTe surface. Thin Solid Films 2009, 517, 2896–2899. [Google Scholar] [CrossRef]
- Vaghayenegar, M.; Jacobs, R.N.; Benson, J.D.; Stoltz, A.J.; Almeida, L.A.; Smith, D.J. Correlation of Etch Pits and Dislocations in As-grown and Thermal Cycle-Annealed HgCdTe(211) Films. J. Electron. Mater. 2017, 46, 5007–5019. [Google Scholar] [CrossRef]
- Adkins, J.F.; Naviaux, J.D.; Subhas, A.V.; Dong, S.; Berelson, W.M. The Dissolution Rate of CaCObinf3einf in the Ocean. Annu. Rev. Mar. Sci. 2021, 13, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Hino, T.; Tomiya, S.; Miyajima, T.; Yanashima, K.; Hashimoto, S.; Ikeda, M. Characterization of threading dislocations in GaN epitaxial layers. Appl. Phys. Lett. 2000, 76, 3421–3423. [Google Scholar] [CrossRef]
- Xu, L.; Yu, B.; Yu, G.; Liu, H.; Zhang, L.; Li, X.; Huang, P.; Wang, B.; Wang, S. Study on the morphology of dislocation-related etch pits on pyramidal faces of KDP crystals. CrystEngComm 2021, 23, 2556–2562. [Google Scholar] [CrossRef]
- Yang, J.R.; Cao, X.L.; Wei, Y.F.; He, L. Traces of HgCdTe defects as revealed by etch pits. J. Electron. Mater. 2008, 37, 1241–1246. [Google Scholar] [CrossRef]
- Lantreibecq, A.; Legros, M.; Plassat, N.; Monchoux, J.P.; Pihan, E. Spatial distribution of structural defects in Cz-seeded directionally solidified silicon ingots: An etch pit study. J. Cryst. Growth 2018, 483, 183–189. [Google Scholar] [CrossRef]
- Corke, N.T.; Kawada, A.A.; Sherwood, J.N. Etching of Dislocations in Crystals of Aromatic Hydrocarbons. Nature 1967, 213, 62–63. [Google Scholar] [CrossRef]
- Sato, K.; Okada, M. Etching on large single crystals of stearic acid. Nature 1977, 269, 399–400. [Google Scholar] [CrossRef]
- Mukerji, S.; Kar, T. Etch pit study of different crystallographic faces of L-arginine hydrobromide monohydrate (LAHBr) in some organic acids. J. Cryst. Growth 1999, 204, 341–347. [Google Scholar] [CrossRef]
- Hashimoto, T.; Wu, F.; Speck, J.S.; Nakamura, S. A GaN bulk crystal with improved structural quality grown by the ammonothermal method. Nat. Mater. 2007, 6, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, F.; Tanpo, M.; Miyoshi, N.; Imade, M.; Yoshimura, M.; Mori, Y.; Kitaoka, Y.; Sasaki, T. Growth of GaN single crystals with extremely low dislocation density by two-step dislocation reduction. J. Cryst. Growth 2009, 311, 3019–3024. [Google Scholar] [CrossRef]
- Benson, J.D.; Smith, P.J.; Jacobs, R.N.; Markunas, J.K.; Jaime-Vasquez, M.; Almeida, L.A.; Stoltz, A.; Bubulac, L.O.; Groenert, M.; Wijewarnasuriya, P.S.; et al. Topography and dislocations in (112)B HgCdTe/CdTe/Si. J. Electron. Mater. 2009, 38, 1771–1775. [Google Scholar] [CrossRef]
- Lu, L.; Gao, Z.Y.; Shen, B.; Xu, F.J.; Huang, S.; Miao, Z.L.; Hao, Y.; Yang, Z.J.; Zhang, G.Y.; Zhang, X.P.; et al. Microstructure and origin of dislocation etch pits in GaN epilayers grown by metal organic chemical vapor deposition. J. Appl. Phys. 2008, 104, 123525. [Google Scholar] [CrossRef] [Green Version]
- Sadrabadi, P.; Durst, K.; Goken, M.; Blum, W. Quantification of dislocation structures at high resolution by atomic force microscopy of dislocation etch pits. Philos. Mag. Lett. 2009, 89, 391–398. [Google Scholar] [CrossRef]
- Hatayama, T.; Shimizu, T.; Kouketsu, H.; Yano, H.; Uraoka, Y.; Fuyuki, T. Thermal etching of 4H-SiC(0001) Si faces in the mixed gas of chlorine and oxygen. Jpn. J. Appl. Phys. 2009, 48, 066516. [Google Scholar] [CrossRef]
- Kallinger, B.; Polster, S.; Berwian, P.; Friedrich, J.; Mller, G.; Danilewsky, A.N.; Wehrhahn, A.; Weber, A.D. Threading dislocations in n- and p-type 4HSiC material analyzed by etching and synchrotron X-ray topography. J. Cryst. Growth 2011, 314, 21–29. [Google Scholar] [CrossRef]
- Habel, F.; Seyboth, M. Determination of dislocation density in epitaxially grown GaN using an HCl etching process. Phys. Status Solidi C Conf. 2003, 2451, 2448–2451. [Google Scholar] [CrossRef]
- Shah, I.A.; van der Wolf, B.M.A.; van Enckevort, W.J.P.; Vlieg, E. Wet chemical etching of silicon {1 1 1}: Etch pit analysis by the Lichtfigur method. J. Cryst. Growth 2009, 311, 1371–1377. [Google Scholar] [CrossRef]
- Shah, I.A.; van der Wolf, B.M.A.; van Enckevort, W.J.P.; Vlieg, E. Wet Chemical Etching of Silicon {111}: Autocatalysis in Pit Formation. J. Electrochem. Soc. 2008, 155, J79. [Google Scholar] [CrossRef]
- Bickermann, M.; Schmidt, S.; Epelbaum, B.M.; Heimann, P.; Nagata, S.; Winnacker, A. Wet KOH etching of freestanding AlN single crystals. J. Cryst. Growth 2007, 300, 299–307. [Google Scholar] [CrossRef]
- Fleck, M.; Zuschlag, A.; Hahn, G. Etch Pit Density Reduction in POCl3 and Atmospheric Pressure Chemical Vapor Deposition-Gettered mc-Si. Phys. Status Solidi Appl. Mater. Sci. 2019, 216, 1900316. [Google Scholar] [CrossRef] [Green Version]
- Persichetti, L.; Fanfoni, M.; De Seta, M.; Di Gaspare, L.; Ottaviano, L.; Goletti, C.; Sgarlata, A. Formation of extended thermal etch pits on annealed Ge wafers. Appl. Surf. Sci. 2018, 462, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Velbel, M.A. Dissolution of olivine during natural weathering. Geochim. Cosmochim. Acta 2009, 73, 6098–6113. [Google Scholar] [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures—A technical review. J. Loss Prev. Process Ind. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Deng, Y.; Wang, Z.; Jiang, Q.; Yang, L.; Zhang, J. Corrosion of rail tracks and their protection. Corros. Rev. 2021, 39, 1–13. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion of Metals. Corrosion 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Campbell, J. The Mechanisms of Metallurgical Failure The origin of Fracture; Matthew Deans: Oxford, UK, 2020. [Google Scholar]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Udono, H.; Kikuma, I. Etch pits observation and etching properties of β-FeSi2. Mater. Sci. Semicond. Process. 2003, 6, 413–416. [Google Scholar] [CrossRef]
- Jung Jung, S.; Lutz, T.; Boland, J.J. Anisotropic etching induced by surface energy driven agglomeration. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29, 051403. [Google Scholar] [CrossRef]
- Kachroo, S.K.; Bamzai, K.K.; Dhar, P.R.; Kotru, P.N.; Wanklyn, B.M. Delineation of defect structures in flux-grown GdFeO3 crystals by etching. Appl. Surf. Sci. 2000, 156, 149–154. [Google Scholar] [CrossRef]
- Seo, J.H.; Ryu, J.-H.; Lee, D.N. Formation of Crystallographic Etch Pits during AC Etching of Aluminum. J. Electrochem. Soc. 2003, 150, B433. [Google Scholar] [CrossRef]
- Bickermann, M.; Schmidt, S.; Epelbaum, B.M.; Heimann, P.; Nagata, S.; Winnacker, A. Defect-selective etching of aluminum nitride single crystals. Phys. Status Solidi Curr. Top. Solid State Phys. 2007, 4, 2609–2612. [Google Scholar] [CrossRef]
- Lang, M.; Glasmacher, U.A.; Moine, B.; Neumann, R.; Wagner, G.A. Etch-pit morphology of tracks caused by swift heavy ions in natural dark mica. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 218, 466–471. [Google Scholar] [CrossRef]
- Liu, C.M.; Chen, J.C.; Huang, Y.C.; Hsieh, H.L. The morphology of etch pits on a sapphire surface. J. Phys. Chem. Solids 2008, 69, 572–575. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, S.; Zhao, B.; Chen, B.; He, Z.; Fan, Q.; Xu, T. Chemical etching orientation of ZnGeP2 single crystals. J. Cryst. Growth 2011, 318, 729–732. [Google Scholar] [CrossRef]
- Huang, H.; Chou, M.M.C.; Gan, D.; Shen, P. A proper solution to study the etch pits on LiAlO2 single crystal. J. Cryst. Growth 2010, 313, 42–46. [Google Scholar] [CrossRef]
- Lin, C.T. Study of growth spirals and screw dislocations on YBa2Cu3O7-δ single crystals. Phys. C Supercond. Its Appl. 2000, 337, 312–316. [Google Scholar] [CrossRef]
- Nijdam, A.J.; Gardeniers, J.G.E.; Gui, C.; Elwenspoek, M. Etching pits and dislocations in Si(111). Sens. Actuators A Phys. 2000, 86, 238–247. [Google Scholar] [CrossRef]
- Vetter, W.M.; Dudley, M. Micropipes in Silicon Carbide Crystals: Do all Screw Dislocations have Open Cores? J. Mater. Res. 2000, 15, 1649–1652. [Google Scholar] [CrossRef]
- Edgar, J.H.; Liu, S.; Hoffman, T.; Zhang, Y.; Twigg, M.E.; Bassim, N.D.; Liang, S.; Khan, N. Defect sensitive etching of hexagonal boron nitride single crystals. J. Appl. Phys. 2017, 122, 225110. [Google Scholar] [CrossRef]
- Kuwano, N.; Tajima, R.; Bohyama, S.; Miyake, H.; Hiramatsu, K.; Shibata, T. Influence of etching condition on surface morphology of AlN and GaN layers. Phys. Status Solidi Appl. Res. 2004, 201, 2755–2759. [Google Scholar] [CrossRef]
- Ichikawa, K.; Kodama, H.; Suzuki, K.; Sawabe, A. Dislocation in heteroepitaxial diamond visualized by hydrogen plasma etching. Thin Solid Films 2016, 600, 142–145. [Google Scholar] [CrossRef]
- Shiojima, K. Atomic force microscopy and transmission electron microscopy observations of KOH-etched GaN surfaces. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2000, 18, 37. [Google Scholar] [CrossRef]
- Yao, Y.; Ishikawa, Y.; Sugawara, Y.; Sato, K.; Danno, K.; Suzuki, H.; Bessho, T.; Yamaguchi, S.; Nishikawa, K. Correlation between etch pits formed by molten KOH+Na2O2 etching and dislocation types in heavily doped n+-4H-SiC studied by X-ray topography. J. Cryst. Growth 2013, 364, 7–10. [Google Scholar] [CrossRef]
- Masuya, S.; Kasu, M. Dislocations in chemical vapor deposition (111) single crystal diamond observed by synchrotron X-ray topography and their relation with etch pits. Diam. Relat. Mater. 2018, 90, 40–46. [Google Scholar] [CrossRef]
- Ohtani, N.; Katsuno, M.; Tsuge, H.; Fujimoto, T.; Nakabayashi, M.; Yashiro, H.; Sawamura, M.; Aigo, T.; Hoshino, T. Behavior of basal plane dislocations in hexagonal silicon carbide single crystals grown by physical vapor transport. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2006, 45, 1738–1742. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Mokuno, Y.; Shikata, S. Characterizations of etch pits formed on single crystal diamond surface using oxygen/hydrogen plasma surface treatment. Diam. Relat. Mater. 2016, 63, 43–46. [Google Scholar] [CrossRef]
- Motzer, C.; Reichling, M. Morphological classification and quantitative analysis of etch pits. J. Appl. Phys. 2010, 108, 113523. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Revealing of planar defects and partial dislocations in large synthetic diamond crystals by the selective etching. J. Cryst. Growth 2007, 306, 458–464. [Google Scholar] [CrossRef]
- Kasu, M.; Oshima, T.; Hanada, K.; Moribayashi, T.; Hashiguchi, A.; Oishi, T.; Koshi, K.; Sasaki, K.; Kuramata, A.; Ueda, O. Crystal defects observed by the etch-pit method and their effects on Schottky-barrier-diode characteristics on (201) β-Ga2O3. Jpn. J. Appl. Phys. 2017, 56, 091101. [Google Scholar] [CrossRef]
- Ueda, T.; Takai, Y.; Shimizu, R.; Yagyu, H.; Matsushima, T.; Souma, M. Cross-sectional transmission electron microscopic observation of etch hillocks and etch pits in LiTaO3 single crystal. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2000, 39, 1200–1202. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhu, L.H.; Sun, S.W.; Yu, H.X.; Xu, C.; Yang, J.R. Relationship of the shape and size between etch pits and corresponding Te inclusions in CdZnTe crystals. J. Cryst. Growth 2019, 512, 90–95. [Google Scholar] [CrossRef]
- Hong, S.K.; Yao, T.; Kim, B.J.; Yoon, S.Y.; Kim, T.I. Origin of hexagonal-shaped etch pits formed in (0001) GaN films. Appl. Phys. Lett. 2000, 77, 82–84. [Google Scholar] [CrossRef]
- Ueda, O.; Ikenaga, N.; Koshi, K.; Iizuka, K.; Kuramata, A.; Hanada, K.; Moribayashi, T.; Yamakoshi, S.; Kasu, M. Structural evaluation of defects in β-Ga2O3 single crystals grown by edge-defined film-fed growth process. Jpn. J. Appl. Phys. 2016, 55, 1202BD. [Google Scholar] [CrossRef]
- Adamczyk, K.; Stokkan, G.; Di Sabatino, M. Guidelines for establishing an etching procedure for dislocation density measurements on multicrystalline silicon samples. MethodsX 2018, 5, 1178–1186. [Google Scholar] [CrossRef]
- Lu, M.; Chang, X.; Li, Z.L.; Yang, Z.J.; Zhang, G.Y.; Zhang, B. Etch pits and threading dislocations in GaN films grown by metal-organic chemical vapour deposition. Chin. Phys. Lett. 2003, 20, 398–400. [Google Scholar] [CrossRef]
- Britt, D.W.; Hlady, V. In-situ atomic force microscope imaging of calcite etch pit morphology changes in undersaturated and 1-hydroxyethylidene-1,1-diphosphonic acid poisoned solutions. Langmuir 1997, 13, 1873–1876. [Google Scholar] [CrossRef] [Green Version]
- Na, K.H.; Pyun, S. Il Effects of SO42−, S2O32− and HSO4− Ion additives on the pitting corrosion of pure aluminium in 1 M NaCl solution at 40–70 °C. J. Solid State Electrochem. 2005, 9, 639–645. [Google Scholar] [CrossRef]
- Yao, Y.-Z.; Ishikawa, Y.; Sugawara, Y.; Saitoh, H.; Danno, K.; Suzuki, H.; Kawai, Y.; Shibata, N. Molten KOH Etching with Na2O2 Additive for Dislocation Revelation in 4H-SiC Epilayers and Substrates. Jpn. J. Appl. Phys. 2011, 50, 075502. [Google Scholar] [CrossRef]
- Lin, C.S.; Li, W.J. Pitting Behavior of Aluminum Foil during Alternating Current Etching in Hydrochloric Acid Containing Sulfate Ions. J. Electrochem. Soc. 2006, 153, C51. [Google Scholar] [CrossRef]
- Hsu, H.C.; Su, Y.K.; Cheng, S.H.; Huang, S.J.; Cao, J.M.; Chen, K.C. Investigation of etch characteristics of non-polar GaN by wet chemical etching. Appl. Surf. Sci. 2010, 257, 1080–1083. [Google Scholar] [CrossRef]
- Shen, G.; Zhao, Y.; Sun, J.; Liu, J.; Xie, H.; Yang, J.; Dong, Z. HCl-H2SO4-H2O solution etching behavior of InAs (1 0 0) surface. J. Cryst. Growth 2020, 547, 125800. [Google Scholar] [CrossRef]
- Jianrong, Y.; Huiming, G.; Xinqiang, C.; Weizheng, F.; Li, H. Dislocation assessment of CdZnTe by chemical etching on both (1 1 1)B and (2 1 1)B faces. J. Cryst. Growth 2002, 234, 337–342. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, Y.; Wu, Y.; Hao, X.; Chen, X.; Qu, S.; Xu, X. Characterization of dislocation etch pits in HVPE-grown GaN using different wet chemical etching methods. J. Alloys Compd. 2010, 504, 186–191. [Google Scholar] [CrossRef]
- Min, L.; Xin, C.; Hui-Zhi, F.; Zhi-Jian, Y.; Hua, Y.; Zi-Lan, L.; Qian, R.; Guo-Yi, Z.; Bei, Z. Etch-pits and threading dislocations in thick LEO GaN films on sapphire grown by MOCVD. Phys. Status Solidi C Conf. 2004, 1, 2438–2440. [Google Scholar] [CrossRef]
- Karan, S.; Sen Gupta, S.; Sen Gupta, S.P. Revelation of dislocation etch pits in mixed crystals of ammonium-potassium sulphate, [K1−x(NH4)x]2SO4. J. Cryst. Growth 2001, 233, 555–560. [Google Scholar] [CrossRef]
- Han, S.-C.; Kim, J.-K.; Kim, J.Y.; Kim, K.-K.; Tampo, H.; Niki, S.; Lee, J.-M. Formation of Hexagonal Pyramids and Pits on V-/VI-Polar and III-/II-Polar GaN/ZnO Surfaces by Wet Etching. J. Electrochem. Soc. 2010, 157, D60. [Google Scholar] [CrossRef]
- Tiwari, R.N.; Chang, L. Etching of GaN by microwave plasma of hydrogen. Semicond. Sci. Technol. 2010, 25, 035010. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.S.; Resch, R.; Dunn, K.; Shuler, P.; Tang, Y.; Koel, B.E.; Yen, T.F. Scanning force microscopy study of etch pits formed during dissolution of a barite (001) surface in CDTA and EDTA solutions. Langmuir 2000, 16, 649–655. [Google Scholar] [CrossRef]
- Karuppasamy, P.; Kamalesh, T.; Anitha, K.; Abdul Kalam, S.; Senthil Pandian, M.; Ramasamy, P.; Verma, S.; Venugopal Rao, S. Synthesis, crystal growth, structure and characterization of a novel third order nonlinear optical organic single crystal: 2-Amino 4,6-Dimethyl Pyrimidine 4-nitrophenol. Opt. Mater. 2018, 84, 475–489. [Google Scholar] [CrossRef]
- Hanada, K.; Moribayashi, T.; Koshi, K.; Sasaki, K.; Kuramata, A.; Ueda, O.; Kasu, M. Origins of etch pits in β-Ga2O3(010) single crystals. Jpn. J. Appl. Phys. 2016, 55, 1202BG. [Google Scholar] [CrossRef] [Green Version]
- Osawa, N.; Fukuoka, K. Pit nucleation behavior of aluminium foil for electrolytic capacitors during early stage of DC etching. Corros. Sci. 2000, 42, 585–597. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Revealing of dislocations in diamond crystals by the selective etching method. J. Cryst. Growth 2006, 293, 469–474. [Google Scholar] [CrossRef]
- Ono, S.; Habazaki, H. Pit growth behaviour of aluminium under galvanostatic control. Corros. Sci. 2011, 53, 3521–3525. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Zhang, W.; Peng, K. Characterization and formation mechanism of pits on diamond {100} face etched by molten potassium nitrite. Int. J. Refract. Met. Hard Mater. 2018, 71, 129–134. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, X.; Xie, X.; Xu, X. Threading dislocation classification for 4H-SiC substrates using the KOH etching method. CrystEngComm 2018, 20, 978–982. [Google Scholar] [CrossRef]
- Yasuda, N.; Koguchi, Y.; Tsubomatsu, M.; Takagi, T.; Kobayashi, I.; Tsuruta, T.; Morishima, H. Extremely high dose neutron dosimetry using CR-39 and atomic force microscopy. Radiat. Prot. Dosim. 2006, 120, 470–474. [Google Scholar] [CrossRef]
- Kachroo, S.K.; Bamzai, K.K.; Dhar, P.R.; Kotru, P.N.; Wanklyn, B.M. Etch patterns on flux grown DyFeO3 crystal surfaces. Mater. Chem. Phys. 2001, 68, 72–76. [Google Scholar] [CrossRef]
- Gu, Z.; Edgar, J.H.; Coffey, D.W.; Chaudhuri, J.; Nyakiti, L.; Lee, R.G.; Wen, J.G. Defect-selective etching of scandium nitride crystals. J. Cryst. Growth 2006, 293, 242–246. [Google Scholar] [CrossRef]
- Petukhov, V.; Bakin, A.; El-Shaer, A.H.; Mofor, A.C.; Waag, A. Etch-pit density investigation on both polar faces of ZnO substrates. Electrochem. Solid-State Lett. 2007, 10, H357. [Google Scholar] [CrossRef]
- Habuka, H.; Fukumoto, Y.; Kato, T. Off-Orientation Influence on C-Face (0001) 4H-SiC Surface Morphology Produced by Etching Using Chlorine Trifluoride Gas. ECS J. Solid State Sci. Technol. 2013, 2, N3025–N3027. [Google Scholar] [CrossRef]
- Robey, S.W.; Sinniah, K. Initial etching of GaAs (001) during [formula omitted] plasma cleaning. J. Appl. Phys. 2000, 88, 2994–2998. [Google Scholar] [CrossRef]
- Wu, P. Etching study of dislocations in heavily nitrogen doped SiC crystals. J. Cryst. Growth 2010, 312, 1193–1198. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Effect of nitrogen impurity on etching of synthetic diamond crystals. J. Cryst. Growth 2015, 430, 71–74. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, J.; Lee, J.; Chung, H.; Tak, Y. Effects of pretreatment on the aluminium etch pit formation. Corros. Sci. 2009, 51, 1501–1505. [Google Scholar] [CrossRef]
- Farrell, S.; Rao, M.V.; Brill, G.; Chen, Y.; Wijewarnasuriya, P.; Dhar, N.; Benson, J.D.; Harris, K. Comparison of the schaake and benson etches to delineate dislocations in HgCdTe layers. J. Electron. Mater. 2013, 42, 3097–3102. [Google Scholar] [CrossRef]
- Sundararajan, S.P.; Crouse, D.; Lo, Y.-H. Gallium nitride: Method of defect characterization by wet oxidation in an oxalic acid electrolytic cell. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2002, 20, 1339. [Google Scholar] [CrossRef]
- Ono, S.; Habazaki, H. Role of cathodic half-cycle on AC etch process of aluminium. Corros. Sci. 2010, 52, 2164–2171. [Google Scholar] [CrossRef]
- Stallcup, R.E.; Mo, Y.; Scharf, T.W.; Perez, J.M. Formation of nanometer-size high-density pits on epitaxial diamond (100) films. Diam. Relat. Mater. 2007, 16, 1727–1731. [Google Scholar] [CrossRef]
- Wheeler, E.K.; Whitman, P.K.; Land, T.A.; De Yoreo, J.; Thorsness, C.B.; McWhirter, J.H.; Hanna, M.L.; Miller, E.L. Investigation of etch pits on KDP crystals with porous sol-gel coatings. Appl. Phys. A Mater. Sci. Process. 2002, 74, 813–823. [Google Scholar] [CrossRef]
- Ohashi, T.; Sugimoto, W.; Takasu, Y. Catalytic etching of {100}-oriented diamond coating with Fe, Co, Ni, and Pt nanoparticles under hydrogen. Diam. Relat. Mater. 2011, 20, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Ivashchenko, V.E.; Boldyrev, V.V.; Zakharov, Y.A.; Shakhtshneider, T.P.; Ermakov, A.E.; Krasheninin, V.I. The effect of magnetic field on the shape of etch pits of paracetamol crystals. Mater. Res. Innov. 2002, 5, 214–218. [Google Scholar] [CrossRef]
- Weyher, J.L. Characterization of Compound Semiconductors by Etching. In Concise Encyclopedia of Semiconducting Materials & Related Technologies; Pergamon: Oxford, UK, 1992; pp. 37–44. [Google Scholar] [CrossRef]
- McDougall, D.J. Etch pits. In Mineralogy; Frye, K., Ed.; Springer: Boston, MA, USA, 1983; pp. 150–152. [Google Scholar]
- Cao, Y.; Ni, S.; Liao, X.; Song, M.; Zhu, Y. Structural evolutions of metallic materials processed by severe plastic deformation. Mater. Sci. Eng. R Rep. 2018, 133, 1–59. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. (Eds.) Smithells Metals Reference Book; Elsevier: Oxford, UK, 2004; p. 26. [Google Scholar]
- Nicolov, M. Shaped single crystals of CaF2. J. Cryst. Growth 2000, 218, 62–66. [Google Scholar] [CrossRef]
- Javaid, F.; Bruder, E.; Durst, K. Indentation size effect and dislocation structure evolution in (001) oriented SrTiO3 Berkovich indentations: HR-EBSD and etch-pit analysis. Acta Mater. 2017, 139, 1–10. [Google Scholar] [CrossRef]
- Hossain, A.; Bolotnikov, A.E.; Camarda, G.S.; Cui, Y.; Yang, G.; James, R.B. Defects in cadmium zinc telluride crystals revealed by etch-pit distributions. J. Cryst. Growth 2008, 310, 4493–4498. [Google Scholar] [CrossRef]
- Yao, Y.; Sugawara, Y.; Ishikawa, Y.; Okada, N.; Tadatomo, K. Crystallinity Evaluation and Dislocation Observation for an Aluminum Nitride Single-Crystal Substrate on a Wafer Scale. J. Electron. Mater. 2020, 49, 5144–5153. [Google Scholar] [CrossRef]
- Cui, X.P.; Fang, W.Z.; Sun, S.W.; Zhang, C.J.; Xu, H.L.; Yang, J.R. Characteristics of the dislocations in CdZnTe crystals revealed by etch pits. J. Cryst. Growth 2011, 321, 40–44. [Google Scholar] [CrossRef]
- Lisovenko, V.A.; Khutornaya, L.A.; Shpak, M.T.; Velikaya, E.N. Basal dislocations in anthracene crystals. Phys. Status Solidi 1977, 42, 433–437. [Google Scholar] [CrossRef]
- Motoki, K.; Okahisa, T.; Nakahata, S.; Matsumoto, N.; Kimura, H.; Kasai, H.; Takemoto, K.; Uematsu, K.; Ueno, M.; Kumagai, Y.; et al. Preparation of large GaN substrates. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2002, 93, 123–130. [Google Scholar] [CrossRef]
- Wuu, D.S.; Wu, H.W.; Chen, S.T.; Tsai, T.Y.; Zheng, X.; Horng, R.H. Defect reduction of laterally regrown GaN on GaN/patterned sapphire substrates. J. Cryst. Growth 2009, 311, 3063–3066. [Google Scholar] [CrossRef]
- Hu, W.; Die, J.; Wang, C.; Yan, S.; Hu, X.; Du, C.; Jiang, Y.; Deng, Z.; Wang, L.; Jia, H.; et al. The substantial dislocation reduction by preferentially passivating etched defect pits in GaN epitaxial growth. Appl. Phys. Express 2019, 12, 035502. [Google Scholar] [CrossRef]
- Lee, M.; Mikulik, D.; Yang, M.; Park, S. Nearly perfect GaN crystal via pit-assisted growth by HVPE. CrystEngComm 2017, 19, 2036–2041. [Google Scholar] [CrossRef]
- Leonhardt, D.; Han, S.M. New Method to Produce High-Quality Epitaxial Ge on Si Using SiO2-Lined Etch Pits and Epitaxial Lateral Overgrowth for III-V Integration. ECS Trans. 2012, 45, 147–149. [Google Scholar] [CrossRef]
- Sheng, F.F.; Cui, X.P.; Sun, S.W.; Yang, J.R. Etch pits of precipitates in CdZnTe crystals on (1 1 1) B surface. J. Cryst. Growth 2012, 354, 76–80. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z. Identification of SiC polytypes by etched Si-face morphology. Mater. Sci. Semicond. Process. 2009, 12, 113–117. [Google Scholar] [CrossRef]
- Muto, D.; Araki, T.; Naoi, H.; Matsuda, F.; Nanishi, Y. Polarity determination of InN by wet etching. Phys. Status Solidi Appl. Mater. Sci. 2005, 202, 773–776. [Google Scholar] [CrossRef]
- Kasu, M.; Hanada, K.; Moribayashi, T.; Hashiguchi, A.; Oshima, T.; Oishi, T.; Koshi, K.; Sasaki, K.; Kuramata, A.; Ueda, O. Relationship between crystal defects and leakage current in β-Ga2O3 Schottky barrier diodes. Jpn. J. Appl. Phys. 2016, 55, 1202BB. [Google Scholar] [CrossRef]
- Lee, S.W.; Oh, D.C.; Goto, H.; Ha, J.S.; Lee, H.J.; Hanada, T.; Cho, M.W.; Yao, T.; Hong, S.K.; Lee, H.Y.; et al. Origin of forward leakage current in GaN-based light-emitting devices. Appl. Phys. Lett. 2006, 89, 132117. [Google Scholar] [CrossRef]
- Hahn, G.; Fleck, M. Automatic etch pit density analysis in multicrystalline silicon. Comput. Mater. Sci. 2020, 183, 109886. [Google Scholar] [CrossRef]
- Palodhi, K.; Chatterjee, J.; Bhattacharyya, R.; Dey, S.; Ghosh, S.K.; Maulik, A.; Raha, S. Convolution based hybrid image processing technique for microscopic images of etch-pits in Nuclear Track Detectors. Radiat. Meas. 2020, 130, 106219. [Google Scholar] [CrossRef]
- Wzorek, M.; Czerwinski, A.; Ratajczak, J.; Dylewicz, R.; Katcki, J. Selective etching of dislocations in GaN and quantitative SEM analysis with shape-reconstruction method. Micron 2009, 40, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Sorai, M.; Ohsumi, T.; Ishikawa, M.; Tsukamoto, K. Feldspar dissolution rates measured using phase-shift interferometry: Implications to CO2 underground sequestration. Appl. Geochem. 2007, 22, 2795–2809. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; García-Ruíz, J.M.; Tsukamoto, K.; Patiño-Lopez, L.D.; Satoh, H. Ultraslow growth rates of giant gypsum crystals. Proc. Natl. Acad. Sci. USA 2011, 108, 15721–15726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, F.; Kuchimaru, T.; Kato, Y.; Iida, T. Digital image analysis of etch pit formation in CR-39 track detector. Jpn. J. Appl. Phys. 2008, 47, 269–272. [Google Scholar] [CrossRef]
- Kurganskaya, I.; Luttge, A. A comprehensive stochastic model of phyllosilicate dissolution: Structure and kinematics of etch pits formed on muscovite basal face. Geochim. Cosmochim. Acta 2013, 120, 545–560. [Google Scholar] [CrossRef]
- Stübner, K.; Jonckheere, R.; Ratschbacher, L. Revelation of nuclear tracks and dislocations: A Monte Carlo simulation of mineral etching. Geochim. Cosmochim. Acta 2008, 72, 3184–3199. [Google Scholar] [CrossRef]
- Ono, S.; Uchibori, K.; Asoh, H. Control of nano/microstructure and pit initiation sites on aluminium surface by use of self-assembled spheres. Surf. Interface Anal. 2010, 42, 264–268. [Google Scholar] [CrossRef]
- Brevnov, D.A. Electrochemical etching of patterned Al (100) foils in HCl. J. Micromech. Microeng. 2008, 18, 2–7. [Google Scholar] [CrossRef]
- Chen, H.M.; Suen, Y.W.; Chen, S.J.; Luo, G.L.; Lai, Y.P.; Chen, S.T.; Lee, C.H.; Kuan, C.H. Effect of surface Si redistribution on the alignment of Ge dots grown on pit-patterned Si(001) substrates. Nanotechnology 2014, 25, 475301. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Chen, J.C. Growth of Mg-Al spinel microcrystals on a sapphire surface using a solution-precipitation method. Appl. Phys. Lett. 2006, 89, 7–10. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Jiang, Q.; Ma, X.; Zhang, Q.; Fu, X.; Fan, L. Defect-Related Etch Pits on Crystals and Their Utilization. Crystals 2022, 12, 1549. https://doi.org/10.3390/cryst12111549
Lu D, Jiang Q, Ma X, Zhang Q, Fu X, Fan L. Defect-Related Etch Pits on Crystals and Their Utilization. Crystals. 2022; 12(11):1549. https://doi.org/10.3390/cryst12111549
Chicago/Turabian StyleLu, Dongzhu, Quantong Jiang, Xiumin Ma, Qichao Zhang, Xiaole Fu, and Liang Fan. 2022. "Defect-Related Etch Pits on Crystals and Their Utilization" Crystals 12, no. 11: 1549. https://doi.org/10.3390/cryst12111549
APA StyleLu, D., Jiang, Q., Ma, X., Zhang, Q., Fu, X., & Fan, L. (2022). Defect-Related Etch Pits on Crystals and Their Utilization. Crystals, 12(11), 1549. https://doi.org/10.3390/cryst12111549






