Recovery of Scandium, Aluminum, Titanium, and Silicon from Iron-Depleted Bauxite Residue into Valuable Products: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experiments
2.2.1. High-Pressure Hydrochloric Acid Leaching of IDBR
2.2.2. Alumina Precipitation
2.2.3. Scandium Solvent Extraction
2.2.4. Preparation of Amorphous Silica and Titanium Concentrate
2.3. Analysis Methods
3. Results
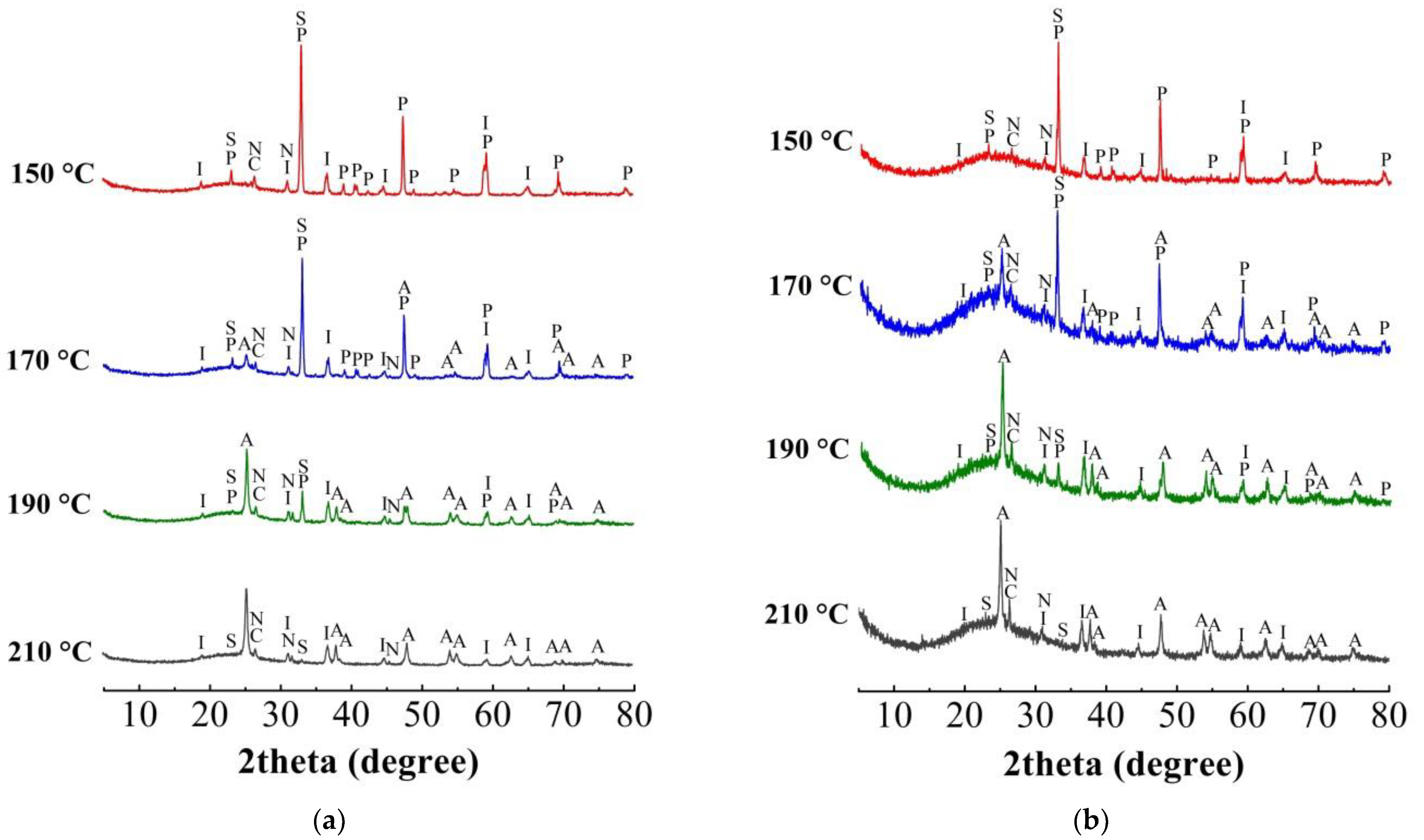
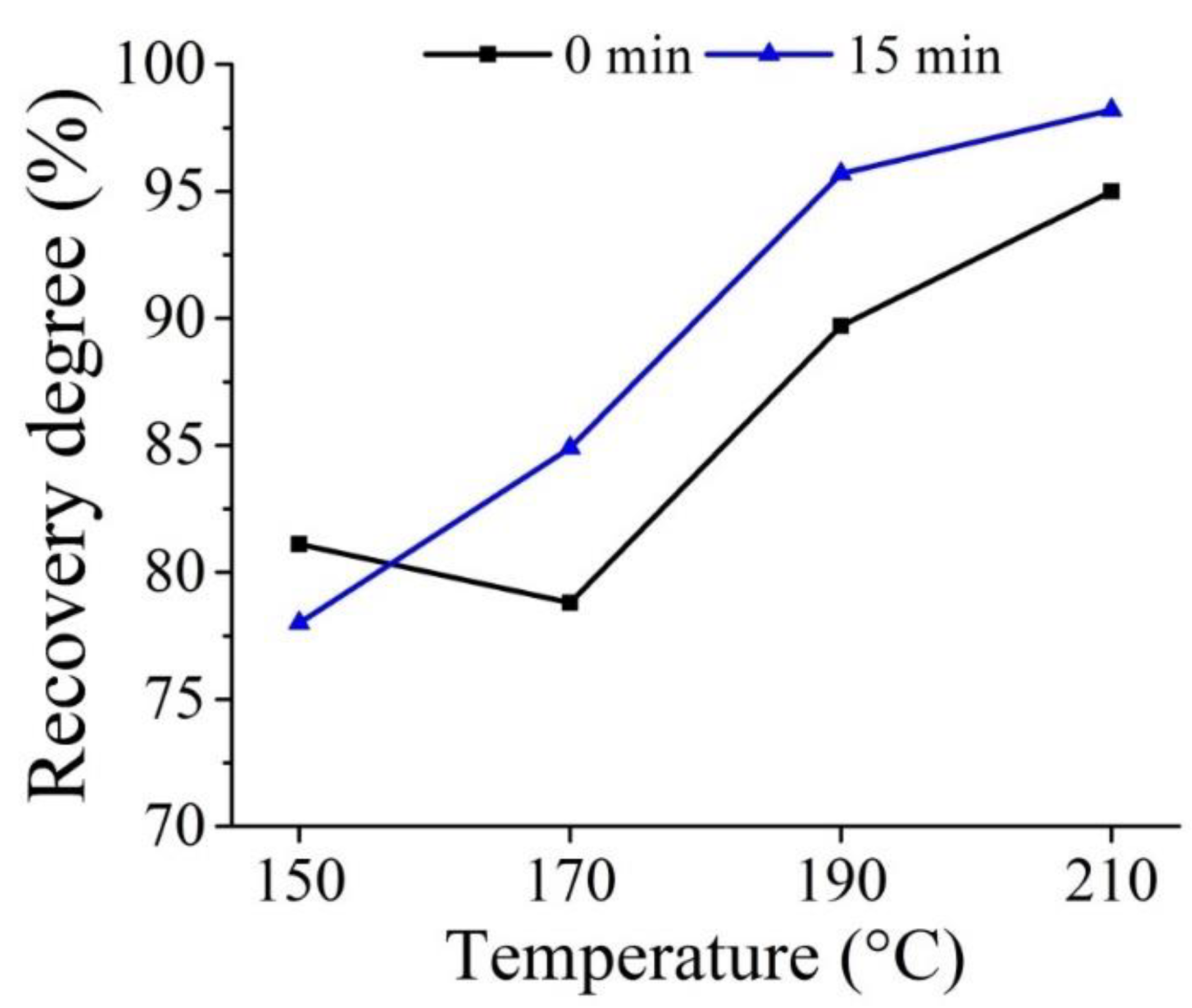
3.1. High-Pressure Hydrochloric Acid Leaching of IDBR
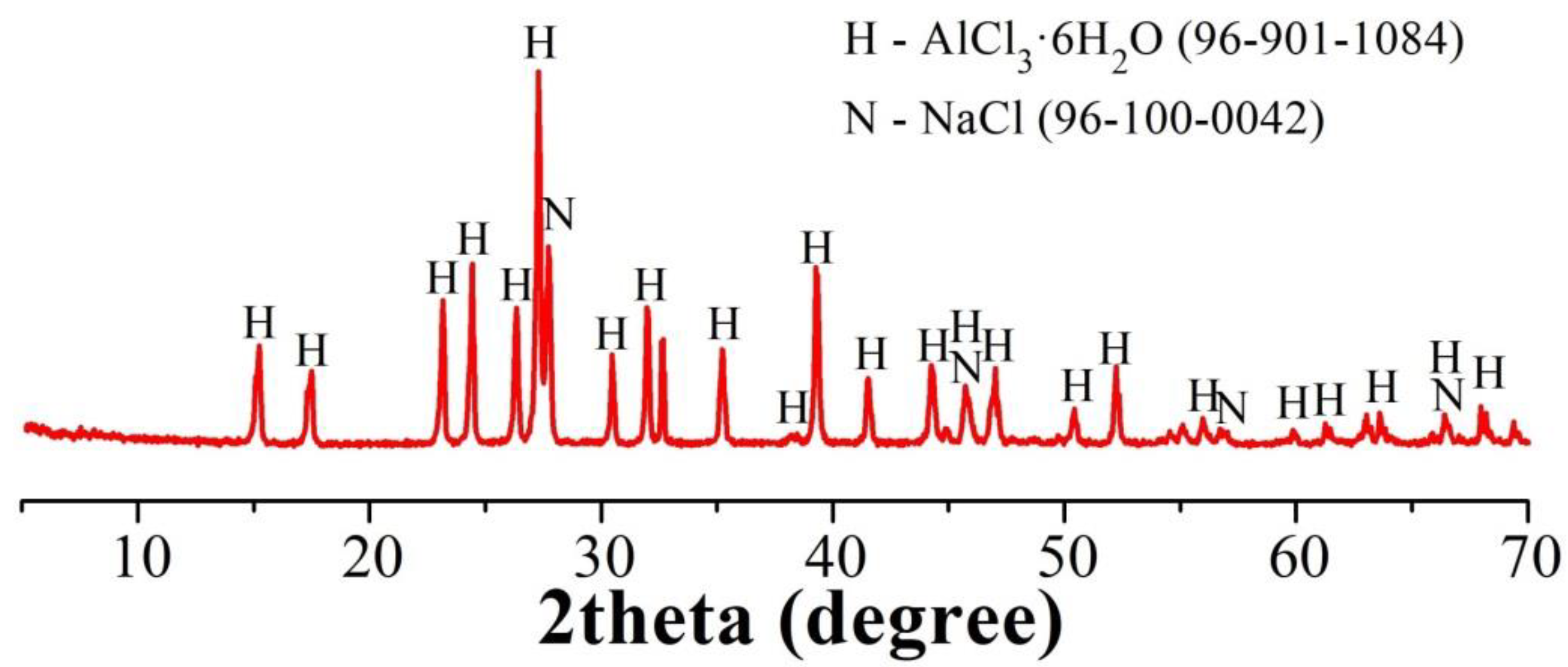
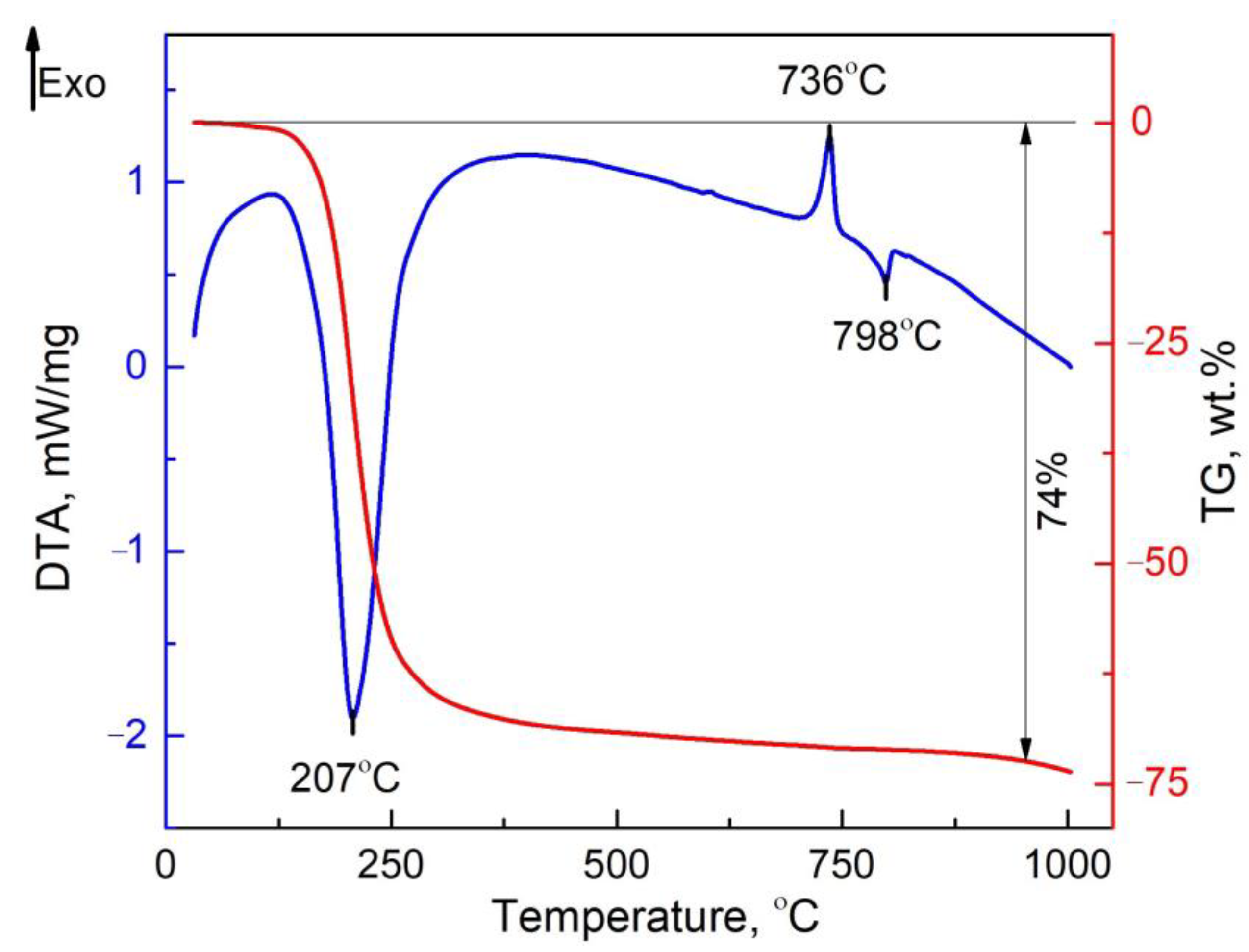
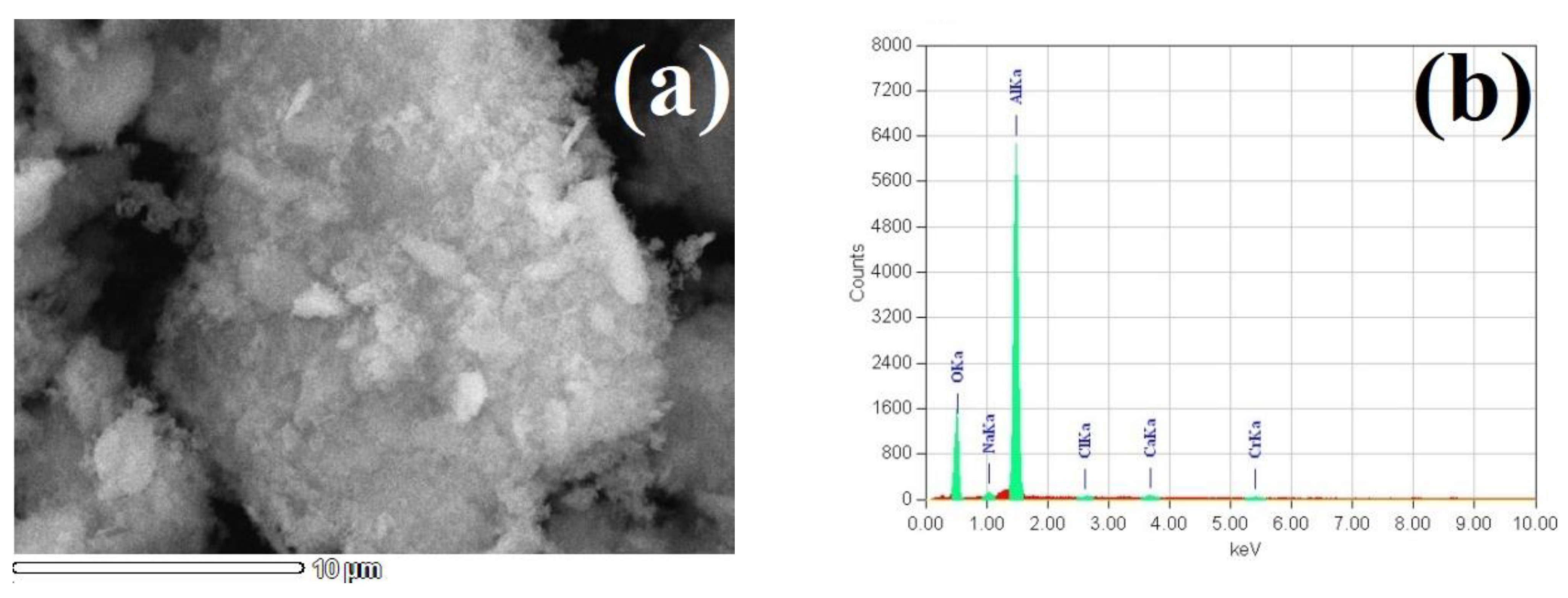
3.2. Alumina Precipitation
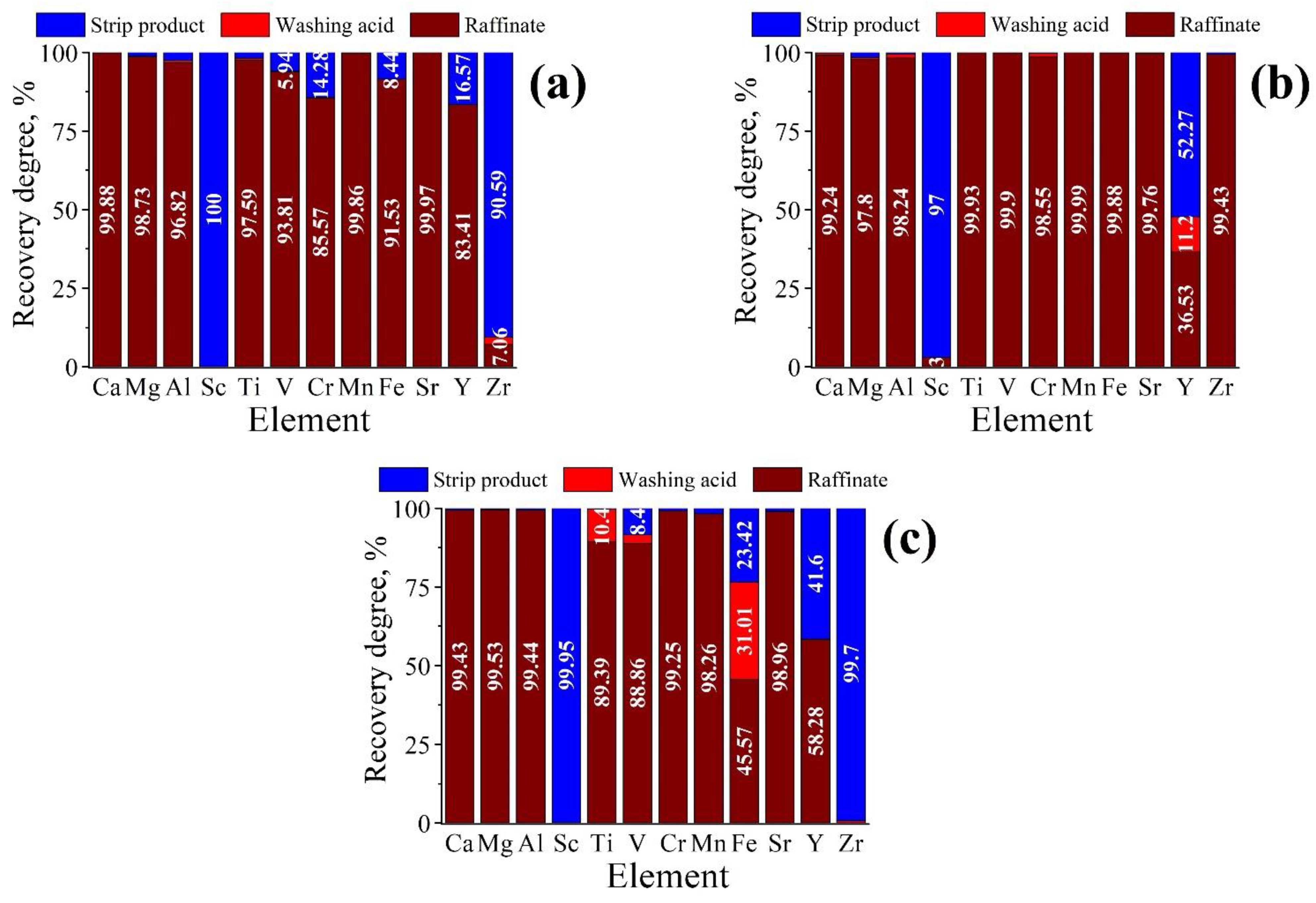
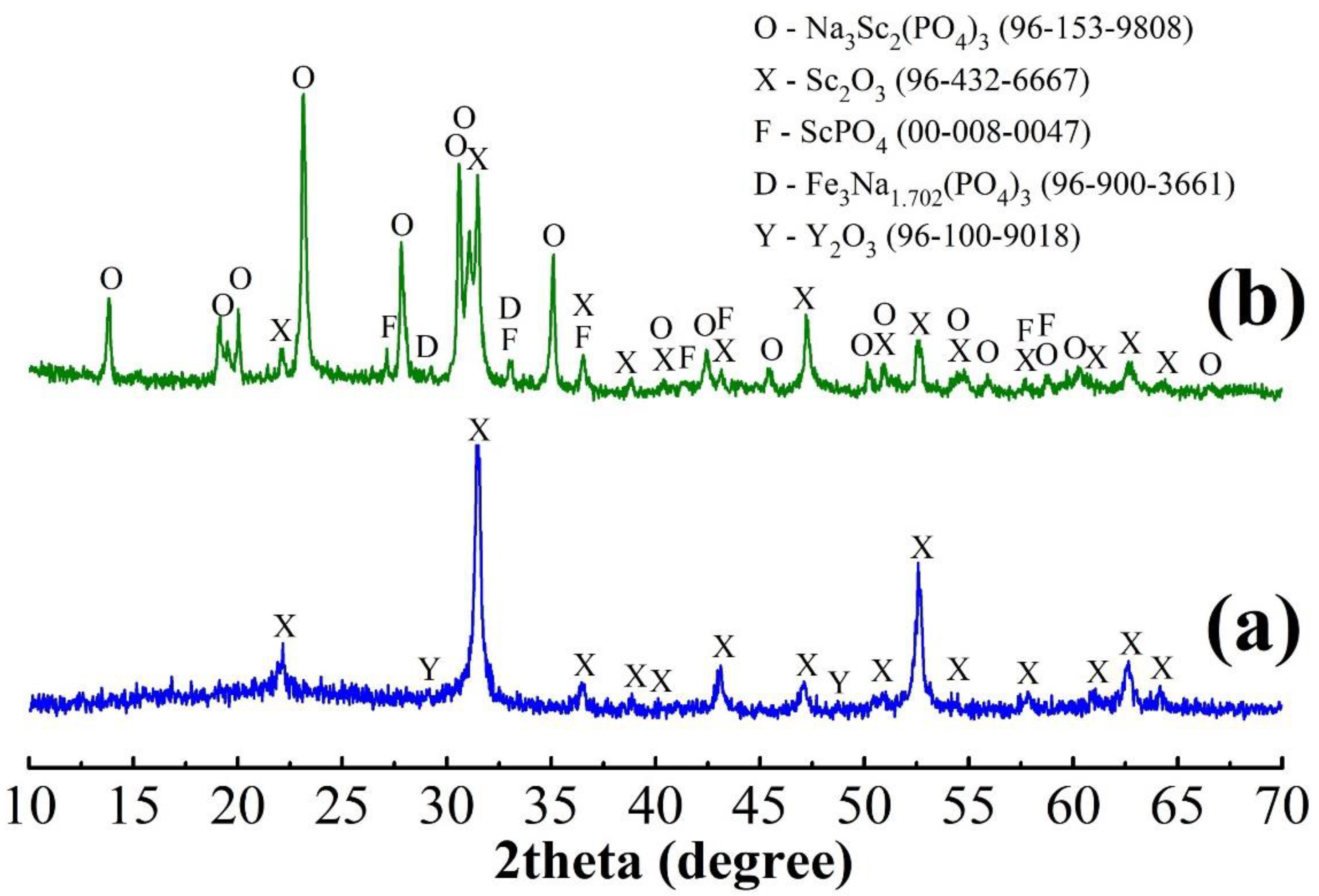
3.3. Scandium Solvent Extraction
- Accumulation of scandium by several technological cycles;
- Multiple reuses of the extractant amount;
- Increasing the O/A ratio at the extraction stage;
- Decreasing the O/A ratio at the stripping stage.
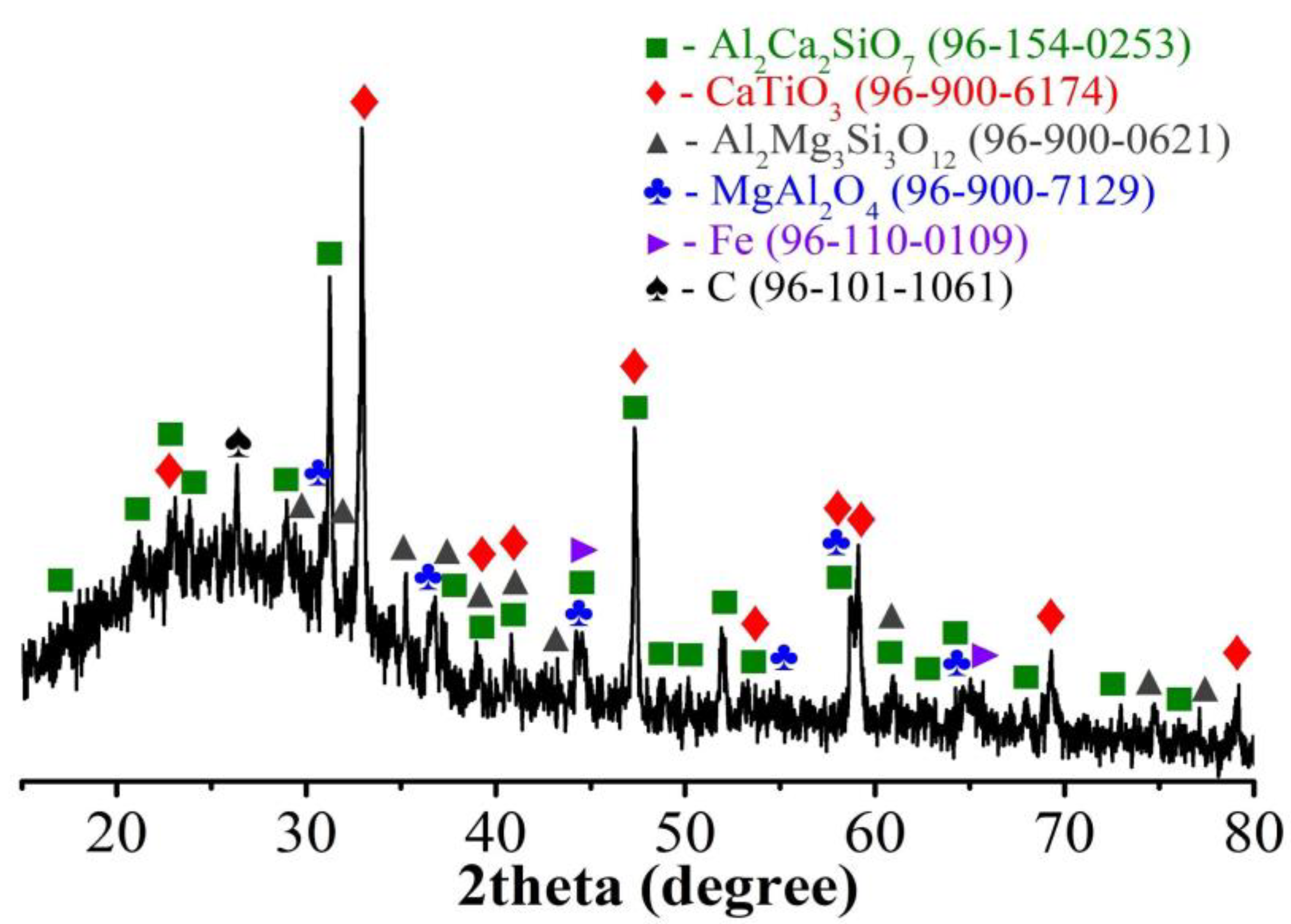
3.4. Obtaining Titanium Concentrate
3.5. Preparation of Amorphous Silica Product (White Carbon)
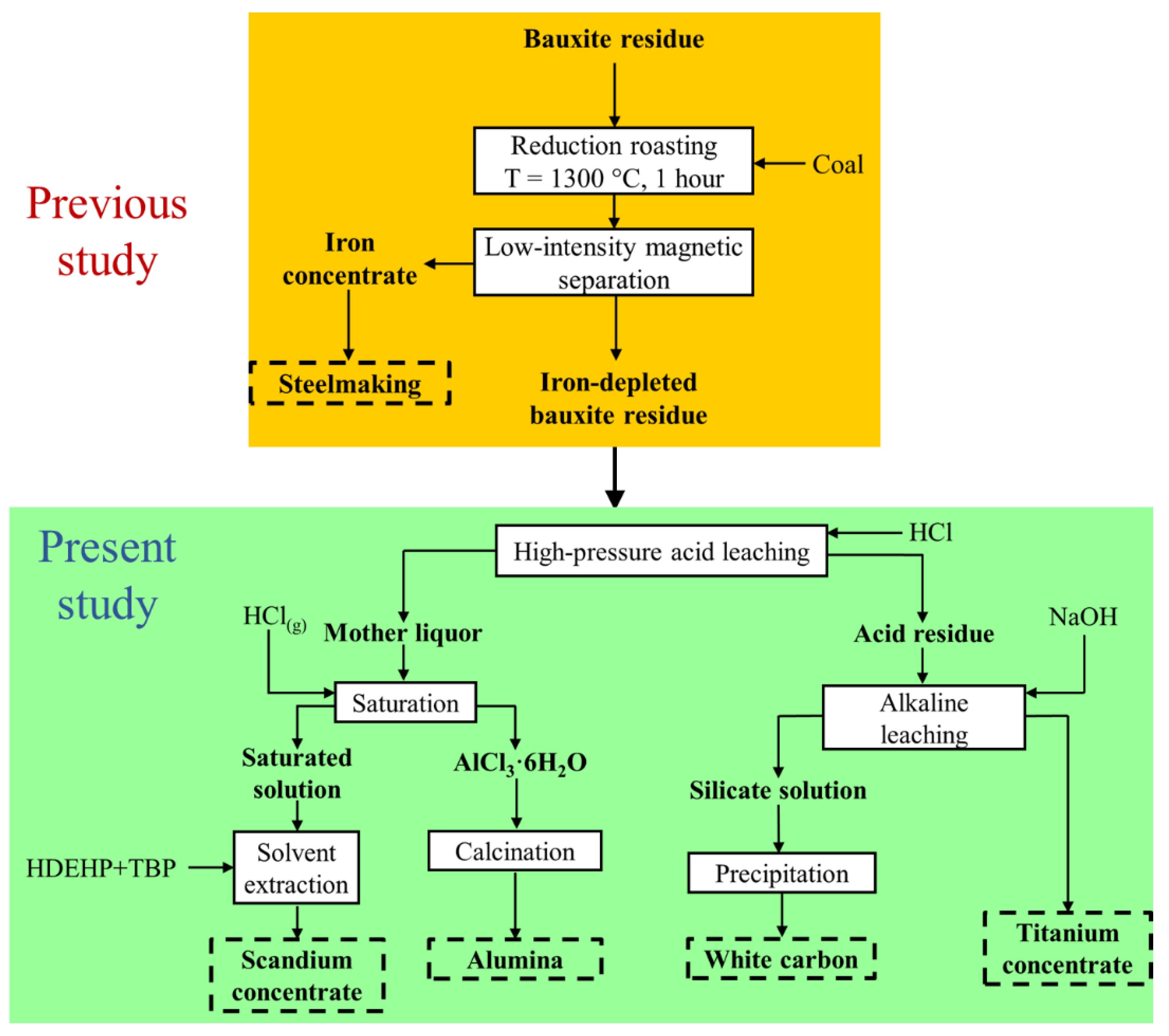
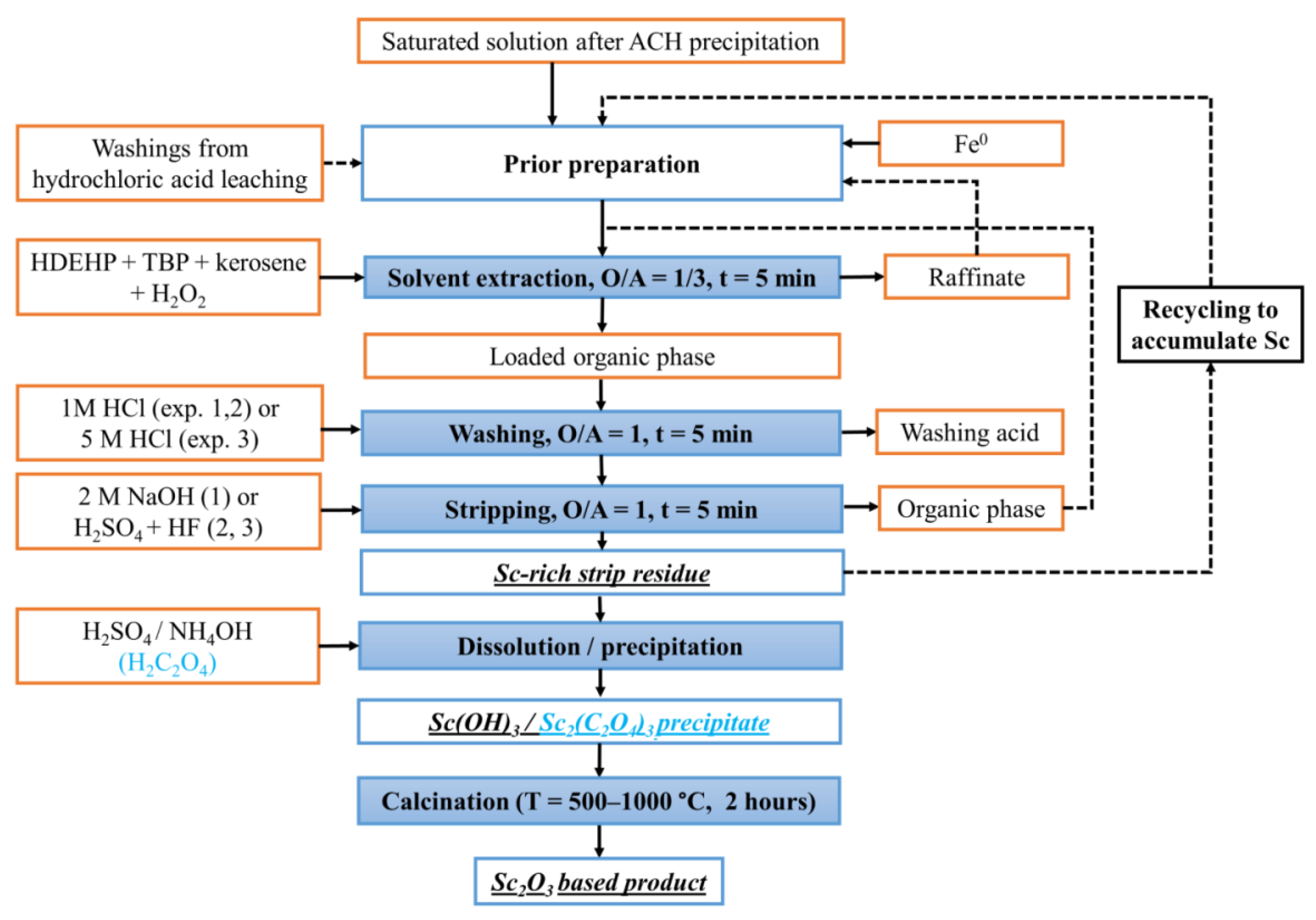
3.6. Flowsheet Characterization
4. Discussion
5. Conclusions
- The high-pressure hydrochloric leaching using 10% HCl at an L/S ratio of 11 for 15 min led to 79% Sc and 79.9% Al dissolution, while 91.4% Ti and 99.8% Si remained in the acid residue;
- The saturation of the acid leaching liquor by gaseous HCl in an argon flow for 30 min brought to ACH precipitation; after the ACH calcination at 800 °C and a subsequent water washing, the impure alumina of 93.5% Al2O3 was obtained, which was suitable for refinement by the conventional Bayer process;
- Solvent extraction of scandium from the acid leaching solution after the ACH precipitation and preliminary iron reduction from Fe3+ to Fe2+ was carried out using 10% HDEHP and 2% TBP in kerosene at an O/A ratio of 1/3 for 10 min with subsequent sequential stripping by oxalate mixture and 25 M HF at O/A ratio of 1/1 for 5 min; as a result, a valuable concentrate of 94% Sc2O3 was obtained;
- Alkaline leaching of the acid residue using 5 M NaOH at an L/S ratio of 12 for 90 min with following silica precipitation by adjusting pH to 8.5, its filtration and drying led to the preparation of white carbon of 77% SiO2 with a slight deviation from relevant standards.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
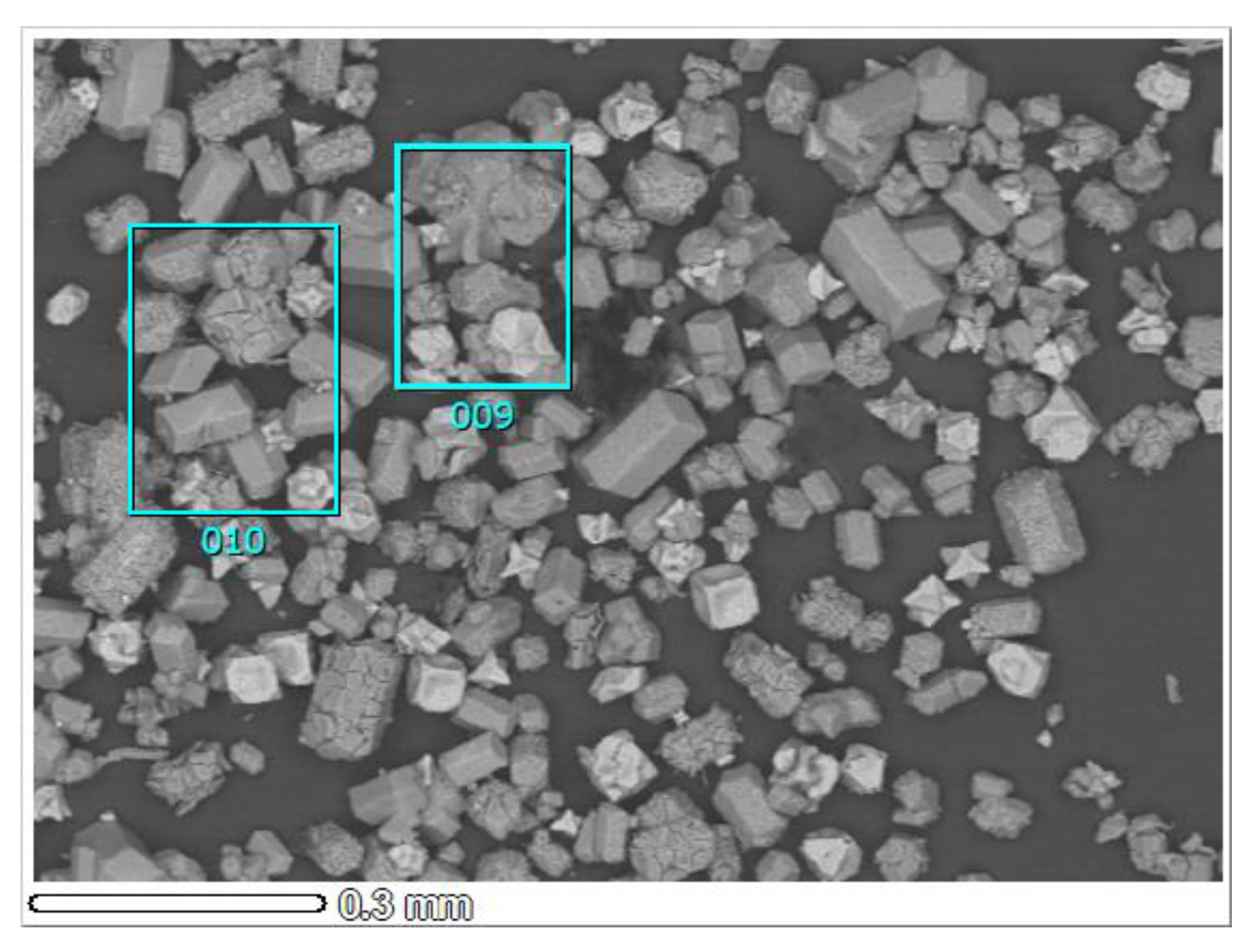
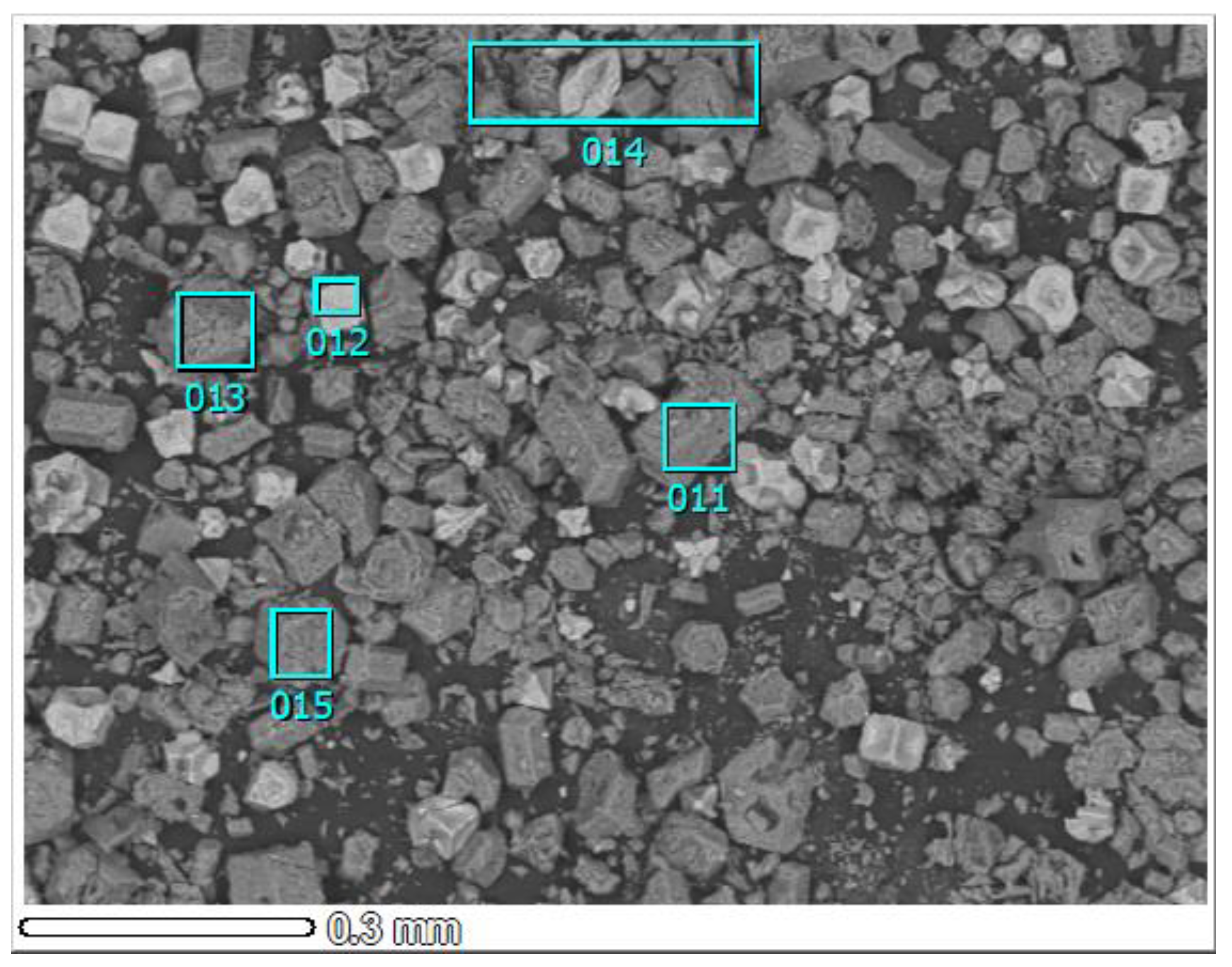
| Memo | 009 | 010 | 011 | 012 | 013 | 014 | 015 |
|---|---|---|---|---|---|---|---|
| O | 25.7 | 28.8 | 31.8 | 5.5 | 35.6 | 26.7 | 34.9 |
| Na | 6.8 | 4.3 | 0.9 | 27.6 | 0.5 | 7.0 | 0.3 |
| Al | 11.6 | 13.3 | 51.2 | 3.1 | 48.1 | 36.7 | 51.1 |
| Cl | 54.3 | 51.9 | 11.7 | 63.8 | 10.8 | 24.8 | 9.7 |
| Ca | 0.5 | 0.7 | 0.0 | 0.0 | 0.4 | 0.6 | 0.0 |
| Cr | 1.1 | 1.0 | 4.4 | 0.0 | 4.2 | 3.8 | 4.0 |
| Fe | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.4 | 0.0 |
| Total (wt.%) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |

References
- West, J. Decreasing Metal Ore Grades: Are They Really Being Driven by the Depletion of High-Grade Deposits? J. Ind. Ecol. 2011, 15, 165–168. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Pistilli, M. Why Scandium Could Be a Huge Opportunity. Available online: https://investingnews.com/daily/resource-investing/critical-metals-investing/scandium-investing/scandium-production-the-problem-and-the-opportunity/ (accessed on 2 December 2021).
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of Rare Earths from Bauxite Residue (Red Mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Gaustad, G.; Williams, E.; Leader, A. Rare Earth Metals from Secondary Sources: Review of Potential Supply from Waste and Byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar] [CrossRef]
- Reddy, P.S.; Reddy, N.G.; Serjun, V.Z.; Mohanty, B.; Das, S.K.; Reddy, K.R.; Rao, B.H. Properties and Assessment of Applications of Red Mud (Bauxite Residue): Current Status and Research Needs. Waste Biomass Valoriz. 2021, 12, 1185–1217. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive Utilization Status of Red Mud in China: A Critical Review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Healy, S. Sustainable Bauxite Residue Management Guidance. Available online: https://international-aluminium.org/wp-content/uploads/2021/10/BRManagementGuidance.pdf (accessed on 1 October 2022).
- Qi, Y. The Neutralization and Recycling of Red Mud—A Review. J. Phys. Conf. Ser. 2021, 1759, 012004. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite Residue Issues: III. Alkalinity and Associated Chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Alam, S.; Das, B.K.; Das, S.K. Dispersion and Sedimentation Characteristics of Red Mud. J. Hazard. Toxic. Radioact. Waste 2018, 22, 04018025. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, F.; Kong, X.; Wu, C.; Huang, L.; Huang, N.; Hartley, W. A Review of the Characterization and Revegetation of Bauxite Residues (Red Mud). Environ. Sci. Pollut. Res. 2016, 23, 1120–1132. [Google Scholar] [CrossRef]
- Di Carlo, E.; Boullemant, A.; Courtney, R. A Field Assessment of Bauxite Residue Rehabilitation Strategies. Sci. Total Environ. 2019, 663, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Feng, R.; Yu, H.; Pan, J.; Bian, J. Environmental Safety Analysis of Red Mud-Based Cemented Backfill on Groundwater. Int. J. Environ. Res. Public Health 2021, 18, 8094. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chen, J.; Han, L.; Wang, M.; Yang, B.; Du, P.; Li, F. Spatial distribution of heavy metals, salinity and alkalinity in soils around bauxite residue disposal area. Sci. Total Environ. 2018, 628–629, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Curtin, T.; Courtney, R. Effectiveness of a constructed wetland for treating alkaline bauxite residue leachate: A 1-year field study. Environ. Sci. Pollut. Res. 2017, 24, 8516–8524. [Google Scholar] [CrossRef] [PubMed]
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall 2016, 2, 316–331. [Google Scholar] [CrossRef] [Green Version]
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, Á.; Hoffer, A.; Imre, K.; Nyiró-Kósa, I.; Csákberényi-Malasics, D.; Tóth, Á.; Czitrovszky, A.; et al. The Red Mud Accident in Ajka (Hungary): Characterization and Potential Health Effects of Fugitive Dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Major Tailings Dam Failure in China’s Henan Province. Available online: https://watchers.news/2016/08/16/major-tailings-dam-failure-in-china-s-henan-province/ (accessed on 28 September 2021).
- Zeng, H.; Lyu, F.; Sun, W.; Zhang, H.; Wang, L.; Wang, Y. Progress on the Industrial Applications of Red Mud with a Focus on China. Minerals 2020, 10, 773. [Google Scholar] [CrossRef]
- Lyu, F.; Hu, Y.; Wang, L.; Sun, W. Dealkalization Processes of Bauxite Residue: A Comprehensive Review. J. Hazard Mater. 2021, 403, 123671. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden Values in Bauxite Residue (Red Mud): Recovery of Metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Dong, W.; Chen, Y.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Jiang, Y. Investigating the Effect of Ferrous Ion on the Digestion of Diasporic Bauxite in the Bayer Process. Hydrometallurgy 2015, 152, 183–189. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Bogdanova, E.A.; Chufarov, A.Y.; Kellerman, D.G.; Medyankina, I.S.; Yatsenko, S.P. A Promising Process for Transformation of Hematite to Magnetite with Simultaneous Dissolution of Alumina from Red Mud in Alkaline Medium. Hydrometallurgy 2020, 196, 105438. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A Review on Comprehensive Utilization of Red Mud and Prospect Analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of Valuable Elements from Red Mud with a Focus on Using Liquid Media—A Review. Recycling 2021, 6, 38. [Google Scholar] [CrossRef]
- Kang, J.; Gao, L.; Zhang, M.; Pu, J.; He, L.; Ruan, R.; Omran, M.; Peng, J.; Chen, G. Synthesis of Rutile TiO2 Powder by Microwave-Enhanced Roasting Followed by Hydrochloric Acid Leaching. Adv. Powder Technol. 2020, 31, 1140–1147. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Chufarov, A.Y.; Suntsov, A.Y.; Yatsenko, S.P. High Purity Scandium Extraction from Red Mud by Novel Simple Technology. Hydrometallurgy 2021, 202, 105597. [Google Scholar] [CrossRef]
- Rivera, R.M.; Xakalashe, B.; Ounoughene, G.; Binnemans, K.; Friedrich, B.; van Gerven, T. Selective Rare Earth Element Extraction Using High-Pressure Acid Leaching of Slags Arising from the Smelting of Bauxite Residue. Hydrometallurgy 2019, 184, 162–174. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Yan, J. Pressure Leaching of High Silica Pb-Zn Oxide Ore in Sulfuric Acid Medium. Hydrometallurgy 2010, 104, 235–240. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Tang, S.; Zeng, M.; Bai, P.; Chen, L. Selective Recovery of Vanadium and Scandium by Ion Exchange with D201 and Solvent Extraction Using P507 from Hydrochloric Acid Leaching Solution of Red Mud. Chemosphere 2017, 175, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, G.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Solvent Extraction Behavior of Metal Ions and Selective Separation Sc3+ in Phosphoric Acid Medium Using P204. Sep. Purif. Technol. 2019, 209, 175–181. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, C.Y. Separation and Purification of Scandium by Solvent Extraction and Related Technologies: A Review. J. Chem. Technol. Biotechnol. 2011, 86, 1237–1246. [Google Scholar] [CrossRef]
- Zhu, X.; Niu, Z.; Li, W.; Zhao, H.; Tang, Q. A Novel Process for Recovery of Aluminum, Iron, Vanadium, Scandium, Titanium and Silicon from Red Mud. J. Environ. Chem. Eng. 2020, 8, 103528. [Google Scholar] [CrossRef]
- Singh, R.K.; Dhadke, P.M. Extraction and Separation Study of Scandium (III) from Perchlorate Media by D2EHPA and PC 88A. Bull. Chem. Technol. Maced. 2002, 22, 1–11. [Google Scholar]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Selective Separation of Sc(III) and Zr(IV) from the Leaching of Bauxite Residue Using Trialkylphosphine Acids, Tertiary Amine, Tri-Butyl Phosphate and Their Mixtures. Sep. Purif. Technol. 2021, 279, 119798. [Google Scholar] [CrossRef]
- Korovin, V.; Shestak, Y. Scandium Extraction from Hydrochloric Acid Media by Levextrel-Type Resins Containing Di-Isooctyl Methyl Phosphonate. Hydrometallurgy 2009, 95, 346–349. [Google Scholar] [CrossRef]
- Shirokova, A.G.; Pasechnik, L.A.; Yatsenko, S.P. Synthesis and Certain Properties of Extraction Microencapsulated Systems. Russ. J. Appl. Chem. 2013, 86, 675–679. [Google Scholar] [CrossRef]
- Salman, A.D.; Juzsakova, T.; Mohsen, S.; Abdullah, T.A.; Le, P.-C.; Sebestyen, V.; Sluser, B.; Cretescu, I. Scandium Recovery Methods from Mining, Metallurgical Extractive Industries, and Industrial Wastes. Materials 2022, 15, 2376. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of Scandium from Synthetic Red Mud Leach Solutions by Solvent Extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Pyagay, I.; Medyankina, I.; Skachkova, O.; Yatsenko, A.; Pasechnik, L.; Skachkov, V.; Sabirzyanov, N. Extraction of Yttrium from Acidic Solutions [Ekstrakcionnoe Izvlechenie Ittriya Iz Kislyh Rastvorov]. Him. Tekhnologiya 2016, 17, 403–407. (In Russian) [Google Scholar]
- Salman, A.D.; Juzsakova, T.; Jalhoom, M.G.; Abdullah, T.A.; Le, P.-C.; Viktor, S.; Domokos, E.; Nguyen, X.C.; La, D.D.; Nadda, A.K.; et al. A Selective Hydrometallurgical Method for Scandium Recovery from a Real Red Mud Leachate: A Comparative Study. Environ. Pollut. 2022, 308, 119596. [Google Scholar] [CrossRef]
- Ditze, A.; Kongolo, K. Recovery of Scandium from Magnesium, Aluminium and Iron Scrap. Hydrometallurgy 1997, 44, 179–184. [Google Scholar] [CrossRef]
- Zinoveev, D.; Grudinsky, P.; Zhiltsova, E.; Grigoreva, D.; Volkov, A.; Dyubanov, V.; Petelin, A. Research on High-Pressure Hydrochloric Acid Leaching of Scandium, Aluminum and other Valuable Components from the Non-Magnetic Tailings Obtained from Red Mud after Iron Removal. Metals 2021, 11, 469. [Google Scholar] [CrossRef]
- Grudinsky, P.; Zinoveev, D.; Yurtaeva, A.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Iron Recovery from Red Mud Using Carbothermic Roasting with Addition of Alkaline Salts. J. Sustain. Metall. 2021, 7, 858–873. [Google Scholar] [CrossRef]
- Qureshi, I.H.; McClendon, L.T.; LaFleur, P.D. Extraction Studies of the Group IIIB-VIIB Elements and the Lanthanides Utilizing Bis(2-Ethyl-Hexyl) Orthophosphoric Acid. Radiochim. Acta 1969, 12, 107–111. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, S.; Meng, J.; Zhang, S.; Zhang, W.; Wang, S.; Chen, Y.; Wang, X.; Wei, Y. Purification of Scandium from Concentrate Generated from Titanium Pigments Production Waste. J. Rare Earths 2021, 39, 194–200. [Google Scholar] [CrossRef]
- Li, S.C.; Kim, S.C.; Kang, C.S. Recovery of Scandium from KOH Sub-Molten Salt Leaching Cake of Fergusonite. Miner. Eng. 2019, 137, 200–206. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Match! Version 3.12; Software for Phase Analysis Using Powder Diffraction; Crystal Impact: Bonn, Germany, 2021. [Google Scholar]
- Vaitkus, A.; Merkys, A.; Grazulis, S. Validation of the Crystallography Open Database Using the Crystallographic Information Framework. J. Appl. Cryst. 2021, 54, 661–672. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Differ. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Wen, X.; Chen, J.; Tang, Q.; Dong, W.; Zhong, J. Effect of Sodium Borate on the Preparation of TiN from Titanomagnetite Concentrates by Carbothermic Reduction—Magnetic Separation and Acid Leaching Process. Minerals 2019, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Major Metals from Bauxite Residue (Red Mud) by Alkali Roasting, Smelting, and Leaching. J. Sustain. Metall. 2017, 3, 393–404. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; He, H.; Sun, Z.; Li, A. Silica Extraction from Bauxite Reaction Residue and Synthesis Water Glass. Green Process. Synth. 2021, 10, 268–283. [Google Scholar] [CrossRef]
- Ivanov, V.V.; Kirik, S.D.; Shubin, A.A.; Blokhina, I.A.; Denisov, V.M.; Irtugo, L.A. Thermolysis of Acidic Aluminum Chloride Solution and Its Products. Ceram. Int. 2013, 39, 3843–3848. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Pang, X.; Yasinskiy, A.; Tan, Y.; Yu, J.; Wang, Z.; Shi, Z. Thermodynamics of the Decomposition of Aluminum Chloride Hexahydrate to Prepare Alumina. J. Mater. Res. Technol. 2021, 15, 6640–6646. [Google Scholar] [CrossRef]
- Anwar, J.; Frenkel, D.; Noro, M.G. Calculation of the Melting Point of NaCl by Molecular Simulation. J. Chem. Phys. 2003, 118, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Suss, A.; Senyuta, A.; Kravchenya, M.; Smirnov, A.; Panov, A. The Quality of Alumina Produced by the Hydrochloric Acid Process and Potential for Improvement. In Proceedings of the International Committee for Study of Bauxite, Alumina & Aluminium (ICSOBA), Dubai, United Arab Emirates, 30 November–1 December 2015. [Google Scholar]
- Valeev, D.; Shoppert, A.; Mikhailova, A.; Kondratiev, A. Acid and Acid-Alkali Treatment Methods of Al-Chloride Solution Obtained by the Leaching of Coal Fly Ash to Produce Sandy Grade Alumina. Metals 2020, 10, 585. [Google Scholar] [CrossRef]
- Senyuta, A.S.; Panov, A.V.; Milshin, O.N.; Slobodyanyuk, E.A.; Smirnov, A.A. Method for Production of Metallurgical Alumina (Variants). Patent RU2647041, 30 September 2016. issued: 13 March 2018. [Google Scholar]
- Huskić, I.; Arhangelskis, M.; Friščić, T. Solvent-Free Ageing Reactions of Rare Earth Element Oxides: From Geomimetic Synthesis of New Metal-Organic Materials towards a Simple, Environmentally Friendly Separation of Scandium. Green Chem. 2020, 22, 4364–4375. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Ning, S.; Zhong, Y.; Wang, X.; Fujita, T.; Wei, Y. Highly Efficient Recovery and Purification of Scandium from the Waste Sulfuric Acid Solution from Titanium Dioxide Production by Solvent Extraction. J. Environ. Chem. Eng. 2021, 9, 106226. [Google Scholar] [CrossRef]
- Sadykhov, G.B. Fundamental Problems and Prospects for the Use of Titanium Raw Materials in Russia. Izv. Ferr. Metall. 2020, 63, 178–194. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Bolívar, J.P.; Garcia-Tenorio, R.; Vaca, F. A Review of the Production Cycle of Titanium Dioxide Pigment. Mater. Sci. Appl. 2014, 5, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Boudreault, R.; Fournier, J.; Primeau, D.; Labrecque-Gilbert, M.-M. Processes for Treating Red Mud. US Patent 20150275330, 15 April 2015. issued: 1 October 2015. [Google Scholar]
- Dosmukhamedov, N.K.; Kaplan, V.A.; Daruesh, G.S. Innovative Technology of Integrated Processing of Ash from Coal Combustion. Ugol’ Russ. Coal J. 2020, 1, 58–63. [Google Scholar] [CrossRef]
- Valeev, D.; Bobylev, P.; Osokin, N.; Zolotova, I.; Rodionov, I.; Salazar-Concha, C.; Verichev, K. A Review of the Alumina Production from Coal Fly Ash, with a Focus in Russia. J. Clean. Prod. 2022, 363, 132360. [Google Scholar] [CrossRef]
- Valeev, D.V.; Lainer, Y.A.; Mikhailova, A.B.; Dorofievich, I.V.; Zheleznyi, M.V.; Goldberg, M.A.; Kutsev, S.V. Reaction of Bauxite with Hydrochloric Acid under Autoclave Conditions. Metallurgist 2016, 60, 204–211. [Google Scholar] [CrossRef]
- Grudinsky, P.; Zinoveev, D.; Pankratov, D.; Semenov, A.; Panova, M.; Kondratiev, A.; Zakunov, A.; Dyubanov, V.; Petelin, A. Influence of Sodium Sulfate Addition on Iron Grain Growth during Carbothermic Roasting of Red Mud Samples with Different Basicity. Metals 2020, 10, 1571. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; van Gerven, T. Recovery of Rare Earths and Other Valuable Metals from Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]




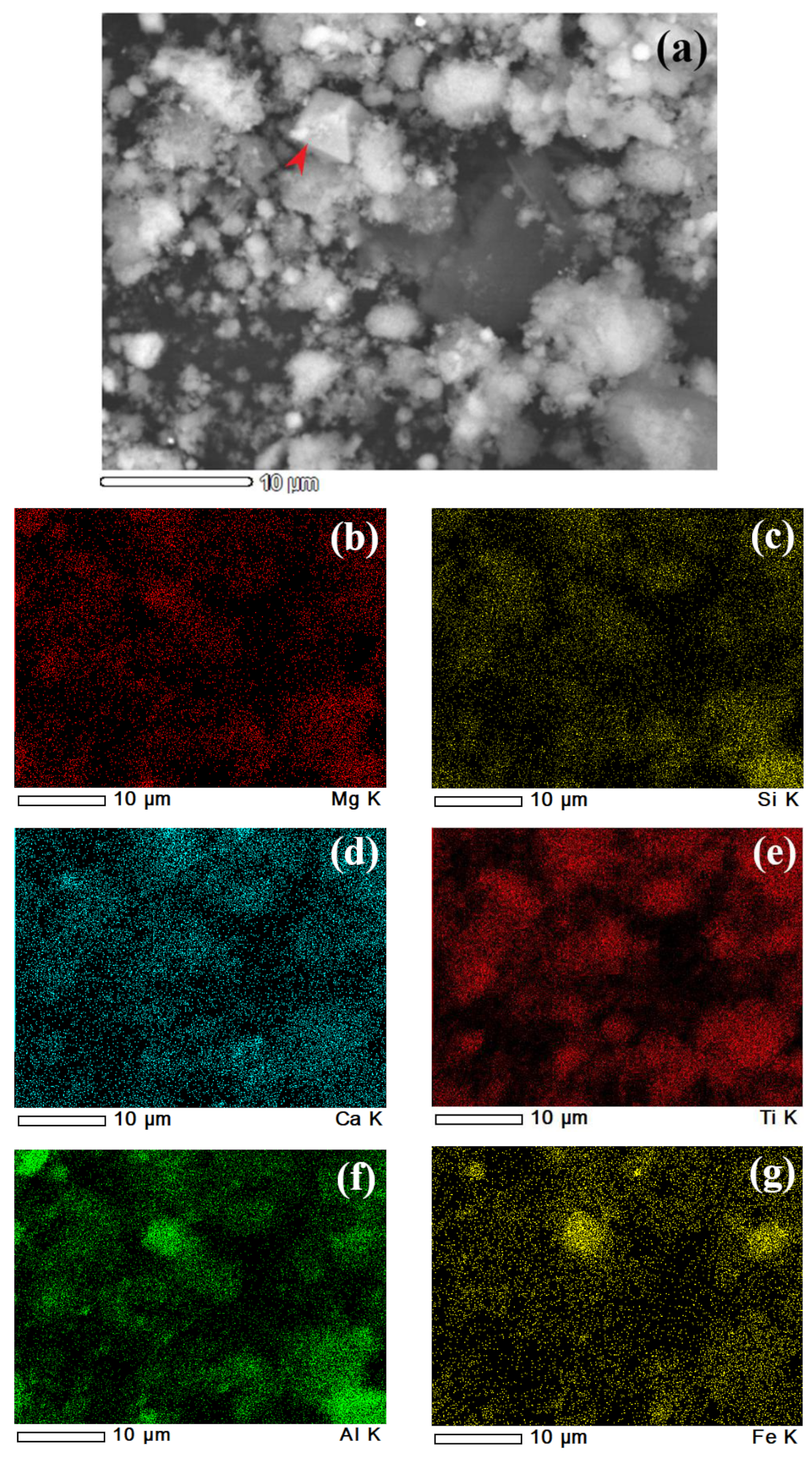
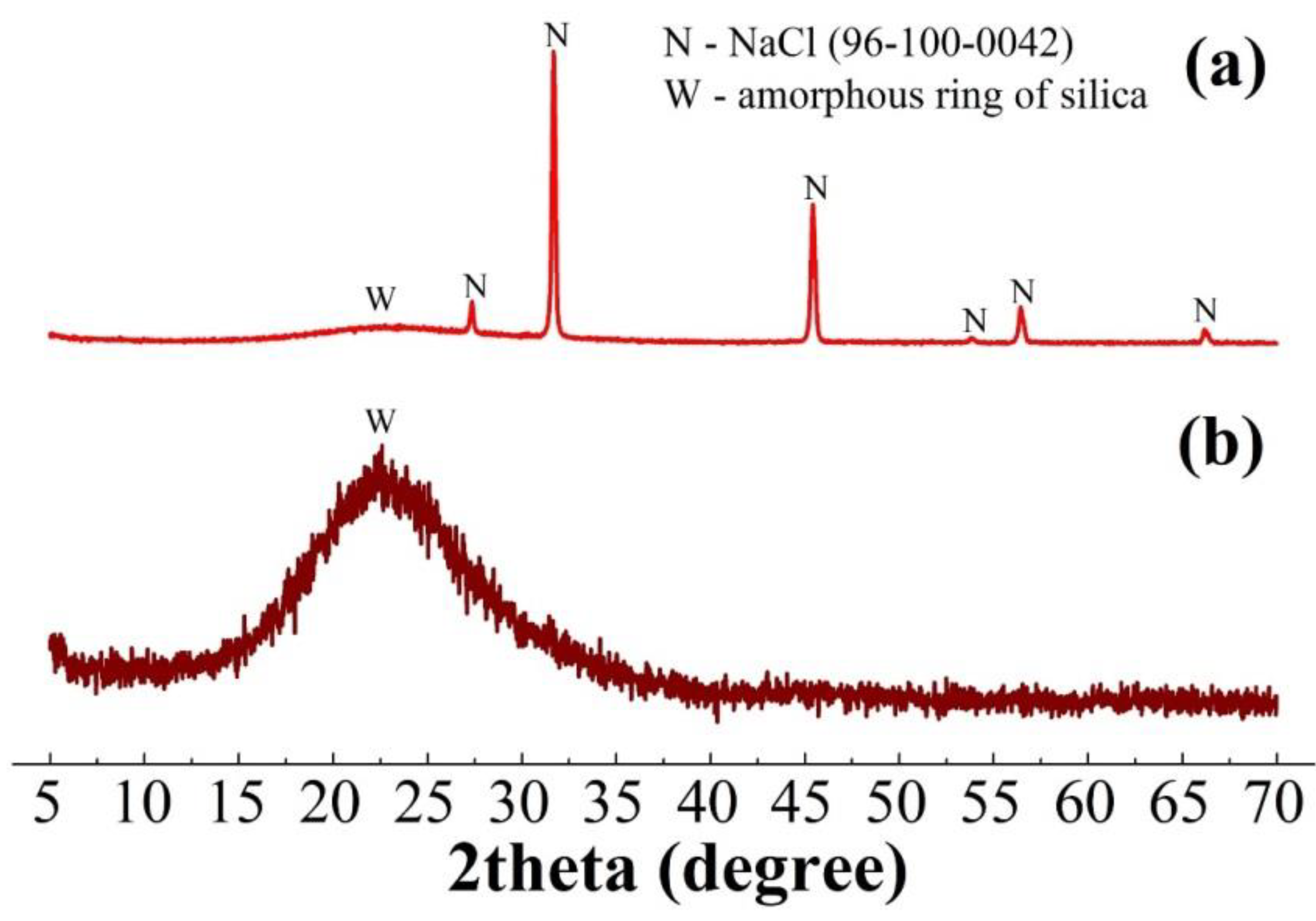
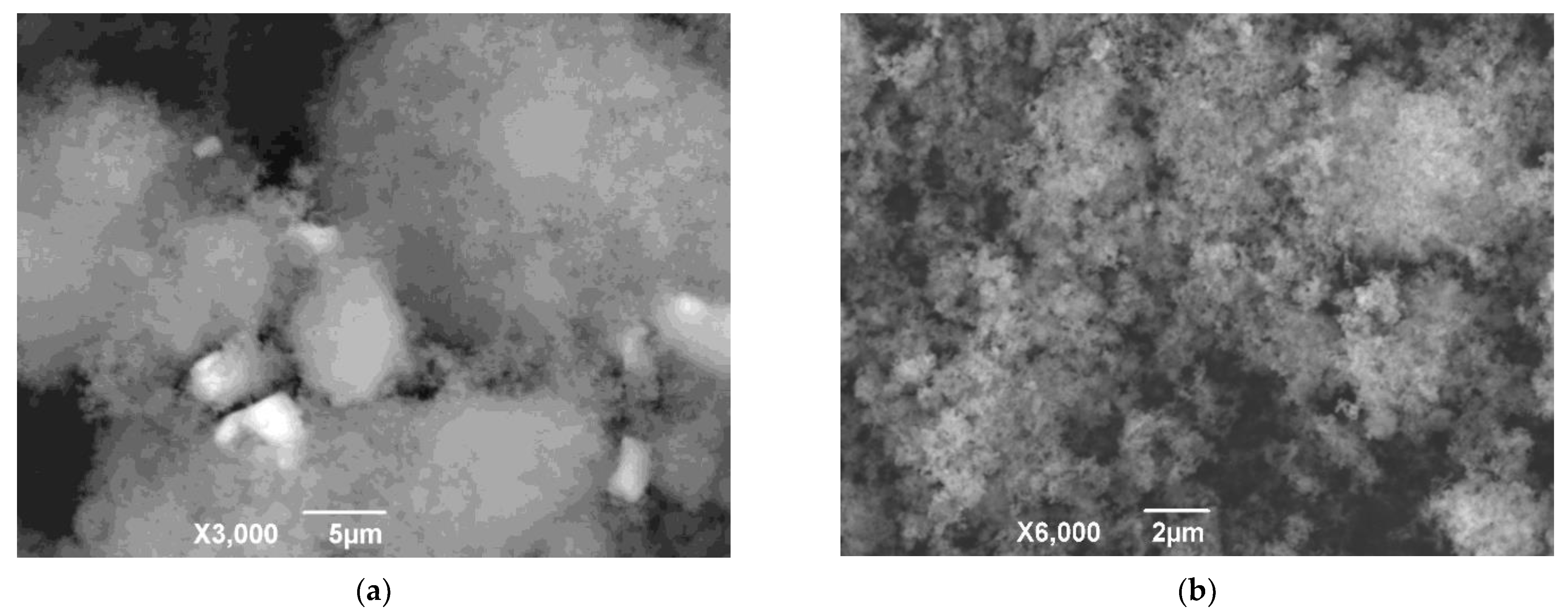

| Sample | Al | Si | Ca | Ti | Fe | Mg | Mn | Na | P | S | Cr | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bauxite residue | 6.76 | 4.07 | 6.62 | 2.8 | 34.8 | 0.4 | 0.2 | 2.45 | 0.38 | 0.48 | 0.08 | 0.014 |
| IDBR | 15.02 | 10.06 | 14.77 | 6.20 | 4.34 | 0.91 | 0.70 | 5.12 | 0.22 | 0.91 | 0.18 | 0.032 |
| Stage | Exp. 1 | Exp. 2 | Exp. 3 |
|---|---|---|---|
| Preparation of organic phase | 5 mL 10% HDEHP + 1 mL 2% TBP + 45 mL kerosene | ||
| Treatment of organic phase | 10 mL of organic phase | ||
| +10 mL 0.5 M H2SO4 | +5 mL 2.5 M HCl | ||
| Preparation of aqueous phase | 20 mL of diluted solution | 30 mL of diluted solution | 15 mL of diluted solution + 20 mL 0.77 g/L Sc |
| +10 mL 0.77 g/L Sc | |||
| Treatment of the aqueous phase | 30 mL of the aqueous phase | 40 mL of the aqueous phase | 35 mL of the aqueous phase |
| +0.05 g Fe | |||
| Solvent extraction (10 min) | 25 mL of aqueous phase + 5 mL 3% H2O2 | 30 mL of aqueous phase + 2 mL 3% H2O2 | |
| +10 mL of organic phase | |||
| Acid washing of loaded organic phase (10 min) | 1) 10 mL of loaded organic phase + 10 mL 1 M HCl; 2) 10 mL of loaded organic phase + 10 mL water. | 1) 10 mL of loaded organic phase + 5 mL 5 M HCl; 2) 10 mL of loaded organic phase + 10 mL water. | |
| Stripping | 10 mL 2 M NaOH t1 (stirring) = 5 min; t2 (stand-by) = 10 min. | 5 mL 25 M HF + 5 mL 0.5 M H2SO4 t1 (stirring) = 10 min; t2 (layering) = 20 min. | 1) 10 mL 10% H2C2O4 + 2% (NH4)2C2O4 t1 (stirring) = 5 min; 2) 2 mL 25 M HF + 8 mL 0.5 M H2SO4 t2 (stirring) = 10 min |
| Dissolution | 5 M H2SO4 | 5 M H2SO4 | – |
| Precipitation of Sc(OH)3/Sc2(C2O4)3 | 10% H2C2O4 addition | NH4OH addition | filtration without any reagent addition |
| Calcination in air | no precipitate | 500 °C | 1000 °C |
| Al | Si | Ca | Ti | Fe | Mg | Mn | Na | Cl | P | S | C | Ni | Cr | Sr | Y | Zr | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.31 | 21.6 | 1.91 | 12.2 | 3.72 | 1.24 | 0.27 | 0.57 | 2.37 | 0.41 | 0.25 | 2.7 | 0.10 | 0.34 | 0.10 | 0.03 | 0.57 | 0.014 |
| Al | Fe | Si | Na | Ca | Ti | Sc | Zr | Cr | Mg | V | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10,800 | 2068 | 13.3 | 3920 | 15670 | 35.8 | 14.8 | 0.1 | 437 | 401 | 47.2 | 34.8 | 302 |
| Al | Mg | Si | Ca | Sc | Ti | V | Cr | Mn | Fe | Zn | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.7 | 0.04 | 0.9 | 0.12 | 0.004 | 0.04 | 0.007 | 0.29 | 0.01 | 0.1 | 0.004 | 0.004 |
| Elements | Obtained Al2O3 | Al2O3 According to UC RUSAL Standard [58] |
|---|---|---|
| Al2O3 | 93.0 | >98.5 |
| Na2O | 0.19 | <0.1–0.2 |
| MgO | 0.23 | <0.005 |
| SiO2 | 0.86 | <0.01 |
| CaO | 0.35 | <0.05 |
| TiO2 | 0.043 | <0.001 |
| V2O5 | 0.021 | <0.001 |
| Cr2O3 | 1.08 | <0.001 |
| MnO2 | 0.047 | <0.001 |
| Fe2O3 | 0.57 | <0.01 |
| ZnO | 0.012 | <0.005 |
| Specific surface area, m2/g | 50.6 | 70–80 |
| Element | Ca | Mg | Al | Sc | Ti | V | Cr | Mn | Fe | Sr | Y | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. 1 | 764 | 34.7 | 600 | 316 | 6.7 | 2.8 | 2.1 | 24.7 | 375 | 43.2 | 5 | 0.01 |
| Exp. 2 | 818 | 37.1 | 643 | 262 | 7.1 | 2.9 | 2.2 | 26.5 | 402 | 46.3 | 5 | 0.01 |
| Exp. 3 | 491 | 22.3 | 386 | 491 | 4.3 | 1.8 | 1.3 | 15.9 | 241 | 27.8 | 3.2 | 0.01 |
| Al | Si | Ca | Ti | Fe | Mg | Mn | Na | P | S | C | Ni | Cr | Sr | Y | Zr | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.01 | 4.43 | 3.56 | 24.9 | 4.42 | 2.76 | 0.37 | 0.28 | 0.40 | 0.40 | 4.9 | 0.20 | 0.85 | 0.14 | 0.05 | 0.75 | 0.018 |
| Attribute | Obtained SiO2 | SiO2 According to Russian State Standard GOST 18307–78 | |
|---|---|---|---|
| BS–30 Grade | BS–50 Grade | ||
| SiO2 | 77 | >85 | >76 |
| CaO + MgO | 0.18 | <0.5 | <7 |
| Na2O | 0.04 | <0.9 | <1.8 |
| Fe2O3 | 0.16 | not rated | <0.03 |
| Al2O3 | 1.51 | not rated | <0.10 |
| Moisture, wt.% | 3.35 | <6.5 | <6.0 |
| pH of aqueous extract | 10 | 8–10 | 9–10.5 |
| Loss of ignition, wt.% | 5.06 | 4.5–7.5 | 7–10 |
| Specific surface area, m2/g | 50.9 | 25–45 | 35–55 |
| Product | Scandium Concentrate | Alumina | Titanium Concentrate | White Carbon |
|---|---|---|---|---|
| Element | Sc | Al | Ti | Si |
| Content, wt.% | 94 | 49.23 | 24.92 | 36 |
| KIDBR | 3032 | 3.28 | 4.02 | 3.58 |
| KBR | 6714 | 7.28 | 8.90 | 8.85 |
| Attribute | The Orbite Process [65] | The Developed Process |
|---|---|---|
| Iron removal stage | Leaching, hydrolysis, and precipitation | Carbothermic reduction and magnetic separation |
| Number of acid leaching stages | Two | One |
| Acid concentration and consumption | Strong acid and high consumption | Dilute acid and low consumption |
| Ti and Si separation | Hydrochloric acid leaching | Alkaline leaching |
| Production of white carbon | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudinsky, P.; Pasechnik, L.; Yurtaeva, A.; Dyubanov, V.; Zinoveev, D. Recovery of Scandium, Aluminum, Titanium, and Silicon from Iron-Depleted Bauxite Residue into Valuable Products: A Case Study. Crystals 2022, 12, 1578. https://doi.org/10.3390/cryst12111578
Grudinsky P, Pasechnik L, Yurtaeva A, Dyubanov V, Zinoveev D. Recovery of Scandium, Aluminum, Titanium, and Silicon from Iron-Depleted Bauxite Residue into Valuable Products: A Case Study. Crystals. 2022; 12(11):1578. https://doi.org/10.3390/cryst12111578
Chicago/Turabian StyleGrudinsky, Pavel, Liliya Pasechnik, Anfisa Yurtaeva, Valery Dyubanov, and Dmitry Zinoveev. 2022. "Recovery of Scandium, Aluminum, Titanium, and Silicon from Iron-Depleted Bauxite Residue into Valuable Products: A Case Study" Crystals 12, no. 11: 1578. https://doi.org/10.3390/cryst12111578






