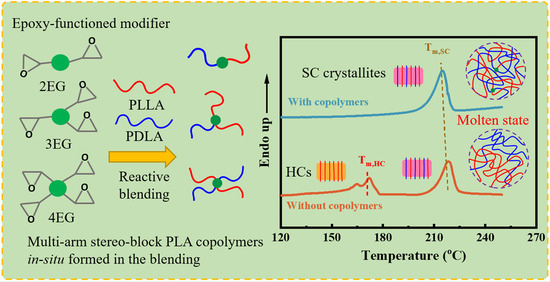
Substantially Enhanced Stereocomplex Crystallization of Poly(L-lactide)/Poly(D-lactide) Blends by the Formation of Multi-Arm Stereo-Block Copolymers
Abstract
1. Introduction
2. Materials and Methods
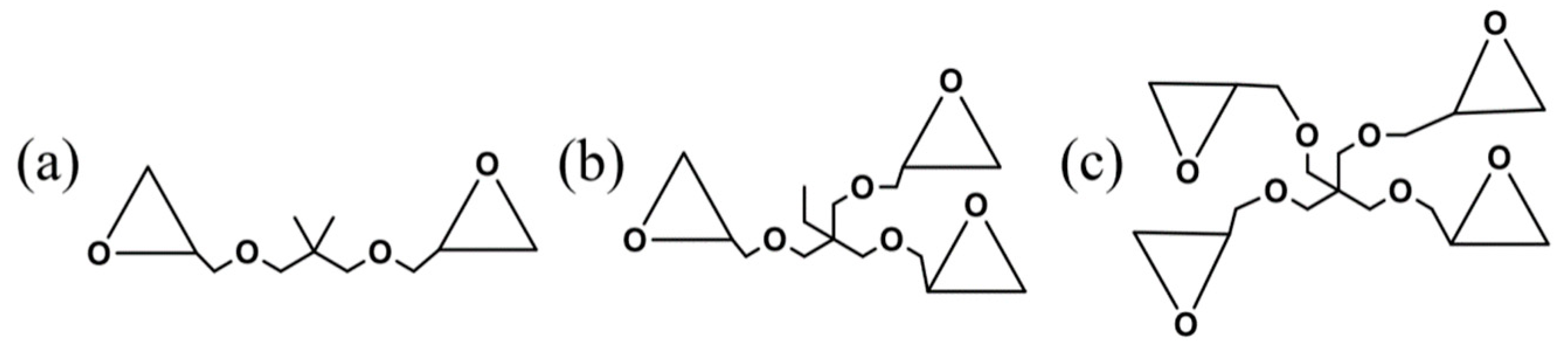
2.1. Materials
2.2. Sample Preparation
2.3. Characterization and Measurements
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.2. Rheological Testing
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Wide-Angle X-ray Diffraction (WAXD)
3. Results and Discussion
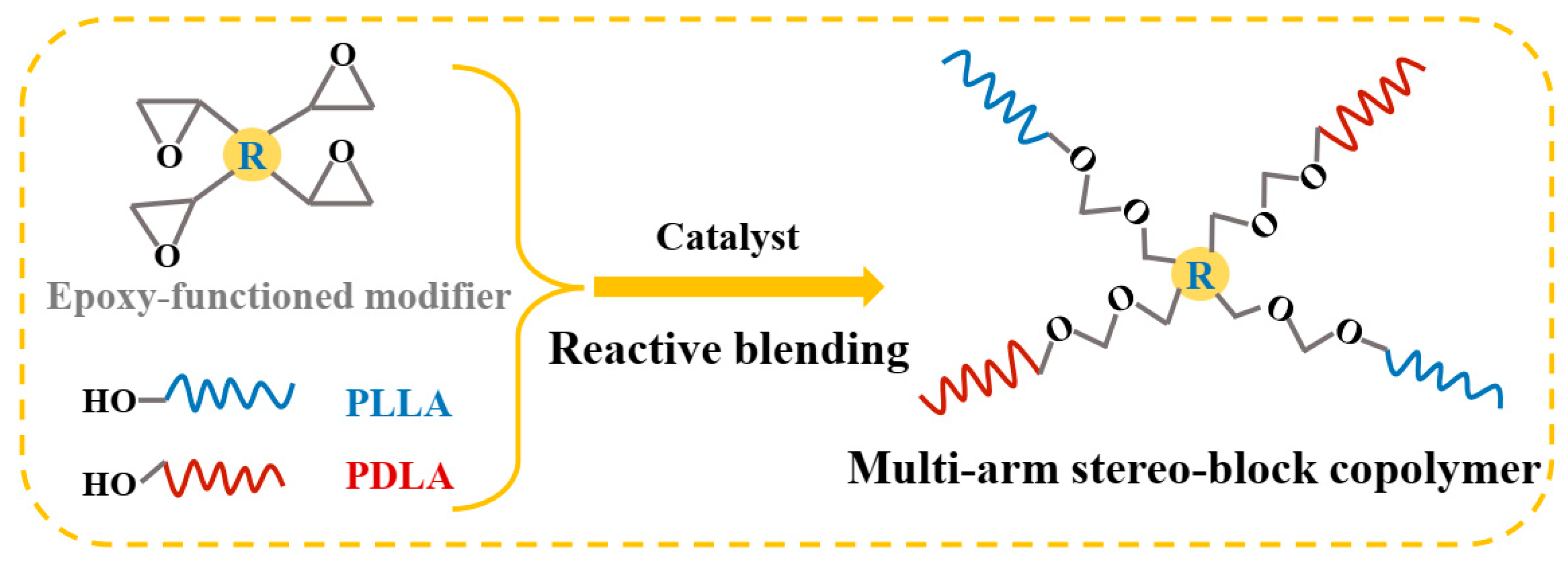
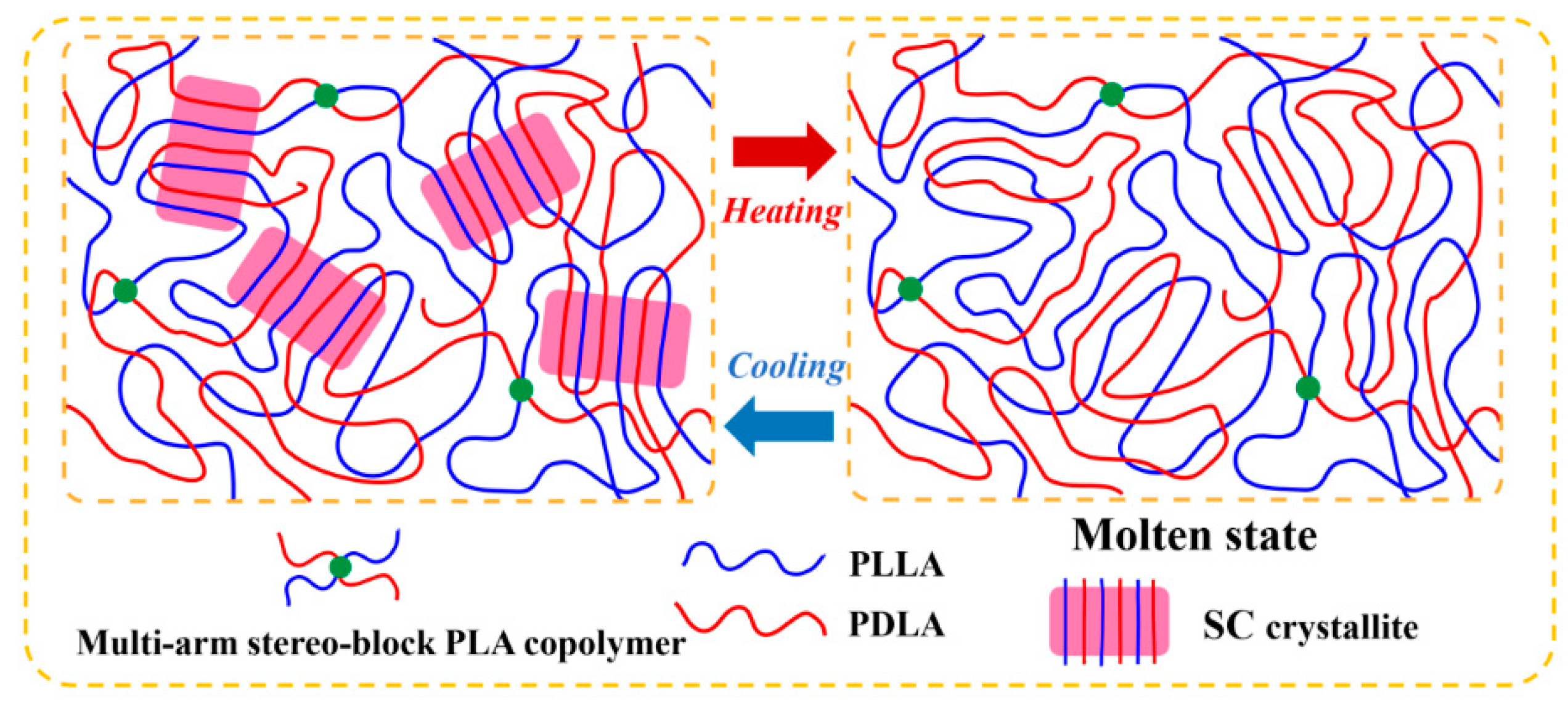
3.1. Generation of Multi-Arm Stereo-Block PLA Copolymers in PLLA/PDLA Blends
3.2. Non-Isothermal Crystallization of PLLA/PDLA Blends
3.3. Isothermal Crystallization of PLLA/PDLA Blends
3.4. Crucial Role of the Arm Number of Multi-Arm Stereo-Block PLA Copolymers in Enhancing SC Crystallizability of PLLA/PDLA Blends
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, X.; Wang, D. Tailoring crystallization: Towards high-performance poly(lactic acid). Adv. Mater. 2014, 26, 6905–6911. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Hwang, J.J.; Liu, H.J.; Zheng, A.M. A characteristic study of polylactic acid/organic modified montmorillonite (PLA/OMMT) nanocomposite materials after hydrolyzing. Crystals 2021, 11, 376. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Michalski, A.; Brzezinski, M.; Lapienis, G.; Biela, T. Star-shaped and branched polylactides: Synthesis, characterization, and properties. Prog. Polym. Sci. 2019, 89, 159–212. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and application. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Pan, G.; Xu, H.; Ma, B.; Wizi, J.; Yang, Y. Polylactide fibers with enhanced hydrolytic and thermal stability via complete stereo-complexation of poly (L-lactide) with high molecular weight of 600000 and lower-molecular-weight poly (D-lactide). J. Mater. Sci. 2018, 53, 5490–5500. [Google Scholar] [CrossRef]
- Gupta, A.; Katiyar, V. Cellulose functionalized high molecular weight stereocomplex polylactic acid biocomposite films with improved gas barrier, thermomechanical properties. ACS Sustain. Chem. Eng. 2017, 5, 6835–6844. [Google Scholar] [CrossRef]
- Oyama, H.T.; Abe, S. Stereocomplex poly (lactic acid) alloys with superb heat resistance and toughness. ACS Sustain. Chem. Eng. 2015, 3, 3245–3252. [Google Scholar] [CrossRef]
- Liu, Z.W.; Luo, Y.L.; Bai, H.W.; Zhang, Q.; Fu, Q. Remarkably enhanced impact toughness and heat resistance of poly(L-lactide)/thermoplastic polyurethane blends by constructing stereocomplex crystallites in the matrix. ACS Sustain. Chem. Eng. 2016, 4, 111–120. [Google Scholar] [CrossRef]
- Brizzolara, D.; Cantow, H.J.; Diederichs, K.; Keller, E.; Domb, A.J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromolecules 1996, 29, 191–197. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. 9. stereocomplexation from the melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Pan, P.J.; Han, L.L.; Bao, J.N.; Xie, Q.; Shan, G.R.; Bao, Y.Z. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly (L-lactic acid)/poly (D-lactic acid) racemic blends: Molecular weight effects. J. Phys. Chem. B 2015, 119, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, C.; Bian, Y.; Dong, Q.; Zhao, H.; Zhang, X.; Xu, M.; Dong, L. Miscibility, thermal properties and polymorphism of stereocomplexation of high-molecular-weight polylactide/poly (D, L-lactide) blends. Thermochim. Acta 2014, 580, 53–62. [Google Scholar] [CrossRef]
- Kakuta, M.; Hirata, M.; Kimura, Y. Stereoblock polylactides as high-performance bio-based polymers. Polym. Rev. 2009, 49, 107–140. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Jiang, W.R.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Fu, Q. Stereocomplex formation of high-molecular-weight polylactide: A low temperature approach. Polymer 2012, 53, 5449–5454. [Google Scholar] [CrossRef]
- Tsuji, H.; Tashiro, K.; Bouapao, L.; Hanesaka, M. Synchronous and separate homo-crystallization of enantiomeric poly (l-lactic acid)/poly (d-lactic acid) blends. Polymer 2012, 53, 747–754. [Google Scholar] [CrossRef]
- Tsuji, H.; Iguchi, K.; Arakawa, Y. Stereocomplex- and homo-crystallization behavior, structure, morphology, and thermal properties of crystalline and amorphous stereo diblock copolymers, enantiomeric poly (l-lactide)-b-poly (dl-lactide) and Poly (d-lactide)-b-Poly (dl-lactide). Polymer 2021, 213, 123226. [Google Scholar] [CrossRef]
- Bai, H.W.; Liu, H.L.; Bai, D.Y.; Zhang, Q.; Wang, K.; Deng, H.; Chen, F.; Fu, Q. Enhancing the melt stability of polylactide stereocomplexes using a solid-state cross-linking strategy during a melt-blending process. Polym. Chem. 2014, 5, 5985–5993. [Google Scholar] [CrossRef]
- Bai, H.W.; Deng, S.H.; Bai, D.Y.; Zhang, Q.; Fu, Q. Recent advances in processing of stereocomplex-type polylactide. Macromol. Rapid Commun. 2017, 38, 1700454. [Google Scholar] [CrossRef]
- Hirata, M.; Kimura, Y. Thermo mechanical properties of stereoblock poly (lactic acid) s with different PLLA/PDLA block compositions. Polymer 2008, 49, 2656–2661. [Google Scholar] [CrossRef]
- Han, L.L.; Xie, Q.; Bao, J.; Shan, G.; Bao, Y.; Pan, P.J. Click chemistry synthesis, stereocomplex formation, and enhanced thermal properties of well-defined poly (l-lactic acid)-b-poly (d-lactic acid) stereo diblock copolymers. Polym. Chem. 2017, 8, 1006–1016. [Google Scholar] [CrossRef]
- Zhou, D.; Shao, J.; Sun, J.; Bian, X.; Xiang, S.; Li, G.; Chen, X. Effect of the different architectures and molecular weights on stereocomplex in enantiomeric polylactides-b-MPEG block copolymers. Polymer 2017, 123, 49–54. [Google Scholar] [CrossRef]
- Shao, J.; Sun, J.; Bian, X.; Cui, Y.; Li, G.; Chen, X. Investigation of poly(lactide) stereocomplexes: 3-armed poly (L-lactide) blended with linear and 3-armed enantiomers. J. Phys. Chem. B 2012, 116, 9983–9991. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Tsuji, H. Crystallization behavior and physical properties of linear 2-arm and branched 4-arm poly (l-lactide)s: Effects of branching. Polymer 2013, 54, 2422–2434. [Google Scholar] [CrossRef]
- Han, L.L.; Shan, G.R.; Bao, Y.Z.; Pan, P.J. Exclusive stereocomplex crystallization of Linear and multiarm star-shaped high-molecular-weight stereo diblock poly(lactic acid)s. J. Phys. Chem. B 2015, 119, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, Z.; Pan, G.; Yang, Y. Enhancing the recrystallization ability of bio-based polylactide stereocomplex by in situ construction of multi-block branched conformation. J. Mater. Sci. 2019, 54, 12145–12158. [Google Scholar] [CrossRef]
- Bai, H.W.; Bai, D.Y.; Xiu, H.; Liu, H.L.; Zhang, Q.; Wang, K.; Deng, H.; Chen, F.; Fu, Q.; Chiu, F.C. Towards high-performance poly (L-lactide)/elastomer blends with tunable interfacial adhesion and matrix crystallization via constructing stereocomplex crystallites at the interface. RSC Adv. 2014, 4, 49374–49385. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Xu, P.; Dong, W.; Chen, M.; Lemstra, P.J. Rapid stereocomplexation between enantiomeric comb-shaped cellulose-g-poly (L-lactide) nanohybrids and poly (D-lactide) from the melt. Biomacromolecules 2015, 16, 3723–3729. [Google Scholar] [CrossRef]
- Deng, S.H.; Bai, H.W.; Liu, Z.W.; Zhang, Q.; Fu, Q. Toward supertough and heat-resistant stereocomplex-type polylactide/elastomer blends with impressive melt stability via in situ formation of graft copolymer during one-pot reactive melt blending. Macromolecules 2019, 52, 1718–1730. [Google Scholar] [CrossRef]
- Cheerarot, O.; Baimark, Y. Thermal and mechanical properties of biodegradable star-shaped/linear polylactide stereocomplexes. J. Chem. 2015, 2015, 206123. [Google Scholar] [CrossRef]
- Biela, T. Stereocomplexes of star-shaped poly (R)-lactide s and poly (S)-lactide s bearing various number of arms. Synthesis and thermal properties. Polimery 2007, 52, 106–116. [Google Scholar] [CrossRef][Green Version]
- Bao, J.N.; Han, L.L.; Shan, G.; Bao, Y.; Pan, P.J. Preferential stereocomplex crystallization in enantiomeric blends of cellulose acetate-g-poly (lactic acid) s with comblike topology. J. Phys. Chem. B 2015, 119, 12689–12698. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, R.; Wang, Y. Effect of plasticizer poly (ethylene glycol) on the crystallization properties of stereocomplex-type poly (lactide acid). Wuhan Univ. J. Nat. Sci. 2017, 22, 420–428. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Zhang, R.; Liu, Y. Design high heat-resistant stereocomplex poly (lactide acid) by cross-linking and plasticizing. Adv. Polym. Technol. 2018, 37, 2429–2435. [Google Scholar] [CrossRef]
- Anakabe, J.; Zaldua Huici, A.M.; Eceiza, A.; Arbelaiz, A.; Averous, L. Combined effect of nucleating agent and plasticizer on the crystallization behaviour of polylactide. Polym. Bull. 2017, 74, 4857–4886. [Google Scholar] [CrossRef]
- Wu, B.G.; Yang, W.J.; Niu, D.Y.; Dong, W.F.; Chen, M.Q.; Liu, T.X.; Du, M.L.; Ma, P.M. Stereocomplexed poly(lactide) composites toward engineering plastics with superior toughness, heat resistance and anti-hydrolysis. Chin. J. Polym. Sci. 2020, 38, 1107–1116. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Zhang, X.; Dong, Q.; Dong, L. Effects of molten poly (D, L-lactide) on nonisothermal crystallization in stereocomplex of poly (L-lactide) with poly (D-lactide). Thermochim. Acta 2013, 573, 193–199. [Google Scholar] [CrossRef]
- Xie, Q.; Han, L.L.; Shan, G.; Bao, Y.; Pan, P.J. Promoted stereocomplex formation and two-step crystallization kinetics of poly (L-lactic acid)/poly (D-lactic acid) blends induced by nucleator. Polym. Cryst. 2019, 2, e10057. [Google Scholar] [CrossRef]
- Xie, Q.; Han, L.L.; Shan, G.; Bao, Y.; Pan, P.J. Polymorphic Crystalline Structure and Crystal Morphology of Enantiomeric Poly (lactic acid) Blends Tailored by a Self-Assemblable Aryl Amide Nucleator. ACS Sustain. Chem. Eng. 2016, 4, 2680–2688. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, W.Q.; Zhang, Z.C.; Xu, J.Z.; Bian, F.G.; Zhong, G.J.; Xu, L.; Li, Z.M. An efficient, food contact accelerator for stereocomplexation of high-molecular-weight poly (L-lactide)/poly (D-lactide) blend under nonisothermal crystallization. Polymer 2019, 170, 54–64. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Sun, X.R.; Bao, R.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Role of carbon nanotube grafted poly(l-lactide)-block-poly (d-lactide) in the crystallization of poly (l-lactic acid)/poly (d-lactic acid) blends: Suppressed homocrystallization and enhanced stereocomplex crystallization. Eur. Polym. J. 2016, 83, 42–52. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Wei, X.F.; Xie, B.H.; Yang, M.B. Enhanced formation of stereocomplex crystallites of high molecular weight poly(L-lactide)/poly(D-lactide) blends from melt by using poly (ethylene glycol). ACS Sustain. Chem. Eng. 2014, 2, 2301–2309. [Google Scholar] [CrossRef]
- Samuel, C.; Cayuela, J.; Barakat, I.; Mueller, A.J.; Raquez, J.M.; Dubois, P. Stereocomplexation of polylactide enhanced by poly (methyl methacrylate): Improved processability and thermomechanical properties of stereocomplexable polylactide-based materials. ACS Appl. Mater. Interfaces 2013, 5, 11797–11807. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Bian, Y.; Li, Y.; Han, C.; Dong, L. Miscibility and crystallization behaviors of stereocomplex-type poly (L- and D-lactide)/poly (methyl methacrylate) blends. J. Therm. Anal. Calorim. 2014, 118, 359–367. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Liu, Z.Y.; Xie, B.H.; Yang, M.B. Polymorphism of a high-molecular-weight racemic poly(L-lactide)/poly(D-lactide) blend: Effect of melt blending with poly (methyl methacrylate). RSC Adv. 2015, 5, 19058–19066. [Google Scholar] [CrossRef]
- Pan, P.J.; Bao, J.N.; Han, L.L.; Xie, Q.; Shan, G.; Bao, Y. Stereocomplexation of high-molecular-weight enantiomeric poly (lactic acid) s enhanced by miscible polymer blending with hydrogen bond interactions. Polymer 2016, 98, 80–87. [Google Scholar] [CrossRef]
- Bao, J.N.; Xue, X.; Li, K.; Chang, X.; Xie, Q.; Yu, C.; Pan, P.J. Competing stereocomplexation and homocrystallization of poly (l-lactic acid)/poly (d-lactic acid) racemic mixture: Effects of miscible blending with other polymers. J. Phys. Chem. B 2017, 121, 6934–6943. [Google Scholar] [CrossRef]
- Deng, S.H.; Yao, J.; Bai, H.W.; Xiu, H.; Zhang, Q.; Fu, Q. A generalizable strategy toward highly tough and heat-resistant stereocomplex-type polylactide/elastomer blends with substantially enhanced melt processability. Polymer 2021, 224, 123736. [Google Scholar] [CrossRef]
- Xu, H.; Fang, H.; Bai, J.; Zhang, Y.; Wang, Z. Preparation and characterization of high-melt-strength polylactide with long-chain branched structure through γ-radiation-induced chemical reactions. Ind. Eng. Chem. Res. 2014, 53, 1150–1159. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Zhang, L.; Bai, Y. Crystallization behavior of long-chain branching polylactide. Ind. Eng. Chem. Res. 2012, 51, 13670–13679. [Google Scholar] [CrossRef]
- Bousmina, M.; Ait-Kadi, A.; Faisant, J.B. Determination of shear rate and viscosity from batch mixer data. J. Rheol. 1999, 43, 415–433. [Google Scholar] [CrossRef]
- Ma, P.; Shen, T.; Xu, P.; Dong, W.; Lemstra, P.J.; Chen, M. Superior performance of fully biobased poly (lactide) via stereocomplexation-induced phase separation: Structure versus property. ACS Sustainable. Chem. Eng. 2015, 3, 1470–1478. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yang, W.; Jiang, W.R.; Liu, Z.Y.; Xie, B.H.; Yang, M.B. Polymorphism of racemic poly (L-lactide)/poly (D-lactide) blend: Effect of melt and cold crystallization. J. Phys. Chem. B 2013, 117, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Huang, Y.F.; Ruan, J.; Su, A.C. Extensive development of precursory helical pairs prior to formation of stereocomplex crystals in racemic polylactide melt mixture. Macromolecules 2012, 45, 872–878. [Google Scholar] [CrossRef]
- Feng, L.; Bian, X.; Li, G.; Chen, X. Thermal Properties and Structural Evolution of Poly (l-lactide)/Poly (d-lactide) Blends. Macromolecules 2021, 54, 10163–10176. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, X.; Chen, X.; Deng, S.; Bai, H. Substantially Enhanced Stereocomplex Crystallization of Poly(L-lactide)/Poly(D-lactide) Blends by the Formation of Multi-Arm Stereo-Block Copolymers. Crystals 2022, 12, 210. https://doi.org/10.3390/cryst12020210
Diao X, Chen X, Deng S, Bai H. Substantially Enhanced Stereocomplex Crystallization of Poly(L-lactide)/Poly(D-lactide) Blends by the Formation of Multi-Arm Stereo-Block Copolymers. Crystals. 2022; 12(2):210. https://doi.org/10.3390/cryst12020210
Chicago/Turabian StyleDiao, Xingyuan, Xiaonan Chen, Shihao Deng, and Hongwei Bai. 2022. "Substantially Enhanced Stereocomplex Crystallization of Poly(L-lactide)/Poly(D-lactide) Blends by the Formation of Multi-Arm Stereo-Block Copolymers" Crystals 12, no. 2: 210. https://doi.org/10.3390/cryst12020210
APA StyleDiao, X., Chen, X., Deng, S., & Bai, H. (2022). Substantially Enhanced Stereocomplex Crystallization of Poly(L-lactide)/Poly(D-lactide) Blends by the Formation of Multi-Arm Stereo-Block Copolymers. Crystals, 12(2), 210. https://doi.org/10.3390/cryst12020210