Tinaksite and Tokkoite: X-ray Powder Diffraction, Optical, and Vibrational Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Powder Diffraction
3.2. Spectroscopic Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogov, Y.G.; Rogova, V.P.; Voronkov, A.A.; Moleva, V.A. Tinaksite, NaK2Ca2TiSi7O19(OH), a new mineral. Dokl. Acad. Nauk SSSR 1965, 162, 658–661. [Google Scholar]
- Lazebnik, K.A.; Nikishova, L.V.; Lazebnik, Y.D. Tokkoite—A new mineral of charoitites. Mineral. Zhurnal 1986, 8, 85–89. [Google Scholar]
- Sokolova, M.N.; Zabavnikova, N.I.; Yakovlevskaya, T.A.; Rudnitskaya, E.S. Tinaksite from pegmatites of the apatite deposit Rasvumchorr (Khibiny Massif). Proc. Rus. Miner. Soc. 1975, 104, 39–43. [Google Scholar]
- Kostyleva-Labuntsova, E.E.; Borutzky, B.E.; Sokolova, M.N.; Shlyukova, Z.V.; Dorfman, M.D.; Dudkin, O.B.; Kozyreva, L.V.; Ikorskii, S.V. Mineralogy of Khibiny Massif; Nauka: Moscow, Russia, 1978; Volume 1, p. 228. [Google Scholar]
- Kasatkin, A.V.; Cámara, F.; Chukanov, N.V.; Škoda, R.; Nestola, F.; Agakhanov, A.A.; Belakovskiy, D.I.; Lednyov, V.S. Patynite, NaKCa4[Si9O23], a new mineral from the Patynskiy massif, Southern Siberia, Russia. Minerals 2019, 9, 611. [Google Scholar] [CrossRef] [Green Version]
- Kaneva, E.; Shendrik, R.; Mesto, E.; Bogdanov, A.; Vladykin, N. Spectroscopy and crystal chemical properties of NaCa2[Si4O10]F natural agrellite with tubular structure. Chem. Phys. Lett. 2020, 738, 136868. [Google Scholar] [CrossRef]
- Kaneva, E.; Lacalamita, M.; Mesto, E.; Schingaro, E.; Scordari, F.; Vladykin, N. Structure and modeling of disorder in miserite from the Murun (Russia) and Dara-i-Pioz (Tajikistan) massifs. Phys. Chem. Miner. 2014, 41, 49–63. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Nikishova, L.V. The crystal structure of frankamenite. Miner. Mag. 1996, 60, 897–905. [Google Scholar] [CrossRef]
- Kaneva, E.; Radomskaya, T.; Suvorova, L.; Sterkhova, I.; Mitichkin, M. Crystal chemistry of fluorcarletonite, a new mineral from the Murun alkaline complex (Russia). Eur. J. Miner. 2020, 32, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Kaneva, E.V.; Shendrik, R.Y.; Radomskaya, T.A.; Suvorova, L.F. Fedorite from Murun alkaline complex (Russia): Spectroscopy and crystal chemical features. Minerals 2020, 10, 702. [Google Scholar] [CrossRef]
- Dunn, P.J.; Wilson, W.E. Nomenclature revisions in the apophyllite group: Hydroxyapophyllite, apophyllite, fluorapophyllite. Miner. Rec. 1978, 3, 95–98. [Google Scholar]
- Rozhdestvenskaya, I.V.; Vasilieva, V.A. Cation ordering and structural deformations in pectolite HNaCaSi3O9–serandite HNaMn2Si3O9. J. Struct. Chem. 2014, 55, 1268–1276. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Mugnaioli, E.; Schowalter, M.; Schmidt, M.U.; Czank, M.; Depmeier, W.; Rosenauerd, A. The structure of denisovite, a fibrous nanocrystalline polytypic disordered ‘very complex’ silicate, studied by a synergistic multi-disciplinary approach employing methods of electron crystallography and X-ray powder diffraction. IUCr J. 2017, 4, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Rozhdestvenskaya, I.; Mugnaioli, E.; Czank, M.; Depmeier, W.; Kolb, U.; Reinholdt, A.; Weirich, T. The structure of charoite, (K, Sr, Ba, Mn)15-16(Ca, Na)32[Si70(O,OH180](OH,F)4·nH2O, solved by conventional and automated electron diffraction. Miner. Mag. 2010, 74, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Rozhdestvenskaya, I.; Mugnaioli, E.; Czank, M.; Depmeier, W.; Kolb, U.; Merlino, S. Essential features of the polytypic charoite-96 structure compared to charoite-90. Miner. Mag. 2011, 75, 2833–2846. [Google Scholar] [CrossRef]
- Kaneva, E.V.; Radomskaya, T.A.; Shendrik, R.Y.; Chubarov, V.M.; Amosova, A.A.; Mitichkin, M.A. FTIR, XRF and powder XRD experimental study of charoite: Crystal chemical features of two associated generations. In Minerals: Structure, Properties, Methods of Investigation. Springer Proceedings in Earth and Environmental Sciences; Votyakov, S., Kiseleva, D., Grokhovsky, V., Shchapova, Y., Eds.; Springer: Cham, Switzerland, 2020; pp. 97–104. [Google Scholar] [CrossRef]
- Liebau, F. Structural Chemistry of Silicates: Structure, Bonding, and Classification; Springer: New York, NY, USA, 2012. [Google Scholar]
- Lacalamita, M.; Mesto, E.; Kaneva, E.; Scordari, F.; Pedrazzi, G.; Vladykin, N.; Schingaro, E. Structure refinement and crystal chemistry of tokkoite and tinaksite from the Murun massif (Russia). Miner. Mag. 2017, 81, 251–272. [Google Scholar] [CrossRef]
- Uvarova, Y.A.; Sokolova, E.; Hawthorne, F.C.; Agakhanov, A.A.; Pautov, L.A.; Karpenko, V.Y. The crystalchemistry of senkevichite, CsKNaCa2TiO[Si7O18(OH)], from the Dara-i-Pioz alkaline massif, northern Tajikistan. Can. Miner. 2006, 44, 1341–1348. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Kaneva, E.V. Crystal Structure and Crystal Chemical Studies of Minerals of Alkaline Rocks from Russia, Tajikistan and Mongolia. Ph.D. Thesis, Universitá degli Studi di Bari “Aldo Moro”, Bari, Italy, 2014. [Google Scholar]
- Bruker AXS. Bruker AXS EVAluation of Powder Diffraction Data, Version 14.0.0.0; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- ICDD. The Powder Diffraction File; International Center for Diffraction Data: Newton Square, PA, USA, 2007. [Google Scholar]
- Faber, J.; Fawcett, T. The Powder Diffraction File: Present and future. Acta Crystallogr. 2002, B58, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Cryst. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Kaneva, E.; Bogdanov, A.; Shendrik, R. Structural and vibrational properties of agrellite. Sci. Rep. 2020, 10, 15569. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Tauson, V.L.; Lipko, S.V.; Shendrik, R.Y.; Levitskii, V.I.; Suvorova, L.F.; Chukanov, N.V.; Vigasina, M.F. On the crystal chemistry of sulfur-rich lazurite, ideally Na7Ca(Al6Si6O24)(SO4)(S3)−·nH2O. Amer. Miner. 2021, 106, 226–234. [Google Scholar] [CrossRef]
- Petrunina, A.A.; Ilyukhin, V.V.; Belov, N.V. Crystal structure of tinaksite NaK2Ca2TiSi7O19(OH). Sov. Phys. Dokl. 1971, 16, 338–340. [Google Scholar]
- Bissert, G. Verfeinerung der struktur von tinaksit, Ca2K2NaTiO[Si7O18(OH)]. Acta Crystallogr. 1980, B36, 259–263. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Lazebnik, Y.D.; Lazebnik, K.A. The crystal structure of tokkoite and its relation to the structure of tinaksite. Z. Kristallogr. 1989, 189, 195–204. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Lazebnik, K.A. Features of the crystal structure of minerals of the tinaksite group. Miner. Zhurnal 1991, 13, 3–12. [Google Scholar]
- Huang, E.; Chen, C.H.; Huang, T.; Lin, E.H.; Xu, J. Raman spectroscopic characteristics of Mg-Fe-Ca pyroxenes. Amer. Miner. 2000, 85, 473–479. [Google Scholar] [CrossRef]
- Shendrik, R.; Kaneva, E.; Radomskaya, T.; Sharygin, I.; Marfin, A. Relationships between the structural, vibrational, and optical properties of microporous cancrinite. Crystals 2021, 11, 280. [Google Scholar] [CrossRef]
- Su, Y.; Balmer, M.L.; Bunker, B.C. Raman spectroscopic studies of silicotitanates. J. Phys. Chem. B 2000, 104, 8160–8169. [Google Scholar] [CrossRef]
- Bogdanov, A.; Kaneva, E.; Shendrik, R. New insights into the crystal chemistry of elpidite, Na2Zr[Si6O15]·3H2O and (Na1+yCax□1-x-y)∑=2Zr[Si6O15]·(3-x)H2O, and ab initio modeling of IR spectra. Materials 2021, 14, 2160. [Google Scholar] [CrossRef]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Dong, B.; Liu, Y.; Han, N.; Sun, H.; Xing, F.; Qin, D. Study on the microstructure of cement-based piezoelectric ceramic composites. Constr. Build. Mater. 2014, 72, 133–138. [Google Scholar] [CrossRef]
- Pekov, I.V.; Chukanov, N.V.; Tarassoff, P.; Yamnova, N.A.; Zadov, A.E. Gjerdingenite-Na and gjerdingeniteCa, two new minerals of the labuntsovite group. Can. Miner. 2007, 45, 529–539. [Google Scholar] [CrossRef]
- Almeida, R.M.; Marques, A.C. Characterization of sol–gel materials by infrared spectroscopy. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Cham, Switzerland, 2016; p. 1137. [Google Scholar] [CrossRef]
- Burns, R.G. Mineralogical Application of Crystal Field Theory; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar] [CrossRef] [Green Version]
- Kaneva, E.; Radomskaya, T.; Shendrik, R.; Chubarov, V.; Danilovsky, V. Potassic-hastingsite from the Kedrovy district (East Siberia, Russia): Petrographic description, crystal chemistry, spectroscopy, and thermal behavior. Minerals 2021, 11, 1049. [Google Scholar] [CrossRef]
- Zhou, O.; Dolgov, L.; Srivastava, A.M.; Zhou, L.; Wang, Z.; Shi, J.; Dramićanin, M.D.; Brik, M.G.; Wu, M. Mn2+ and Mn4+ red phosphors: Synthesis, luminescence and applications in WLEDs. A review. J. Mater. Chem. C 2018, 11, 2652–2671. [Google Scholar] [CrossRef]
- Yarovoy, P.N. Laser-Induced Luminescence Identification of Materials. 1996. Available online: https://luminescence.csiro.au/ (accessed on 17 February 2022).
- Rogers, E.G.; Dorenbos, P. Vacuum energy referred Ti3+/4+ donor/acceptor states in insulating and semiconducting inorganic compounds. J. Lumin. 2014, 153, 40–45. [Google Scholar] [CrossRef]
- Naik, R.; Prashantha, S.C.; Nagabhushana, H.; Girish, K.M. Electrochemical, photoluminescence and EPR studies of Fe3+ doped nano forsterite: Effect of doping on tetra and octahedral sites. J. Lumin. 2018, 197, 233–241. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
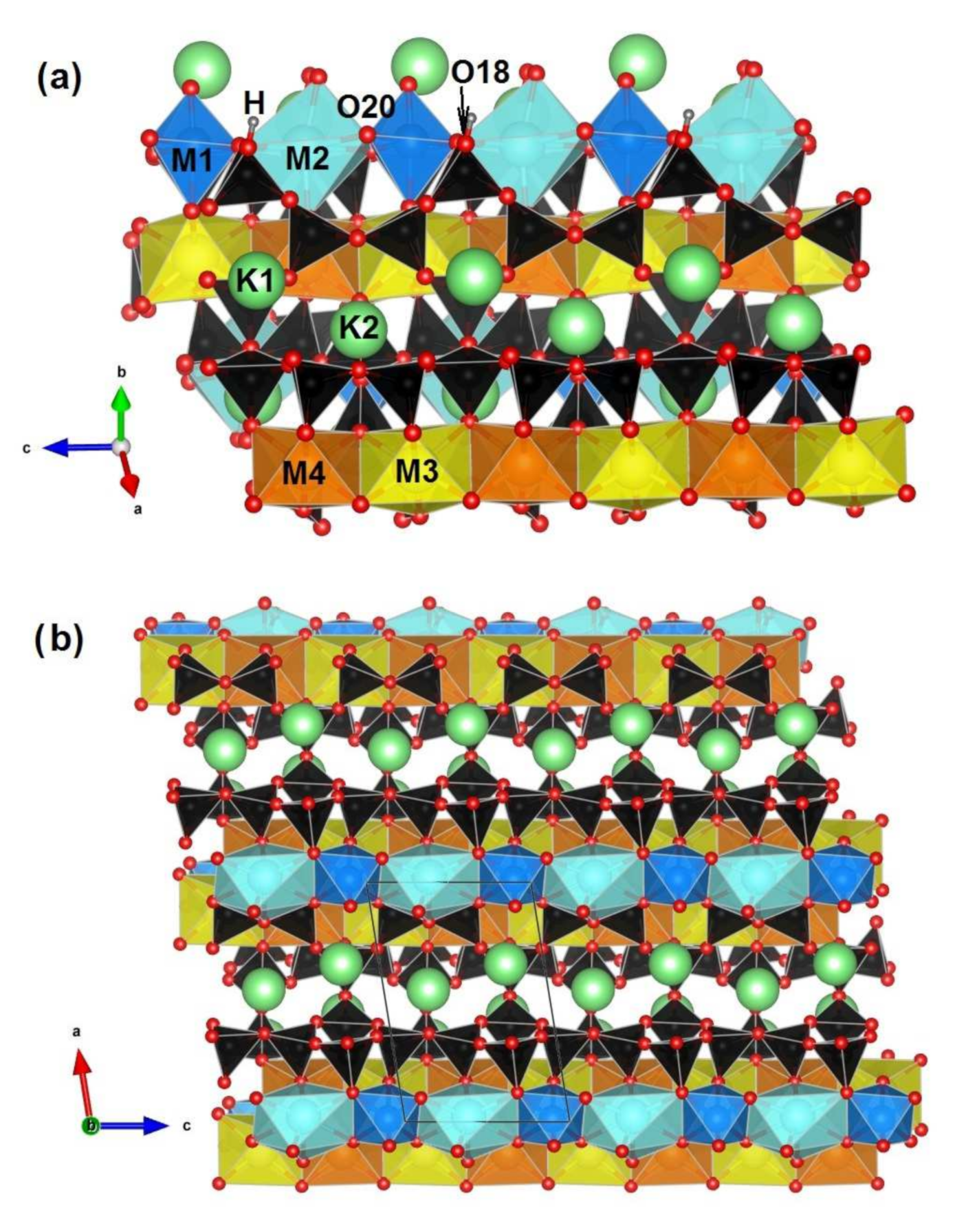



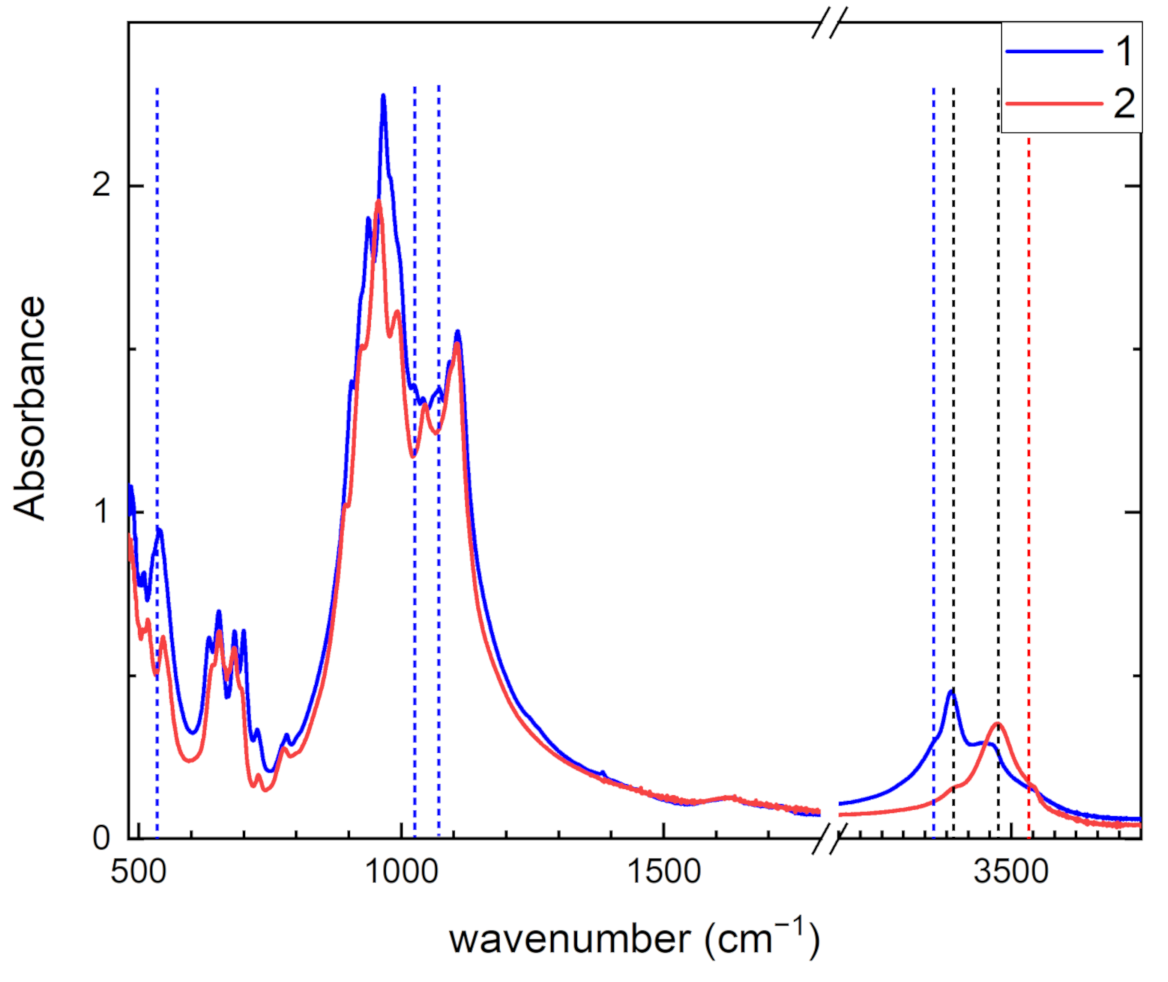
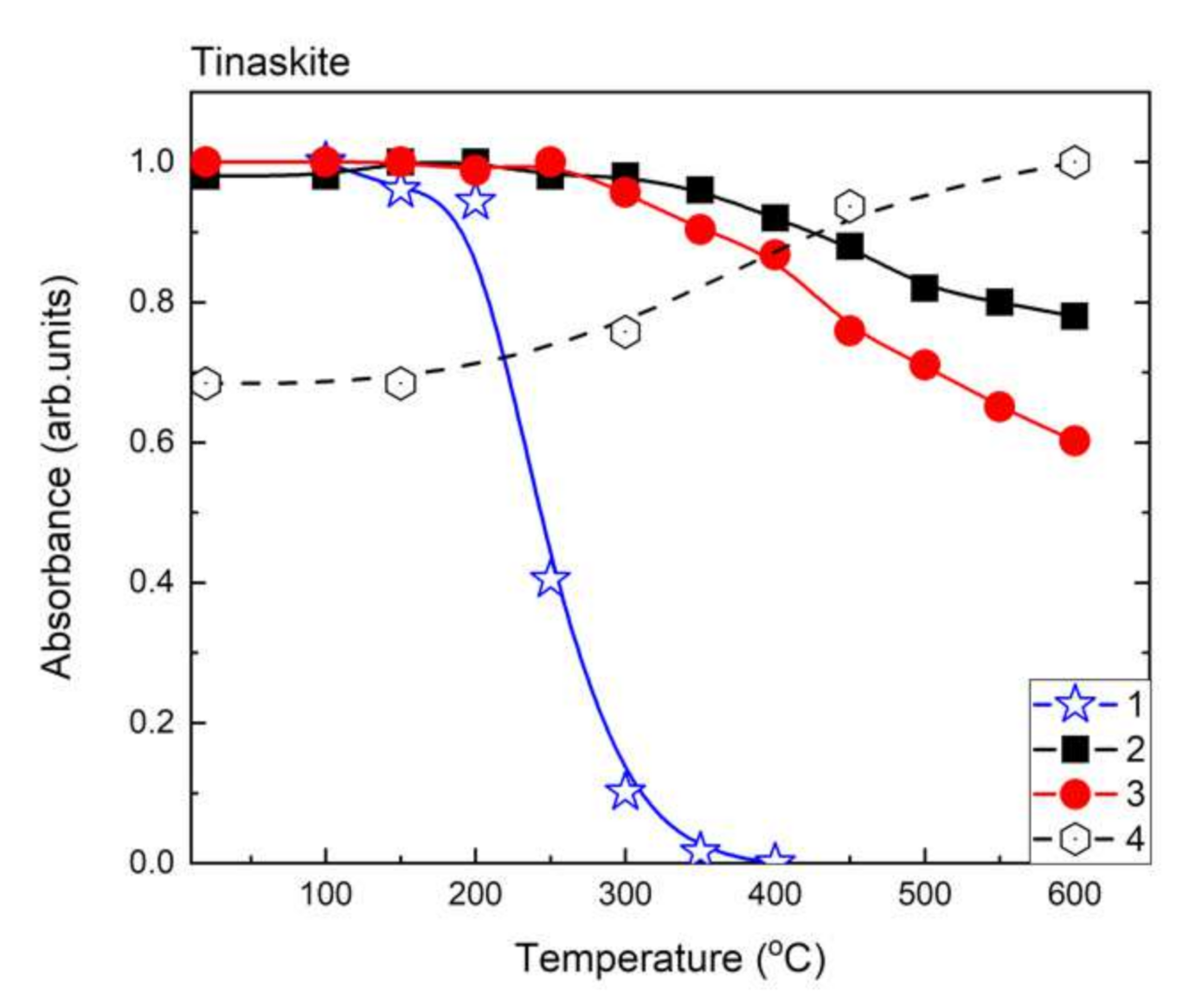
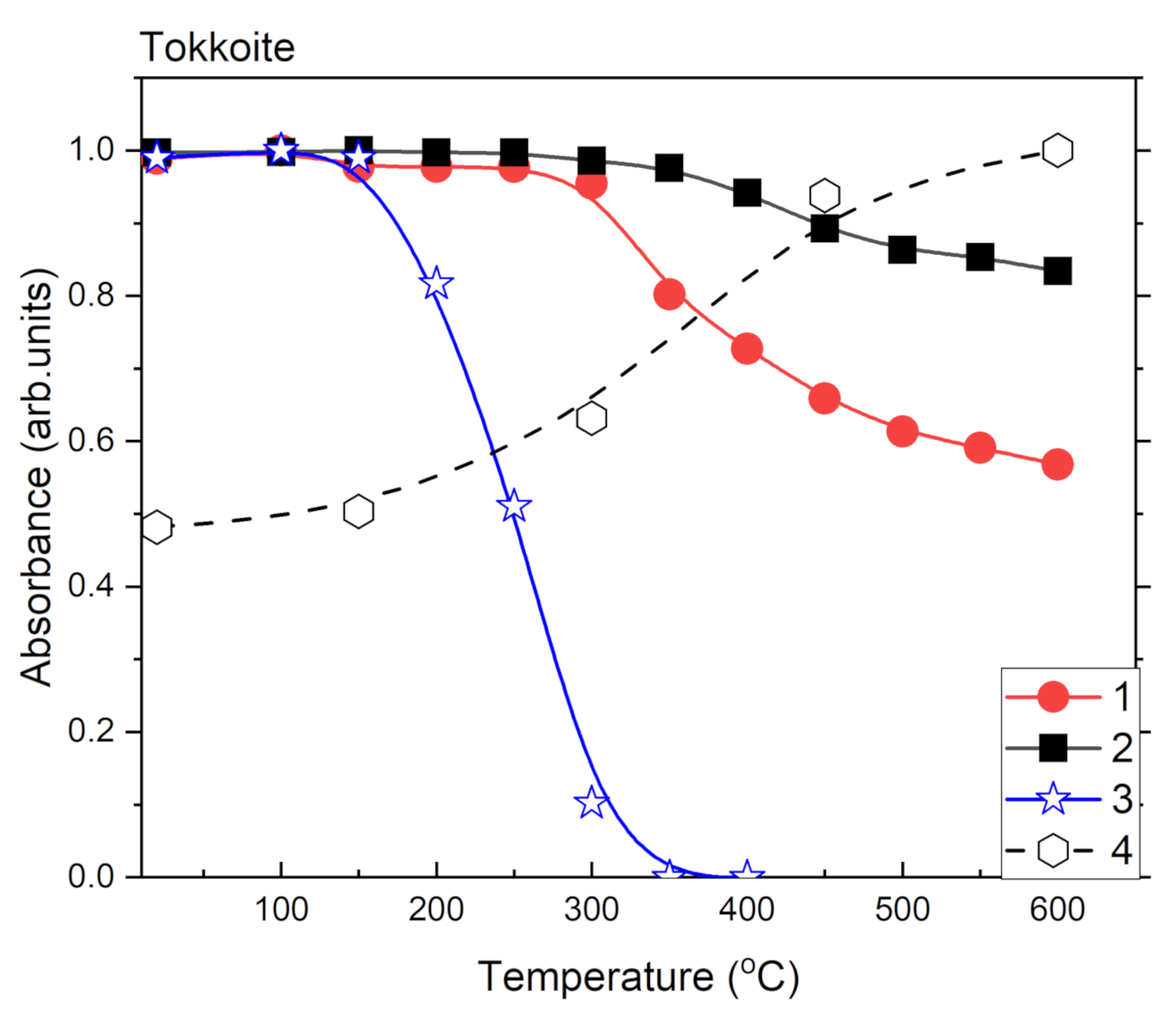
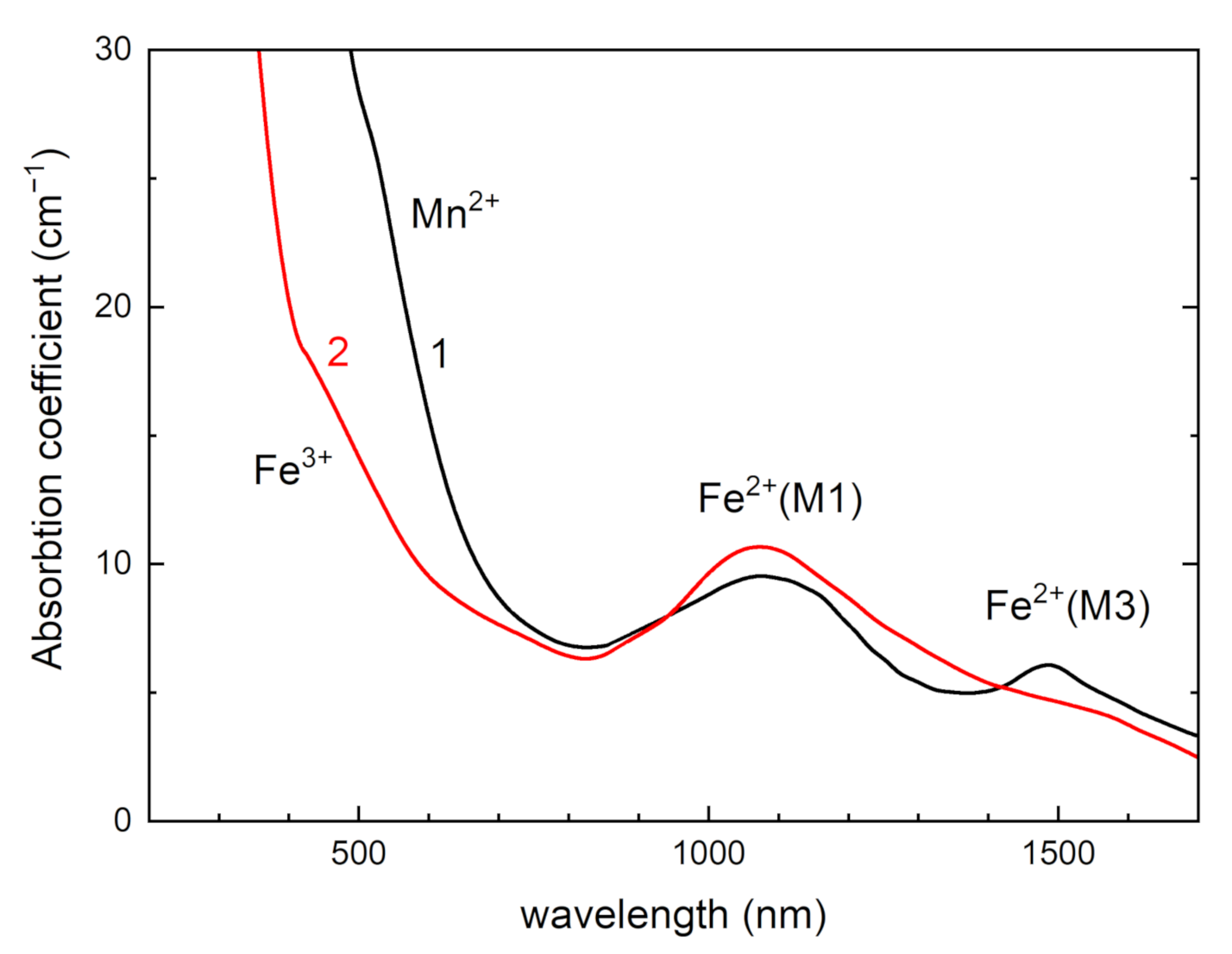
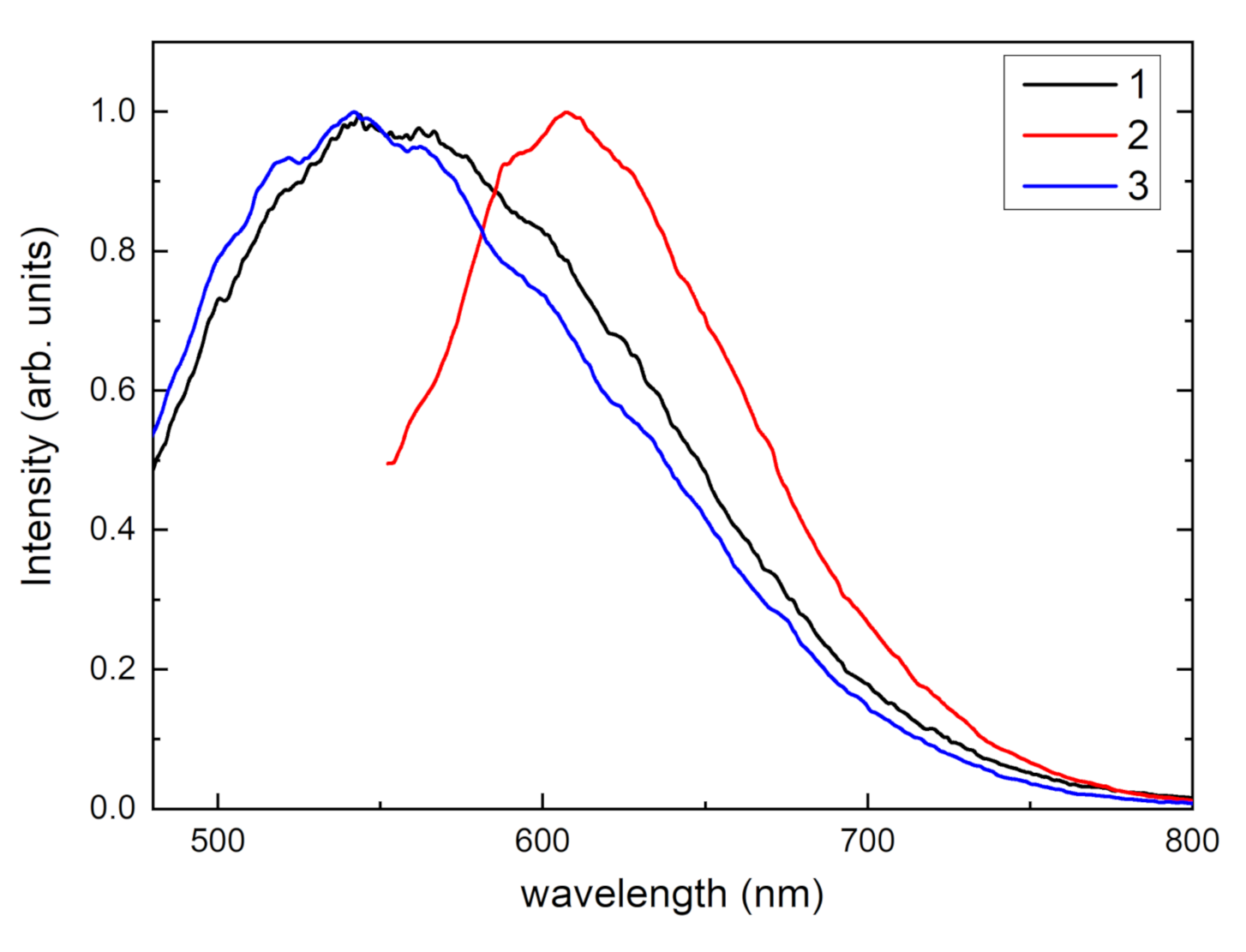
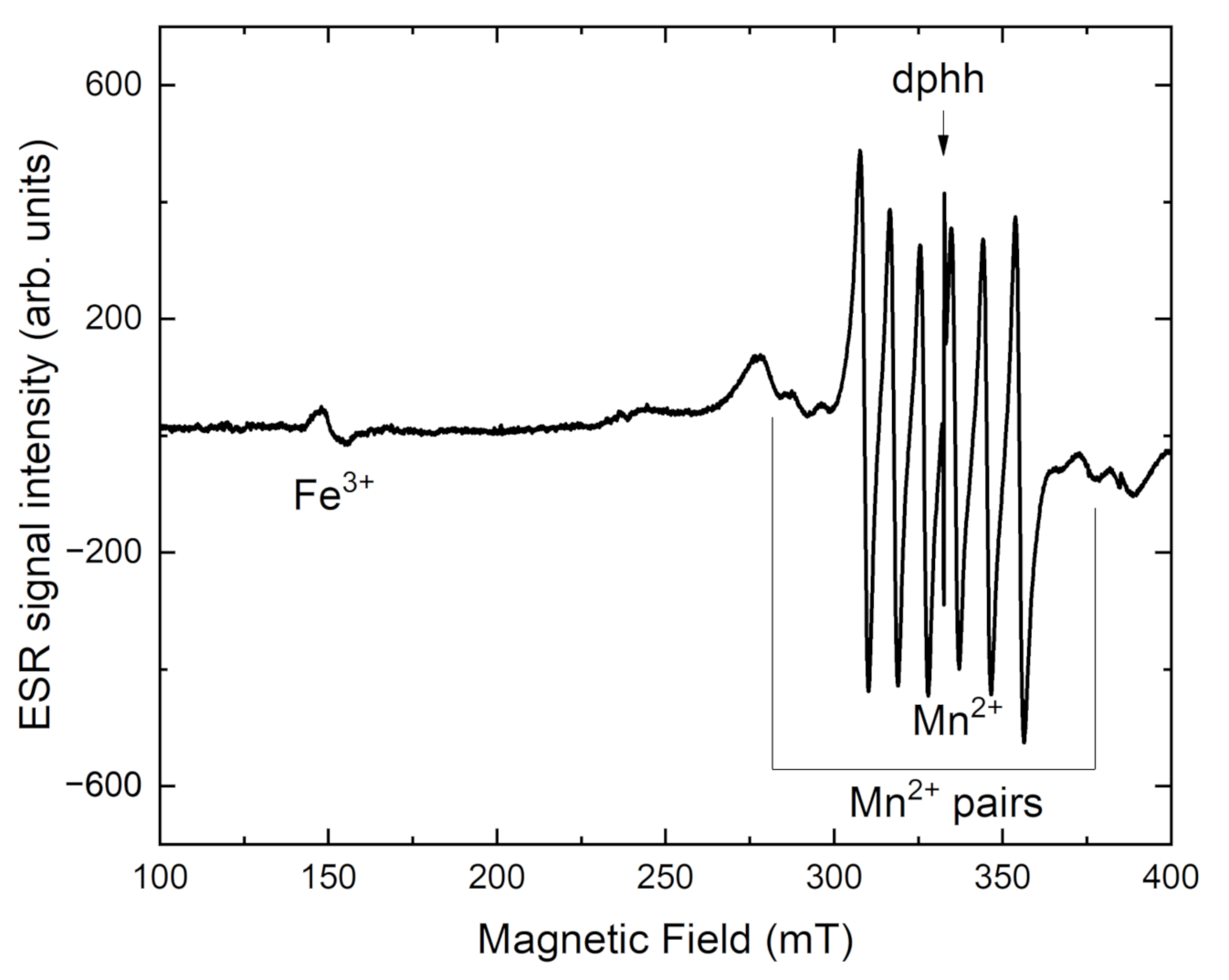

| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Sp. Gr. | Source |
|---|---|---|---|---|---|---|---|
| Tinaksite | |||||||
| 10.371 (6) | 12.167 (6) | 7.060 (5) | 90.91 (2) | 99.37 (3) | 92.79 (3) | This work | |
| 10.375 (2) | 12.190 (2) | 7.057 (1) | 90.75 (3) | 99.25 (3) | 92.81 (3) | [18] | |
| 10.370 (1) | 12.162 (1) | 7.057 (1) | 90.89 (1) | 99.20 (1) | 92.80 (1) | [18] | |
| 10.361 (2) | 12.153 (2) | 7.044 (1) | 90.79 (2) | 99.22 (2) | 92.83 (2) | [31] | |
| 10.350 | 12.170 | 7.050 | 91.00 | 99.33 | 92.50 | PDF 00-018-1382 | |
| 10.369 | 12.177 | 7.052 | 90.00 | 99.00 | 92.00 | PDF 00-054-0646 | |
| 10.350 | 12.170 | 7.050 | 91.00 | 99.33 | 92.50 | P1 | PDF 01-072-1823 |
| 10.377 | 12.166 | 7.059 | 90.91 | 99.30 | 92.76 | PDF 01-071-1758 | |
| Tokkoite | |||||||
| 10.436 (5) | 12.474 (7) | 7.085 (4) | 89.98 (3) | 99.50 (3) | 92.89 (3) | This work | |
| 10.423 (1) | 12.492 (1) | 7.116 (1) | 89.89 (1) | 99.69 (1) | 92.95 (1) | [18] | |
| 10.424 (1) | 12.477 (1) | 7.113 (1) | 89.88 (1) | 99.68 (1) | 92.99 (1) | [18] | |
| 10.423 (1) | 12.462 (1) | 7.106 (1) | 89.98 (1) | 99.68 (1) | 92.95 (1) | [18] | |
| 10.370 | 25.390 | 7.270 | 91.67 | 100.66 | 92.09 | PDF 00-040-0517 | |
| 10.438 | 12.511 | 7.112 | 89.92 | 99.75 | 92.89 | PDF 01-079-1981 | |
| Wavenumber (cm−1) | Assignment | |
|---|---|---|
| Tinaksite | Tokkoite | |
| 485 | Lattice modes involving Si–O–Si bending and Ca–O stretching | |
| 510 | 518 | |
| 527 | TiO6 stretching | |
| 538 | ||
| 546 | O–Si–O bending vibrations | |
| 634 | 641 | |
| 653 | ||
| 682 | ||
| 702 | TiO6 stretching | |
| 727 | Tetrahedral ring vibrations | |
| 777 | ||
| 893 | 905 | |
| 923 | ||
| 937 | Si–O–Ti stretching | |
| 956 | 966 | Stretching vibrations of apical Si–O bonds |
| 994 | ||
| 1025 | Si–O–Ti stretching | |
| 1044 | Stretching vibrations of the Si–O–Si framework | |
| 1067 | Si–O–Ti stretching | |
| 1077 | ||
| 1097 | Stretching vibrations of the Si–O–Si framework | |
| 1108 | ||
| 3320 w | Adsorbed H2O | |
| 3366 s | 3366 w | OH− |
| 3470 m | 3470 s | OH− |
| 3540 w | Adsorbed H2O | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneva, E.; Shendrik, R. Tinaksite and Tokkoite: X-ray Powder Diffraction, Optical, and Vibrational Properties. Crystals 2022, 12, 377. https://doi.org/10.3390/cryst12030377
Kaneva E, Shendrik R. Tinaksite and Tokkoite: X-ray Powder Diffraction, Optical, and Vibrational Properties. Crystals. 2022; 12(3):377. https://doi.org/10.3390/cryst12030377
Chicago/Turabian StyleKaneva, Ekaterina, and Roman Shendrik. 2022. "Tinaksite and Tokkoite: X-ray Powder Diffraction, Optical, and Vibrational Properties" Crystals 12, no. 3: 377. https://doi.org/10.3390/cryst12030377






