Abstract
Nanocrystalline olivine-structured Mg2SiO4 and MgCoSiO4, with an average particle size of 27 nm and 31 nm, respectively, were successfully synthesized from oxide precursors via mechanochemical methods. The two nanocrystalline products were obtained after milling for 360 min and displayed high concentrations of Mg2SiO4 (>94%) and MgCoSiO4 (>95%), together with minor amounts of WC (~3%) contaminant originating as debris abraded off milling balls and chambers. The macroscopic temperature monitoring of the grinding jars during milling trials recorded a peak temperature of 75 °C. A combination of analytical techniques that included XRD, TEM, SAED, and EDS were employed for the characterization of the synthesized products.
1. Introduction
Olivine-type orthosilicates (M,N)2SiO4, where M and N are mainly Fe and Mg, with small amounts of Co, Mn, Ca, and Ni present, represent a fundamental class of minerals of mafic and ultramafic igneous rocks in the crust and upper mantle of the Earth [1,2,3]. While forsterite (Mg2SiO4) and fayalite (Fe2SiO4) are the predominant end members, other binary silicates—i.e., larnite (Ca2SiO4); liebenbergite (Ni2SiO4); tephroite (Mn2SiO4); and Ca-containing ternary silicates, i.e., kirschsteinite (CaFeSiO4), monticellite (CaMgSiO4), and glaucochroite (CaMnSiO4)—are also naturally occurring minerals. The olivine structure is among the most robust crystallographic arrangements and able to accommodate a variety of metal cations, together with different types of anionic groups, i.e., germanate GeO42−, SiS42−, phosphate PO43−, and GeS42− [4,5,6,7].
The naturally occurring solid solution series of olivine (Fe1−xMgx)2SiO4 has been the subject of intensive research for refractories, battery materials, mineralogy, and carbon sequestration [8,9,10,11,12]. Mg2SiO4 exists in three well-established equilibrium polymorphs: orthorhombic Pnma forsterite α-Mg2SiO4, orthorhombic Imma wadsleyite spinelloid β-Mg2SiO4, and cubic Fd-3m spinel ringwoodite γ-Mg2SiO4 [13]. The transformation of α-Mg2SiO4 to β-Mg2SiO4 and γ-Mg2SiO4 occurs at elevated pressure and temperature, yet both stabilize at ambient conditions upon quenching.
Natural olivines incorporate small amounts of Co; the parameters controlling this process, e.g., diffusion rates, and the effects of Co incorporation on the physical properties of olivine have also been studied [14,15]. The end member of the (Mg,Co)2SiO4 solid solution, Co2SiO4, is not a recognized mineral, but can be produced synthetically. It also exists in three polymorphic forms, analogous to Mg2SiO4 phases. Ringwood et al. found that the transformation of Co2SiO4 from the olivine structure to the ringwoodite structure took place at 7 GPa and 700 °C, and Morimoto et al. reported on the syntheses and crystal-chemical relationships of the three Co2SiO4 polymorphs [16,17].
Recently, olivine solid solutions, MgM’SiO4 (where M’ = Fe, Co, Mn), have received renewed interest as potential cathode materials for rechargeable magnesium batteries due to their high theoretical energy densities. The synthesis of these olivine solid solution series utilizes various conventional methods including a combination of modified sol–gel, molten salt, and water-based solution chemistry, all of which involve multi-step synthesis, while some required heating to above 450 °C [18,19,20,21,22,23,24]. For example, Li et al. prepared MgFeSiO4 through a flux method utilizing stoichiometric amounts of MgO, FeC2O4·2H2O, and SiO2 and heat treatment at >800 °C in a controlled Ar atmosphere [19]. Qafoku et al. reported the synthesis of nanosized Fe(II)-rich olivine, fayalite, (Mg0.33Fe0.66)2SiO4, and (Mg0.66Fe0.33)2SiO4, using a metal ion-sucrose solution approach, with calcination at >850 °C under a flow of CO2/CO/Ar balanced gas mixture and the removal of excess iron metal from isopropanol suspensions [23]. Feng et al. prepared Mg1.03Mn0.97SiO4 by milling a stoichiometric mixture of MgO, MnCO3, and SiO2, followed by sintering at 900 °C for 24 h [18]. Mori et al. synthesized MgMnSiO4 using a flux method, starting from a 1:1:1 molar ratio mixture of MgO, MnCO3, and SiO2 ball-milled in ethanol at 300 RPM with subsequent calcination at >500 °C under a flux of 3% H2/Ar balance [24]. Nuli et al. and Zheng et al. synthesized MgCoSiO4 utilizing water-based solution chemistry and a molten salt/mixed solvothermal approach in combination with heat treatment at 1000 °C and 1300 °C, respectively [20,21].
Mechanochemical synthesis by high-energy ball milling has become an important solid-state synthesis method for the production of nanomaterials, with advantages including large-scale capabilities (grams) and low reaction temperatures. The one-pot synthesis of olivine-type Fe2SiO4 highlights the early work of Šepelák et al., starting from Fe2O3, Fe, and SiO2 and milled under Ar inert atmosphere [25]. Mechanochemically synthesized nanocrystalline Co2SiO4 has also been reported recently by our lab using a 2:1 molar ratio mixture of CoO and SiO2 [26]. In an effort to standardize the Mg-Co silicate solution series preparation, we report here the results of our investigation into the mechanochemical synthesis of olivine-type Mg2SiO4 and ternary olivine MgCoSiO4, starting with stoichiometric amounts of MgO, SiO2 and CoO, MgO, SiO2, respectively.
2. Experimental Methods
General Experimental Details. All sample loading was conducted on a benchtop under ambient conditions. Powder XRD analysis of the substrates revealed that the CoO material contained 12.88 wt.% of spinel-structured mixed-valence cobalt oxide, Co3O4 (known as mineral guite) (see Supplementary Materials Figure S1). SiO2 starting material was analyzed in the same manner as the cobalt oxide and was found to consist of pure phase α-quartz with no measurable impurities (see Supplementary Materials Figure S2). Prior to the mechanochemical processing, the MgO starting material was heat-treated at 400 °C for 4 h to dehydrate any Mg(OH)2 that was formed upon reacting with water in the ambient atmosphere. Powder XRD analysis of the sample after heat treatment indicated a high concentration of periclase MgO phase with trace amount of calcite CaCO3 (see Supplementary Materials Figure S3).
Powder X-ray Diffraction (PXRD). The sample was analyzed by high-resolution X-ray powder diffraction on a Bruker D8 Advance diffractometer with a 3 kW CuKα source and a LynxEye XE detector in Bragg–Brentano parafocusing geometry, mounted on a 9-position automated sample changer using either standard puck-type mounts or zero-background Si-wafer mounts when only a small quantity of the processed sample was available. A 0.020 mm Ni filter was used to reduce CuKβ radiation. Typical powder scans were conducted over a range of 5–85 degrees with a step size of 0.02 degrees and an exposure time of 1 s per step.
Rietveld refinement analysis. Rietveld analysis was conducted using Bruker TOPAS 5 [27]. Starting models of MgCoSiO4, Mg2SiO4, SiO2, WC, CoWO4, Co3O4, MgSiO3, MgO, and CaCO3 structures were taken from PDF 04-017-1377, PDF 00-034-0189, PDF 00-046-1045, PDF 04-001-2755, PDF 00-015-0867, PDF 00-042-1467, COD 9007016, PDF 00-045-0946, and PDF 00-066-0867, respectively [28]. Refinement included the optimization of background, phase fractions, unit cell parameters, peak profiles (controlled by grain size and strain models), site occupancies for non-oxygen atoms, and atomic displacement parameters. Fractional atomic coordinates were kept fixed at literature values.
Transmission Electron Microscopy (TEM). In order to determine the elemental composition and to assess the chemical homogeneity of the final milled product, we used an 80–300 keV high-base Titan (FEI Thermo-Fisher) scanning transmission electron microscope equipped with a solid-state Si(Li) energy-dispersive X-ray detector (Genesis 4000, EDAX). Brightfield and darkfield images were acquired in scanning transmission (“STEM”) mode and the crystal structures of grains were assessed using selected area electron diffraction (SAED). Compositions were measured by energy-dispersive X-ray spectroscopy (“EDS”) and quantified using a Cliff–Lorimer thin-film approximation and correction factors (“K factors”) derived from thin-film standards. This procedure assumes that the specimen is sufficiently thin that any X-ray absorption by the specimen is minimal.
Preparation of Mg2SiO4/MgCoSiO4. The synthetic procedure was based on the methodology developed previously by our group for the synthesis of the nanocrystalline Co2SiO4 [26]. A mixture of a 2:1 molar ratio of magnesium oxide (0.62 g) and silicon oxide (0.47 g) and a second mixture of a 1:1:1 molar ratio of magnesium oxide (0.25 g), cobalt oxide (0.47 g), and silicon oxide (0.37 g) were loaded into 2 separate 25 mL tungsten carbide (WC) jars and mechanically milled under dry conditions with two 15 mm diameter WC balls (40:1 ball to powder ratio) oscillating at a frequency of 30 Hz for various durations of time, ranging from 0 to 360 min. Milling experiments performed in air or under a controlled atmosphere (Ar) produced no difference in the processing outcomes.
3. Results and Discussion
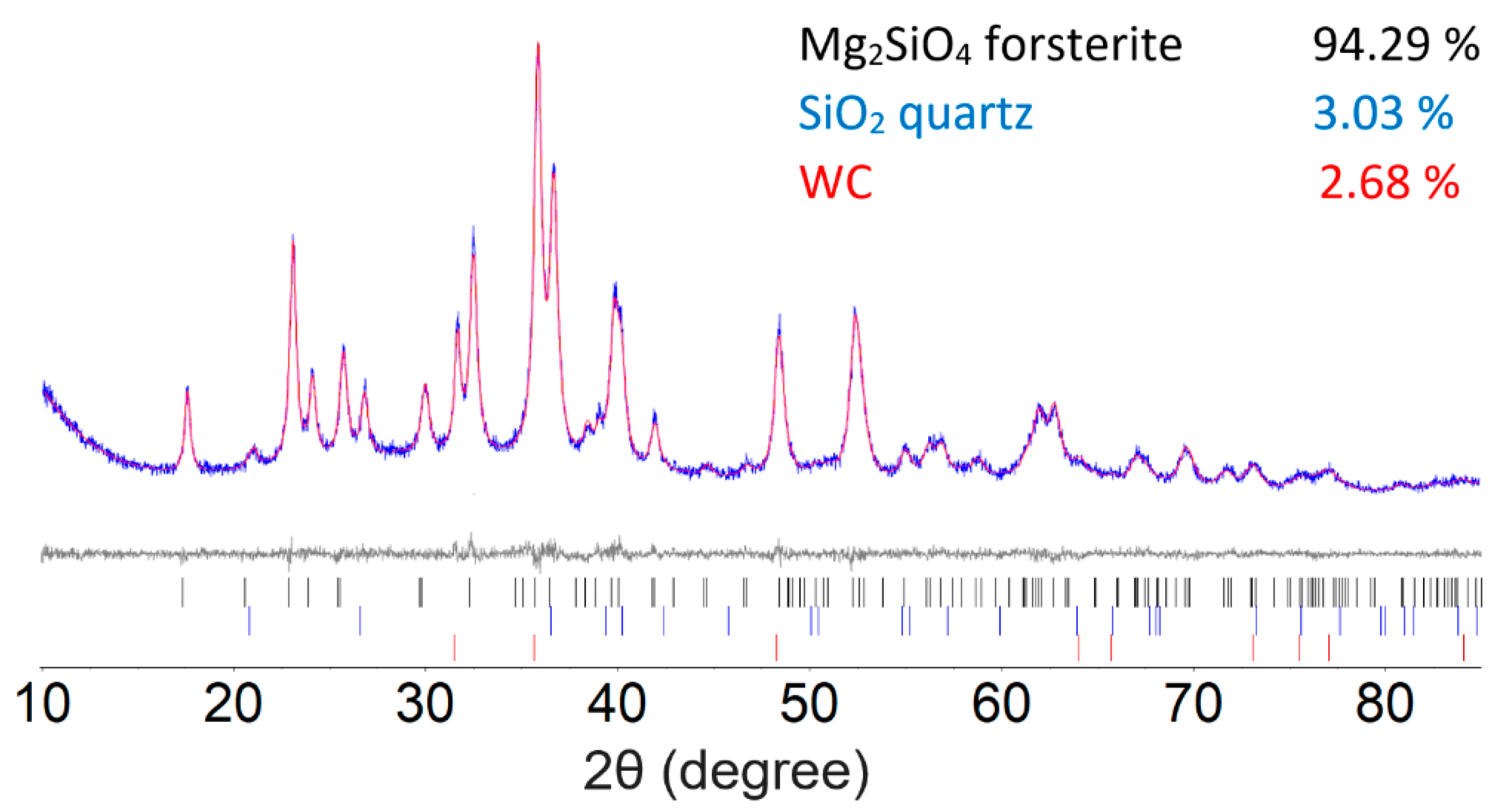
To set up a baseline methodological approach, forsterite Mg2SiO4 was prepared by the high-energy milling of a 2:1 molar ratio mixture of MgO and SiO2 for 180 min. Our synthesis follows the general procedure of Nguyen et al. for the synthesis of olivine Co2SiO4 [26]. Figure 1 illustrates the results of the Rietveld refinement of 180 min milled Mg2SiO4, which converge to a final figure of merit Rwp = 6.524. Small quantities of unreacted quartz SiO2 (3.03%) and qusongite WC (2.68%) that were shaved off the milling media were detected alongside the Mg2SiO4 (94.29%). A good agreement between calculated and measured diffraction patterns was obtained based on the Rietveld refinements. The average crystallite size for the synthesized Mg2SiO4 forsterite, based on the Rietveld refinement, was 26 nm.
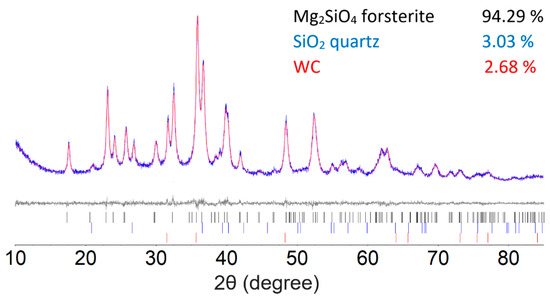
Figure 1.
The Rietveld refinement of the 2:1 molar ratio of MgO and SiO2 after 180 min of milling time. The bottom curve shows the difference between the observed and the calculated intensities. Tick marks below indicate the peak positions for Mg2SiO4, unreacted SiO2, and WC.
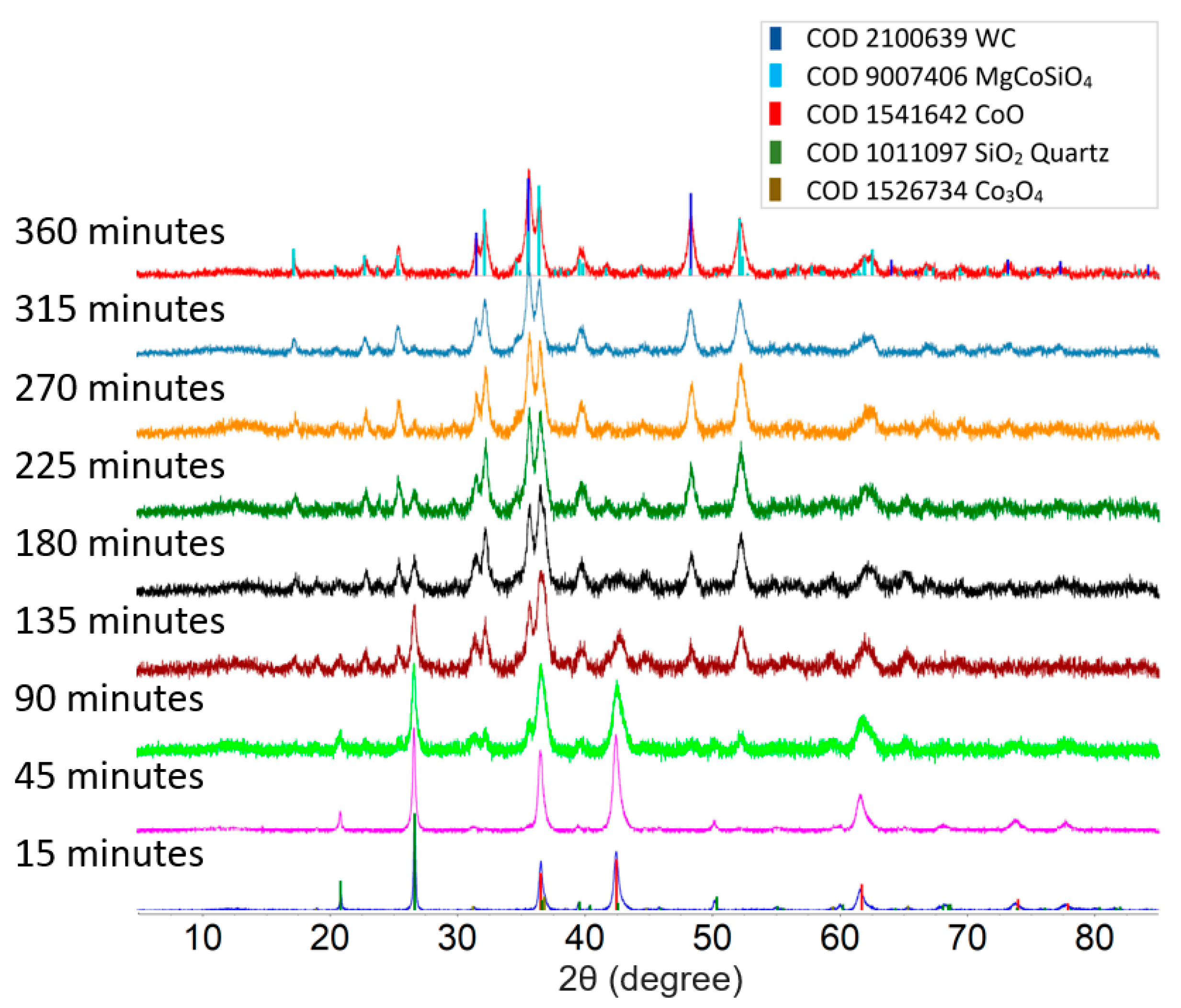
Figure 2 shows a comparison between the powder XRD patterns of the product from the 1:1:1 molar ratio mixture of MgO, CoO, and SiO2 milled for different lengths of time. Short milling times (up to 45 min) only produced changes in the average grain size of the sample and lattice strain, which are indicated by the increasing diffraction peak width, but no new peaks were observed. It was also observed that MgO turns amorphous after 15 min into milling. A weak pattern of orthorhombic olivine MgCoSiO4, indicated by the characteristic peak at 35.7°, was observed after 90 min of milling time. The formation of MgCoSiO4 from this starting mixture was complete after 360 min of milling, as indicated by the complete disappearance of the strong SiO2 quartz diffraction peak at 26.8°. The diffraction peak width of the product phase is similar to that of the peaks of the precursors after 45 min of milling, indicating sub-micrometer grain sizes. WC debris, produced by the wear of the grinding elements, was also detected in the final product.
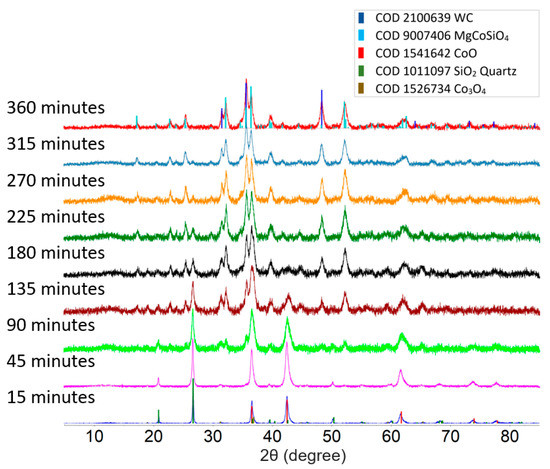
Figure 2.
Powder XRD pattern of 1:1:1 molar ratio of MgO, CoO, and SiO2 mixture after various milling times. MgO turns amorphous after milling for 15 min.
The milling process in an oscillating mill is believed to proceed at only slightly elevated average temperatures (<100 °C), although significant localized heating may occur from individual ball and sample impacts [29,30]. Attempts to monitor the temperature of the milling jar with an attached thermocouple registered that a maximum temperature of 75 °C was reached during the milling trials. We further investigated the effect of temperature on the synthesized MgCoSiO4 olivine by annealing the milled powder at 1000 °C for 12 h.
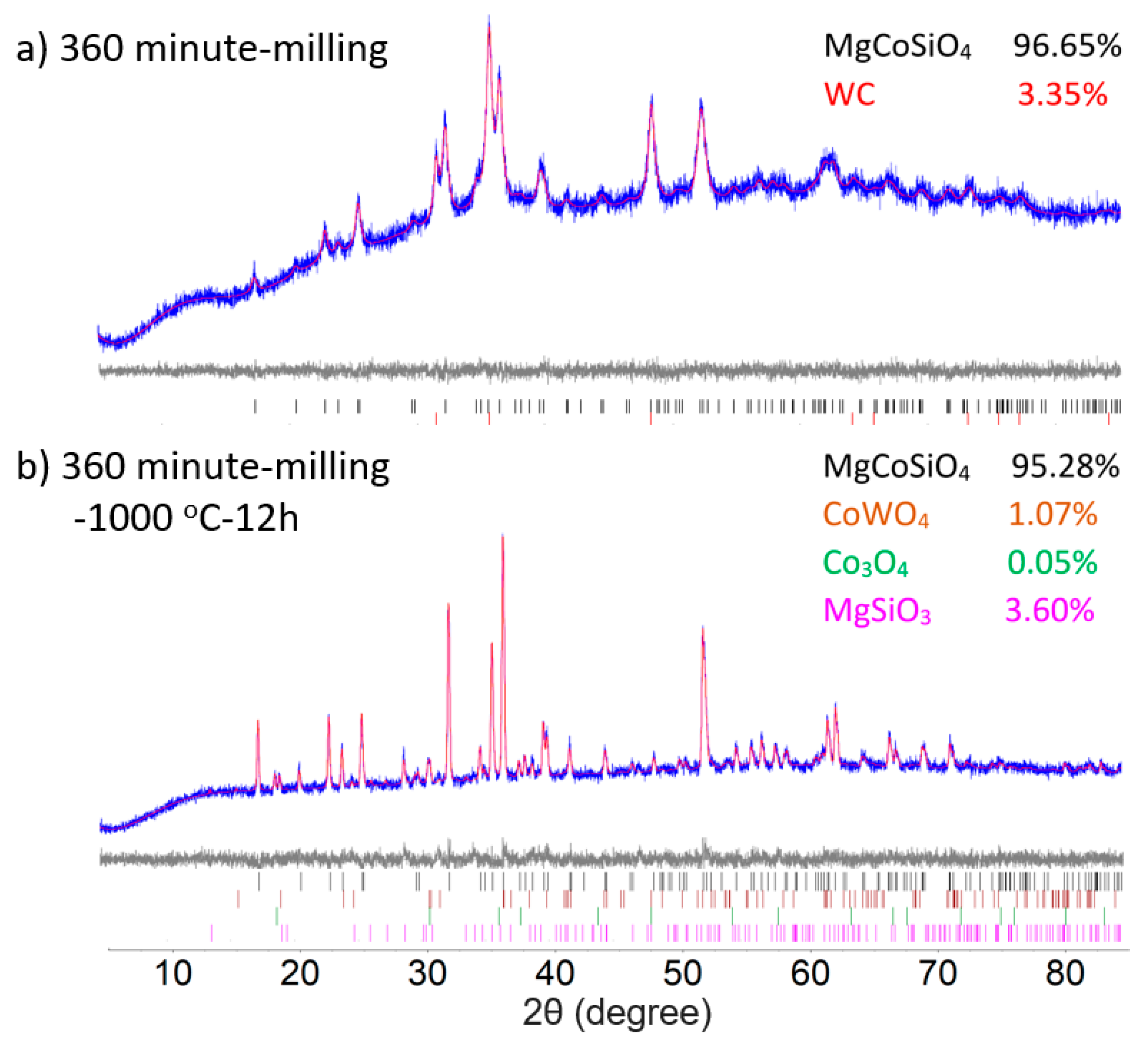
Figure 3 illustrates the results of the Rietveld refinements on the 360 min milled samples with and without subsequent annealing, which converge to final figures of merit Rwp = 2.219 and 2.906, respectively. The Rietveld refinements of powder both before and after the annealing display marked changes in the peak width of the olivine phase, signifying grain growth. Annealing at 1000 °C further induced a side reaction of the WC debris with the olivine MgCoSiO4 sample, resulting in minor impurities of CoWO4 (1.07%) in the final product. Unreacted guite Co3O4 (0.05%) and clinoenstatite MgSiO3 (3.60%) were also detected as minor phases. Unit cell parameters of olivine Mg2−xCoxSiO4 (x = 0, 0.5, 1) show the expected linear trend of composition, confirming the successful diffusion of Mg and Co into the olivine lattice (Table 1). Further analysis of the occupancies on the M1 (edge-sharing tetragonally elongated octahedra) and M2 (corner-sharing trigonally elongated octahedra) metal sites suggests a strong preference for Co2+ over Mg on the M1 site in both annealed and unannealed samples. Annealing after milling further promoted the diffusion of Mg2+ into the M2 site, resulting in an increase in the Mg site occupancy factor from 0.591 to 0.675 on M2. Unit cell parameters of samples with and without annealing are comparable to literature values [17,31,32,33,34,35].
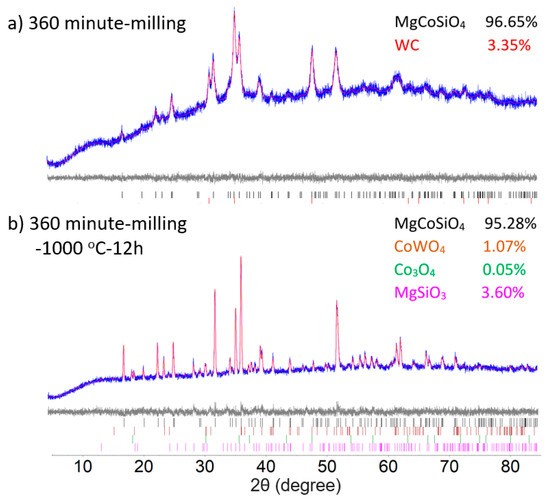
Figure 3.
Rietveld refinement of the (a) 1:1:1 molar ratio of MgO, CoO and SiO2 after (a) 360 min milling time and (b) 360 min milled mixture followed by heating at 1000 °C for 12 h. The bottom curve shows the difference between the observed and the calculated intensities. Tick marks below indicate the peak positions for cobalt silicate and WC/CoWO4.

Table 1.
Unit cell parameters obtained from the Rietveld refinement of the synthesized MgCoSiO4, before and after heating in comparison with literature values.
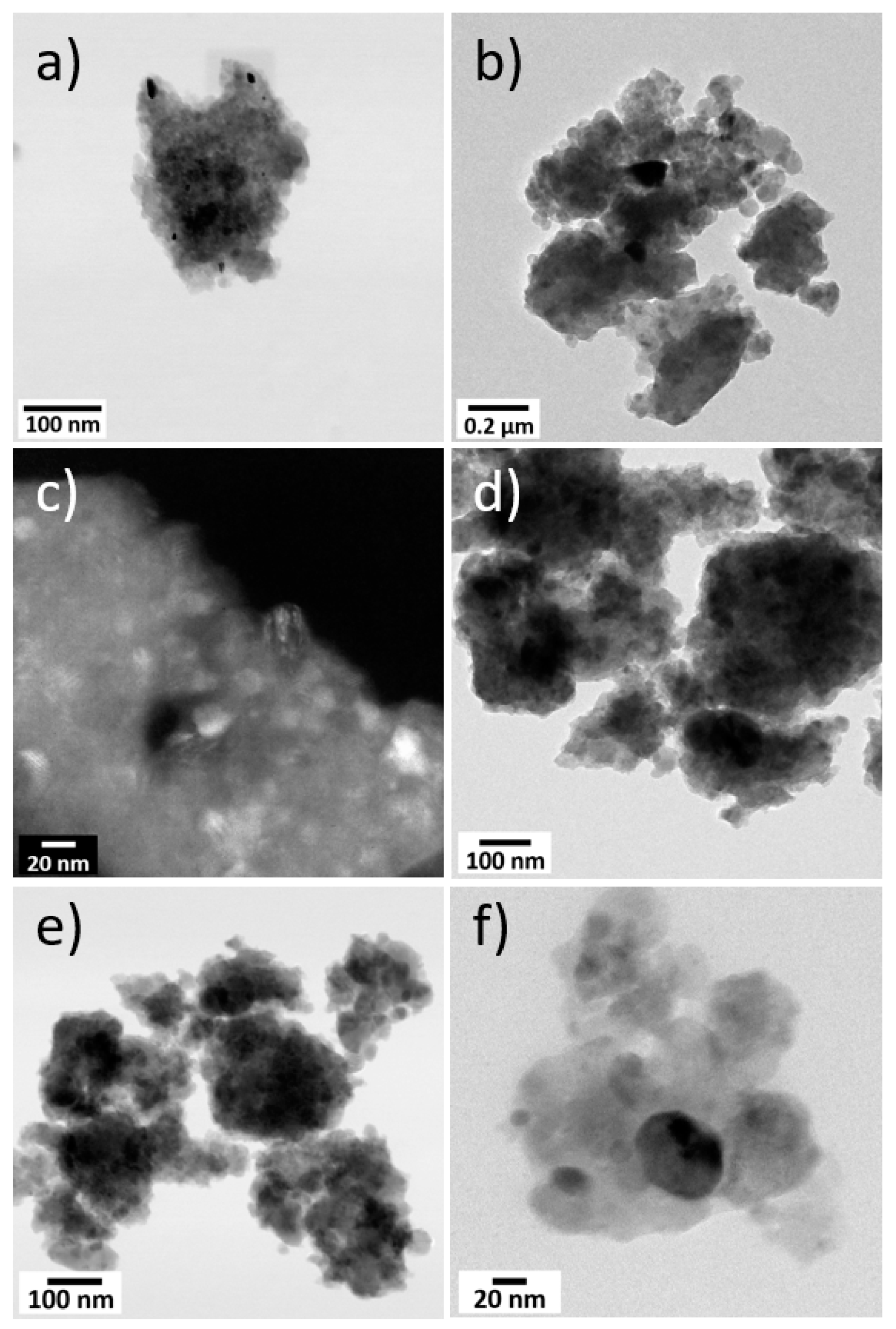
TEM images of the synthesized Mg2SiO4 product, obtained at different magnifications, are compared to synthetic MgCoSiO4 in Figure 4. The particle size for MgCoSiO4 ranged from 23 to 50 nm (average 27 nm), while a greater size variation between 8 to 67 nm (average 31 nm) was observed in Mg2SiO4. Many of the particles appeared to be present as clumps, signifying aggregation during milling. Attempts to break apart these clumps ultrasonically produced little change in the end product.
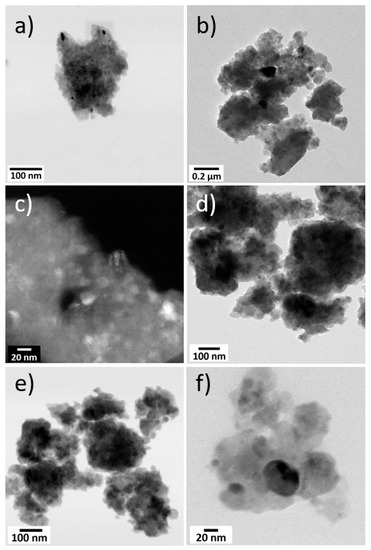
Figure 4.
Brightfield and darkfield STEM images at different magnifications of the synthesized Mg2SiO4 (a–c) and MgCoSiO4 (d–f).
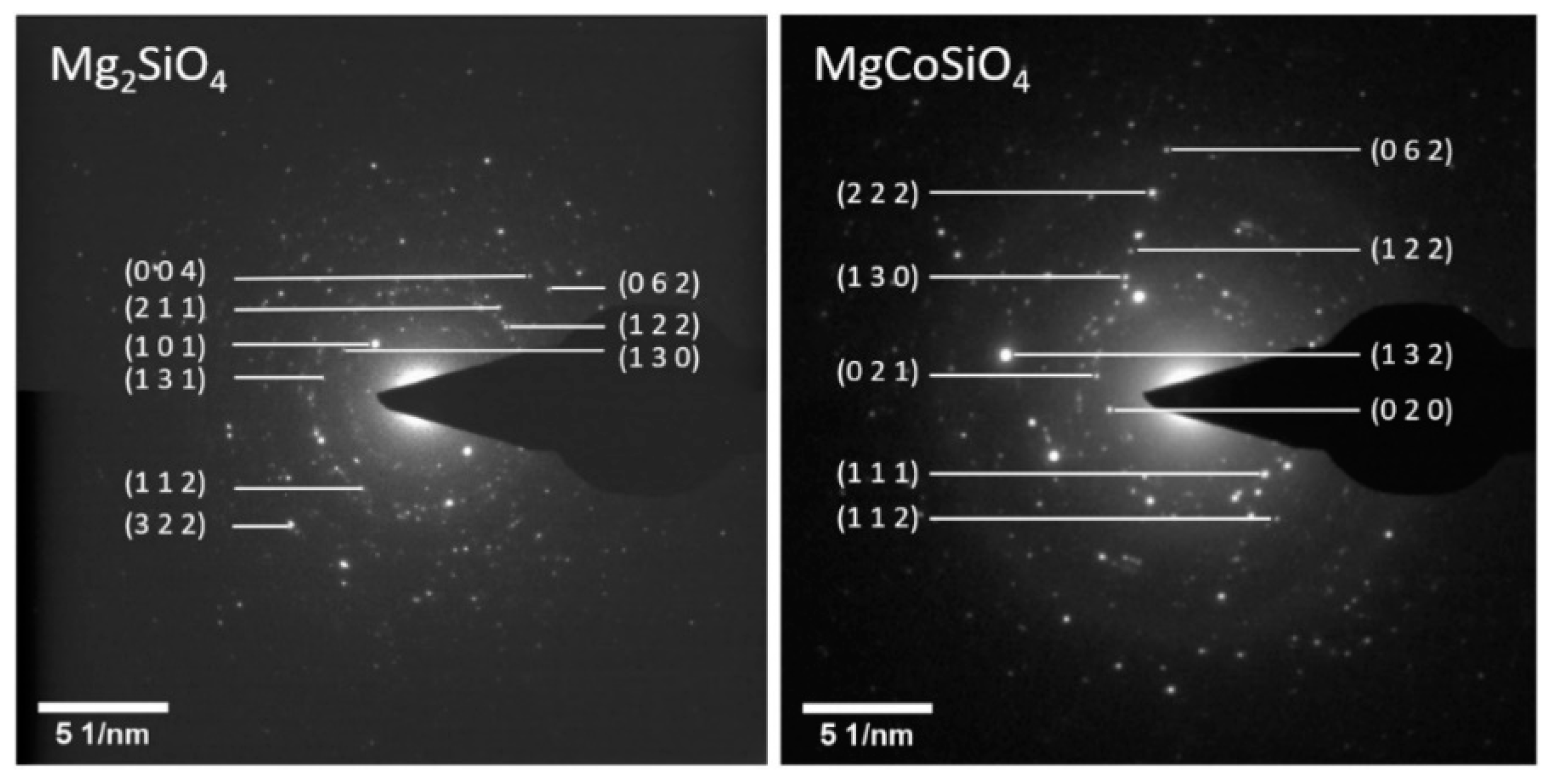
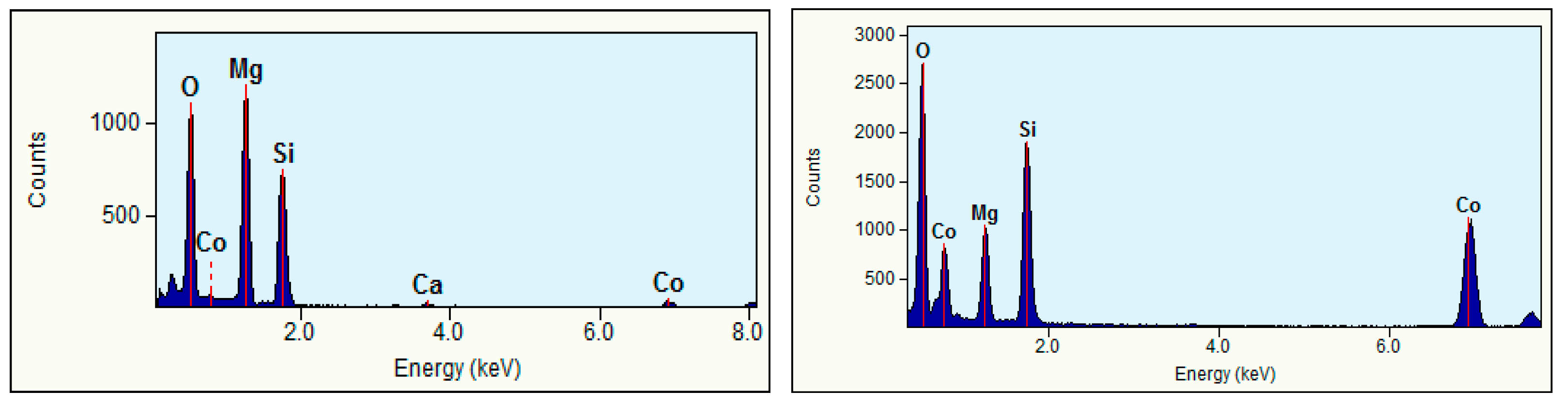
The milled samples (Figure 5) showed high degrees of spottiness in the diffraction rings, consistent with the polycrystalline nature of the as-synthesized Mg2SiO4 and MgCoSiO4. The indexing of the SAED patterns revealed signature orthorhombic olivine crystal structures. The EDS analysis of selected particles of the 180 min milled Mg2SiO4 indicated the presence of (as expected) Mg, Si, and O, as well as trace amounts of Ca (from CaCO3 in the MgO starting material), and Co as a carryover contaminant from previous milling trials (where a small amount of Co embedded itself in the WC grinding components) (Figure 6). Quantitative analysis of elemental mass percentages based on the EDS spectrum suggested an empirical formula of Mg2SiO4. EDS analysis of the MgCoSiO4 sample, although within the margin of error of the measurement, indicated some degree of variation in the Mg:Co:Si proportions. For example, the Mg:Si ratio of forsterite was 2.03:1; the Mg:Co:Si ratios of MgCoSiO4 covered the range of (0.61–0.93):(0.67–0.81):1. The amount of Si detected by XRD was significantly lower than that determined from the EDS analysis of the synthesized MgCoSiO4. This could be due to selective (X-ray) absorption or to a residual amorphous Si-containing component that was not readily detected by XRD analysis [36].
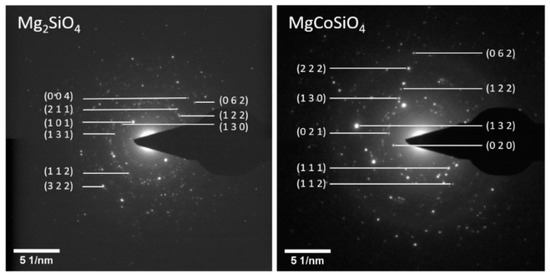
Figure 5.
Selected area electron diffraction (SAED) images of synthesized Mg2SiO4 (left) and MgCoSiO4 (right).
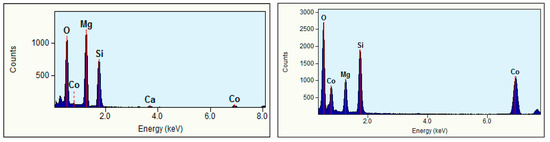
Figure 6.
Representative STEM-EDS spectra of 360 min milled Mg2SiO4 (left) and 360 min milled MgCoSiO4 (right).
4. Conclusions
This report presents a straightforward method for synthesizing nanocrystalline Mg2SiO4 and MgCoSiO4. The ability to synthesize and finetune the chemical series of Mg2+ and Co2+ represents a unique contribution to olivine reactivity research. The synthesis technique was shown to be efficient at ambient temperatures, without contingent inert atmospheric control. Given the nanocrystalline nature of the products and the simple reaction activation by milling, research on a potential application, i.e., the battery performance testing of the mechanochemically synthesized Mg2SiO4 and MgCoSiO4, is currently underway. The contamination of the synthesized olivine with WC, which in turn resulted in the undesirable formation of tungstate when annealing the milled product at an elevated temperature, remains an unresolved concern for further material development. Thus, a robust method for removing WC from mechanically synthesized olivine is necessary and will be addressed in a follow-up publication.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12030369/s1, Figure S1: Rietveld refinement of starting material CoO; Figure S2: Rietveld refinement of starting material SiO2; Figure S3: Rietveld refinement of starting material MgO.
Author Contributions
Conceptualization, P.Q.H.N. and P.D.; methodology, P.Q.H.N., P.D. and J.P.B.; data curation, P.Q.H.N., P.D. and J.P.B.; resources, P.D. and J.P.B.; writing—original draft preparation, P.Q.H.N. and P.D.; writing—review and editing, W.M., D.Z., J.X., R.R. and J.P.B. All authors have read and agreed to the published version of the manuscript.
Funding
Office of Naval Research, Department of Navy’s Historically Black Colleges and Universities/Minority Institutions, the Materials for Thermal and Chemical Extreme program, grant number FOA N00014-19-S-F004 and NSF EAR grant 1829273.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the financial support of this work provided by the Office of Naval Research, Department of Navy’s Historically Black Colleges and Universities/Minority Institutions, the Materials for Thermal and Chemical Extreme program, grant number FOA N00014-19-S-F004. R.R. has been supported by NSF EAR grant 1829273.
Conflicts of Interest
There are no conflict to declare.
References
- Anderson, D.L.; Bass, J.D. Mineralogy and composition of the upper mantle. Geophys. Res. Lett. 1984, 11, 637–640. [Google Scholar] [CrossRef] [Green Version]
- McDonough, W.F.; Rudnick, R.L. Minerology and composition of the upper mantle. Rev. Mineral. 1998, 37, 139–164. [Google Scholar]
- Rudnick, R.L.; Fountain, D.M. Nature and composition of the continental crust: A lower crustal perspective. Rev. Geophys. 1995, 33, 267–309. [Google Scholar] [CrossRef] [Green Version]
- Volkov, N.V.; Mikhashenok, N.V.; Sablina, K.A.; Bayukov, O.A.; Gorev, M.V.; Balaev, A.D.; Pankrats, A.I.; Tugarinov, V.I.; Velikanov, D.A.; Molokeev, M.S.; et al. Magnetic phase diagram of the olivine-type Mg2GeO4 single crystal estimated from magnetic, resonance and thermodynamic properties. J. Phys. Condens. Matter 2013, 25, 136003. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (phosphates, silicates, sulfates) framework as electrode materials for rechargable Li (or Na) batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef]
- Ericsson, T.; Amcoff, O.; Kalinowski, M. Cation preferences in thio-olivines (Fe1−xMgx)2SiS4, x =< 0.30, studied by Mossbauer spectroscopy at room temperature. Neues Jb. f. Mineral. Mh. 1999, 11, 518–528. [Google Scholar]
- Chung, J.-H.; Ohgushi, K.; Ueda, Y. Magnetic phase diagram of the multicritical olivine Mn2SiS4 and Mn2GeS4. J. Magn. Magn. Mater. 2010, 322, 832–837. [Google Scholar] [CrossRef]
- Goldschmidt, V. Olivine and forsterite refractories in Europe. Ind. Eng. Chem. 1938, 30, 32–34. [Google Scholar] [CrossRef]
- Pack, A.; Hoernes, S.; Walther, T.; Bross, R. Behavior of basic refractories at high temperatures in steelmaking processes—thermodynamics and implications for the usability of olivine as refractory material. Eur. J. Mineral. 2003, 15, 193–205. [Google Scholar] [CrossRef]
- Routschka, G. Refractory Materials: Pocket Manual; Design, Properties, Testing; Vulkan-Verlag GmbH: Essen, Germany, 2008. [Google Scholar]
- Guo, H.; Ping, H.; Hu, J.; Song, X.; Zheng, J.; Pan, F. Controllable synthesis of LiFePO4 in different polymorphs and study of the reaction mechanism. J. Mater. Chem. A 2017, 5, 14294–14300. [Google Scholar] [CrossRef]
- Montserrat, F.; Renforth, P.; Hartmann, J.; Leermakers, M.; Knops, P.; Meysman, F.J. Olivine dissolution in seawater: Implications for CO2 sequestration through enhanced weathering in coastal environments. Environ. Sci. Technol. 2017, 51, 3960–3972. [Google Scholar] [CrossRef] [Green Version]
- Katsura, T.; Ito, E. The system Mg2SiO4-Fe2SiO4 at high pressures and temperatures: Precise determination of stabilities of olivine, modified spinel, and spinel. J. Geophys. Res. Solid Earth 1989, 94, 15663–15670. [Google Scholar] [CrossRef]
- Morioka, M. Cation diffusion in olivine—I. Cobalt and magnesium. Geochim. Cosmochim. Acta 1980, 44, 759–762. [Google Scholar] [CrossRef]
- Chakraborty, S. Diffusion coefficients in olivine, wadsleyite and ringwoodite. Rev. Mineral. Geochem. 2010, 72, 603–639. [Google Scholar] [CrossRef]
- Ringwood, A. Olivine-spinel transformation in cobalt orthosilicate. Nature 1963, 198, 79–80. [Google Scholar] [CrossRef]
- Morimoto, N.; Tokonami, M.; Watanabe, M.; Koto, K. Crystal structures of three polymorphs of Co2SiO4. Am. Mineral. 1974, 59, 475–485. [Google Scholar]
- Feng, Z.; Yang, J.; NuLi, Y.; Wang, J.; Wang, X.; Wang, Z. Preparation and electrochemical study of a new magnesium intercalation material Mg1.03Mn0.97SiO4. Electrochem. Commun. 2008, 10, 1291–1294. [Google Scholar] [CrossRef]
- Li, Y.; Nuli, Y.; Yang, J.; Yilinuer, T.; Wang, J. MgFeSiO4 prepared via a molten salt method as a new cathode material for rechargable magnesium batteries. Chin. Sci. Bull. 2011, 56, 386–390. [Google Scholar] [CrossRef] [Green Version]
- NuLi, Y.; Zheng, Y.; Wang, Y.; Yang, J.; Wang, J. Electrochemical intercalation of Mg2+ in 3D hierarchically porous magnesium cobalt silicate and its application as an advanced cathode material in rechargable magnesium batteries. J. Mater. Chem. 2011, 21, 12437–12443. [Google Scholar] [CrossRef]
- Zheng, Y.; NuLi, Y.; Chen, Q.; Wang, Y.; Yang, J.; Wang, J. Magnesium cobalt silicate materials for reversible magnesium ion storage. Electrochim. Acta 2012, 66, 75–81. [Google Scholar] [CrossRef]
- Orikasa, Y.; Masese, T.; Koyama, Y.; Mori, T.; Hattori, M.; Yamamoto, K.; Okado, T.; Huang, Z.-D.; Minato, T.; Tassel, C. High energy density rechargable magnesium battery using earth-abundant and non-toxic elements. Sci. Rep. 2014, 4, 5622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qafoku, O.; Ilton, E.S.; Bowden, M.E.; Kovarik, L.; Zhang, X.; Kukkadapu, R.K.; Engelhard, M.H.; Thompson, C.J.; Schaef, H.T.; McGrail, B.P.; et al. Synthesis of nanometer-sized fayalite and magnesium-iron(II) mixture olivines. J. Colloid Interface Sci. 2018, 515, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Masese, T.; Orikasa, Y.; Huang, Z.-D.; Okado, T.; Kim, J.; Uchimoto, Y. Anti-site mixing governs the electrochemical performances of olivine-type MgMgSiO4 cathodes for rechargable magnesium batteries. Phys. Chem. Chem. Phys. 2016, 18, 13524–13529. [Google Scholar] [CrossRef] [PubMed]
- Šepelák, V.; Myndyk, M.; Fabián, M.; Da Silva, K.L.; Feldhoff, A.; Menzel, D.; Ghafari, M.; Hahn, H.; Heitjans, P.; Becker, K.D. Mechanosynthesis of nanocrystalline fayalite, Fe2SiO4. Chem. Commun. 2012, 48, 11121–11123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.Q.H.; Zhang, D.; Rapp, R.; Bradley, J.P.; Dera, P. Room temperature facile synthesis of olivine-Co2SiO4 nanoparticles utilizing a mechanochemical method. RSC Adv. 2021, 11, 20687–20690. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Gates-Rector, S.; Blanton, T. The powder diffraction file: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Scholze, H.M.; Stolle, A. Temperature progression in a mixer ball mill. Int. J. Ind. Chem. 2016, 7, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Takacs, L.; McHenry, J. Temperature of the milling balls in a shaker and planetary mills. J. Mater. Sci. 2006, 41, 5246–5249. [Google Scholar] [CrossRef]
- Matsui, Y.; Syono, Y. Unit cell dimensions of some synthetic olivine group solid solutions. Geochem. J. 1968, 2, 51–59. [Google Scholar] [CrossRef]
- Akimoto, S.I.; Fujisawa, H. Olivine-spinel solid solution equilibria in the system Mg2SiO4-Fe2SiO4. J. Geophys. Res. 1968, 73, 1467–1479. [Google Scholar] [CrossRef]
- Rinaldi, R.; Gatta, G.; Artioli, G.; Knight, K.; Geiger, C. Crystal chemistry, cation ordering and thermoelastic behaviour of CoMgSiO4 olivine at high temperatue as studied by in situ neutron powder diffraction. Phys. Chem. Miner. 2005, 32, 655–664. [Google Scholar] [CrossRef]
- Miyake, M.; Nakamura, H.; Kojima, H.; Marumo, F. Cation ordering in Co-Mg olivine solid-solution series. Am. Mineral. 1987, 72, 594–598. [Google Scholar]
- Müller-Sommer, M.; Hock, R.; Kirfel, A. Rietveld refinement study of the cation distribution in (Co,Mg)-olivine solid solution. Phys. Chem. Miner. 1997, 24, 17–23. [Google Scholar] [CrossRef]
- Sohor, M.A.H.M.; Mustapha, M.; Mamat, O. The Effect of Milling Duration on Silicon Dioxide Characterization; MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; p. 03007. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).