Intermolecular Interactions of 3,5-bis(4-Methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in a Cocrystal with 1,3-bis(4-Methoxyphenyl)prop-2-en-1-one and Dimethylformamide Solvate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Crystallization
2.2. Crystal Structure Determination
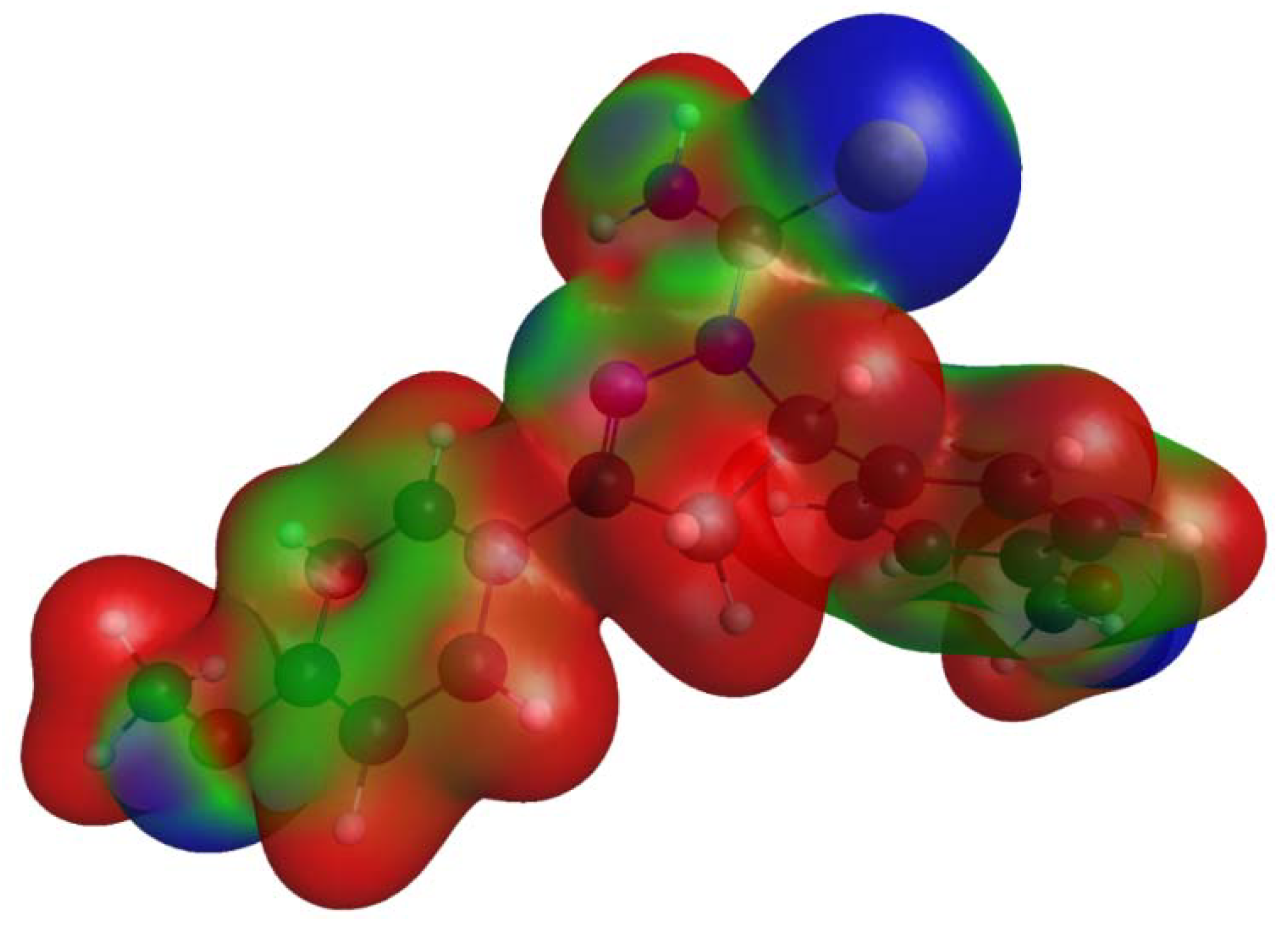
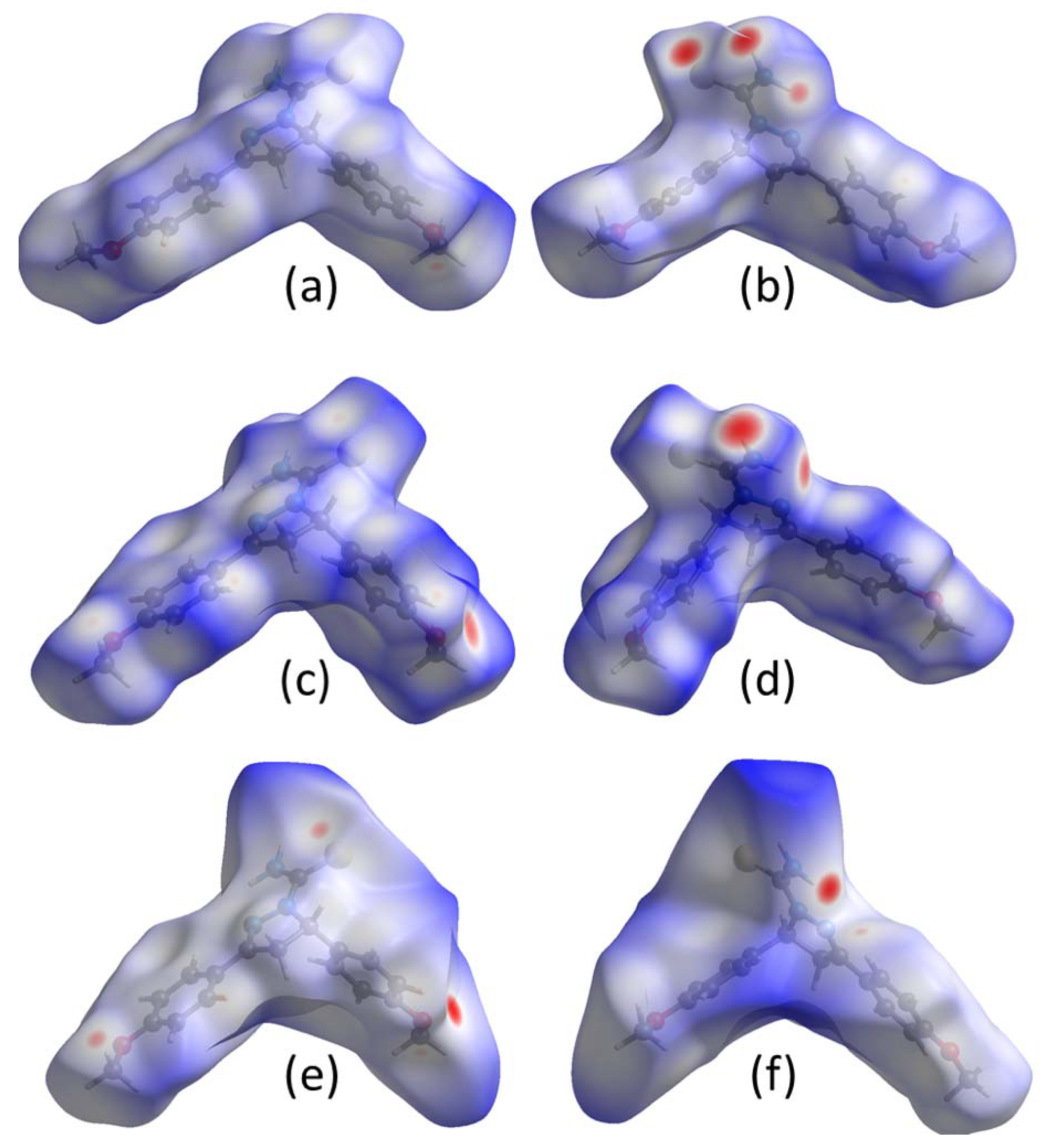
2.3. Electrostatic Potentials and Hirshfeld Surface Calculations
3. Results and Discussion
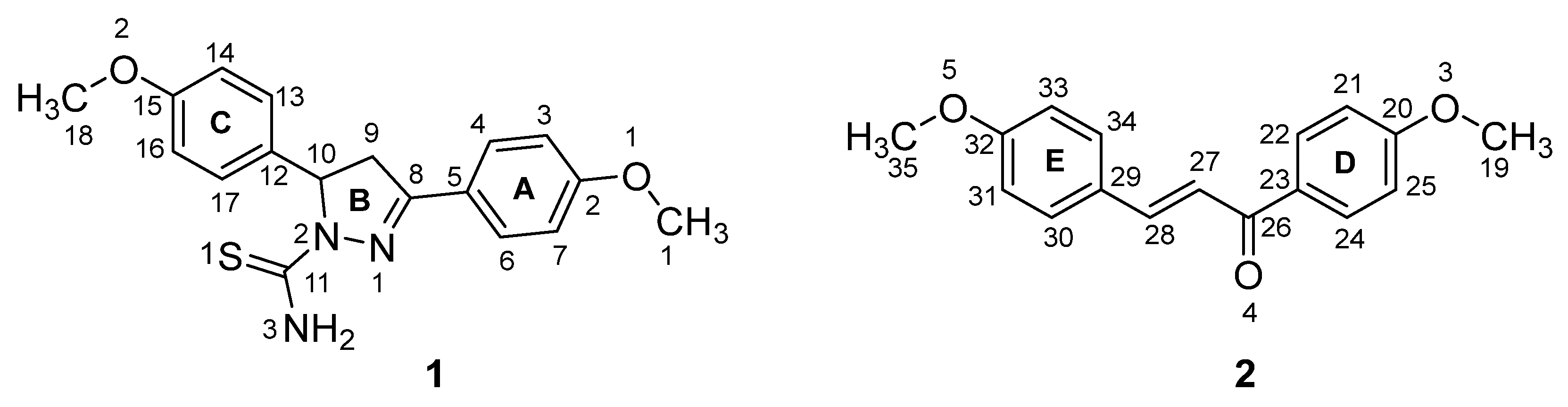
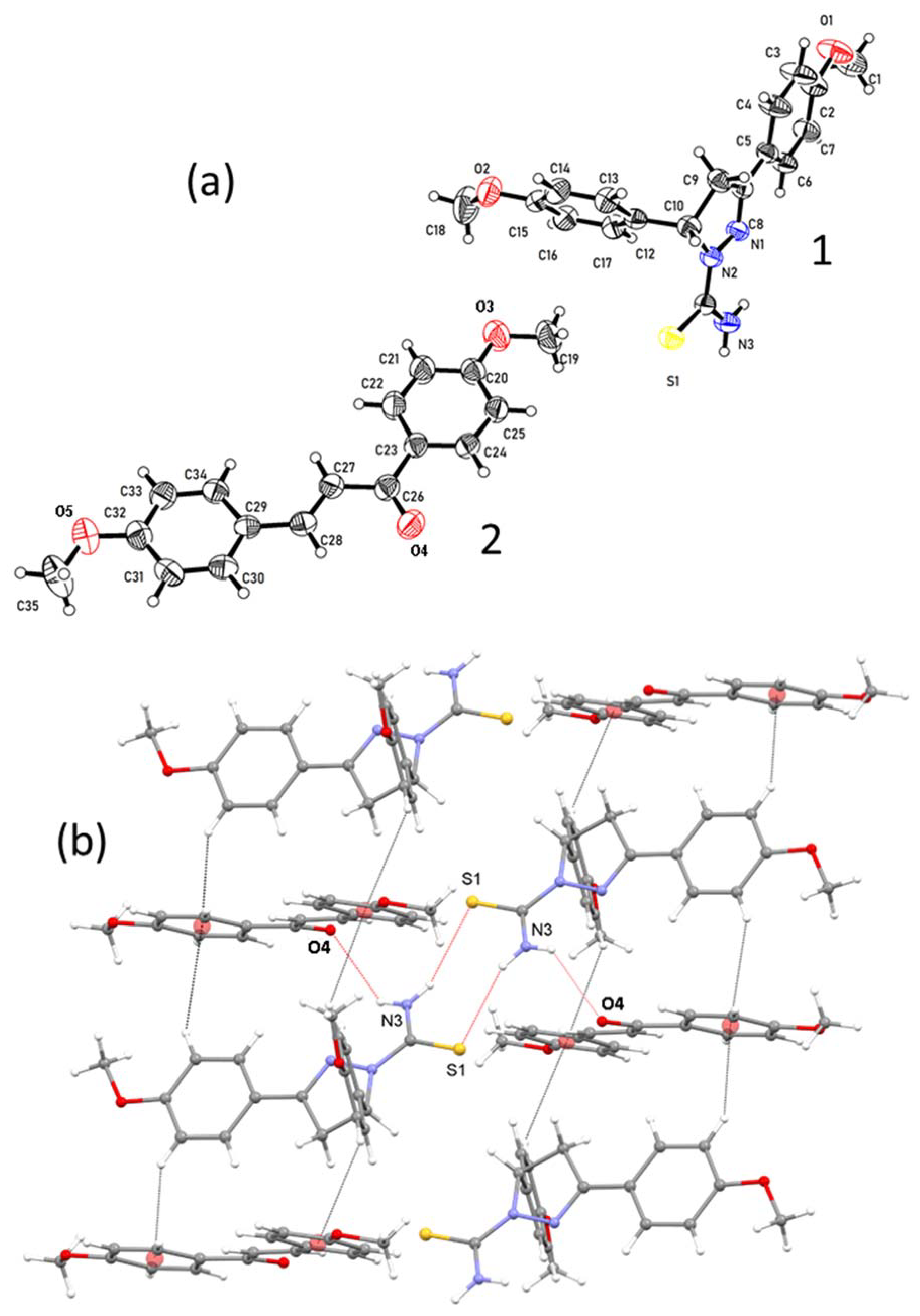
3.1. Crystal Structure of Cocrystal 1–2
3.2. Crystal Structure of Solvate 1–DMF
3.3. Comparison of Intermolecular Contacts for Molecule 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef] [PubMed]
- Braga, D. Crystal engineering, Where from? Where to? Chem. Commun. 2003, 22, 2751–2754. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J. Polymorphism in Molecular Crystals, 2nd ed.; Oxford University Press: New York, NY, USA, 2002; p. 352. [Google Scholar]
- Gavezzotti, A. A solid-state chemist’s view of the crystal polymorphism of organic compounds. J. Pharm. Sci. 2007, 96, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Prohens, R. Solid form and solubility. CrystEngComm 2017, 19, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Lehn, J.M.; Mascal, M.; DeCian, A.; Fischer, J. Molecular recognition directed self-assembly of ordered supramolecular strands by cocrystallization of complementary molecular components. J. Chem. Soc. Chem. Commun. 1990, 6, 479–481. [Google Scholar] [CrossRef]
- Stahly, G.P. A survey of cocrystals reported prior to 2000. Cryst. Growth Des. 2009, 9, 4212–4229. [Google Scholar] [CrossRef]
- Cincic, D.; Friscic, T.; Jones, W. A cocrystallisation-based strategy to construct isostructural solids. New J. Chem. 2008, 32, 1776–1781. [Google Scholar] [CrossRef]
- Bond, A.D. What is a co-crystal? CrystEngComm 2007, 9, 833–834. [Google Scholar] [CrossRef]
- Trask, A.V.; Jones, W. Crystal Engineering of Organic Cocrystals by the Solid-State Grinding Approach. In Organic Solid State Reactions. Topics in Current Chemistry; Toda, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 254, pp. 41–70. [Google Scholar]
- Duggirala, N.K.; Perry, M.L.; Almarsson, O.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Brittain, H.G. Pharmaceutical cocrystals: The coming wave of new drug substances. J. Pharm. Sci. 2013, 102, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R.; Saini, A.; Arora, P.; Bhandari, S. Pharmaceutical cocrystals: A novel approach for oral bioavailability enhancement of drugs. Crit. Rev. Ther. Drug Carrier Syst. 2012, 29, 183–218. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.R.; Gangavaram, S.; Suresh, K.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Novel furosemide cocrystals and selection of high solubility drug forms. J. Pharm. Sci. 2012, 101, 664–680. [Google Scholar] [CrossRef]
- Brittain, H.G. Cocrystal systems of pharmaceutical interest: 2009. Profiles Drug Subst. Excip. Relat. Methodol. 2011, 36, 361–381. [Google Scholar] [CrossRef]
- Kratochvil, B. Cocrystals and their expected pharmaceutical applications. Chem. Listy 2010, 104, 823–830. [Google Scholar]
- Peterson, M.; Bourghol Hickey, M.; Oliveira, M.; Almarsson, O.; Remenar, J. Mixed Co-crystals and Pharmaceutical Compositions. U.S. Patent No. 7,671,093, 02 March 2010. [Google Scholar]
- Kanmazalp, S.D.; Dege, N.; Ilhan, I.O.; Akin, N. Crystal structure and Hirshfeld surface analysis of 3,5-bis(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide. J. Struct. Chem. 2020, 61, 126–132. [Google Scholar] [CrossRef]
- Gökce, H.; Şen, F.; Sert, Y.; Abdel-Wahab, B.F.; Kariuki, B.M.; El-Hiti, G.A. Quantum computational investigation of (E)-1-(4-methoxyphenyl)-5-methyl-N′-(3-phenoxybenzylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molecules 2022, 27, 2193. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; El-Hiti, G.A. Synthesis and structural characterization of isostructural 4-(4-aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles. Crystals 2021, 11, 795. [Google Scholar] [CrossRef]
- Balakit, A.A.; Makki, S.Q.; Sert, Y.; Ucun, F.; Alshammari, M.B.; Thordarson, P.; El-Hiti, G.A. Synthesis, spectrophotometric and DFT studies of new triazole Schiff bases as selective naked-eye sensors for acetate anion. Supramol. Chem. 2020, 32, 519–526. [Google Scholar] [CrossRef]
- Kariuki, B.M.; El-Hiti, G.A. A reversible single-crystal to single-crystal thermal phase transformation of 3-(2-bromo-4-(1-methylethyl)phenyl)-1,1-dimethylurea. Crystals 2017, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, B.F.; Farahat, A.A.; Awad, G.E.A.; El-Hiti, G.A. Synthesis and antimicrobial activity of some novel substituted 3-(thiophen-2-yl)pyrazole-based heterocycles. Lett. Drug Des. Discov. 2017, 14, 1316–1323. [Google Scholar] [CrossRef]
- Baashen, M.A.; Abdel-Wahab, B.F.; El-Hiti, G.A. A simple procedure for the synthesis of novel 3-(benzofur-2-yl)pyrazole-based heterocycles. Chem. Pap. 2017, 71, 2159–2166. [Google Scholar] [CrossRef]
- Ravindra, H.J.; Harrison, W.T.A.; Suresh Kumar, M.R.; Dharmaprakash, S.M. Synthesis, crystal growth, characterization and structure–NLO property relationship in 1,3-bis(4-methoxyphenyl)prop-2-en-1-one single crystal. J. Cryst. Growth 2009, 311, 310–315. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E.; et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef] [Green Version]
- Bode, B.M.; Gordon, M.S. Macmolplt: A graphical user interface for GAMESS. J. Mol. Graph. Model. 1998, 16, 133–138. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystal. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Hurst, D.P.; Titterington, J.A.; Van Wier, S.P.; Adrian, C.; Whitwood; Wood, N.J. Experimental Crystal Structure Determination CCDC 184226. Available online: https://doi.org/10.5517/ccdc.csd.cc1ztznf (accessed on 1 April 2022).
- Shen, P.; Zheng, J.; Zhang, D.; Che, Y. A new organic nonlinear optical crystal-4,4′-dimethoxychalcone. Rengong Jingti Xuebao 1992, 21, 280–285. [Google Scholar]
- Shu, Y.; Ye, K.; Yue, Y.; Sun, J.; Wang, H.; Zhong, J.; Yang, X.; Gao, H.; Lu, R. Fluorine as a robust balancer for tuning the reactivity of topo-photoreactions of chalcones and the photomechanical effects of molecular crystals. CrystEngComm 2021, 23, 5856–5868. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]

| 1–2 | 1–DMF | |
|---|---|---|
| Molecular formula | C18H19N3O2S, C17H16O3 | C18H19N3O2S, C3H7NO |
| T (K) | 293(2) | 293(2) |
| λ (Å) | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic |
| Space group | PĪ | PĪ |
| a (Å) | 9.6162(6) | 9.3300(10) |
| b (Å) | 13.2978(11) | 9.4551(11) |
| c (Å) | 14.2415(11) | 13.6082(13) |
| α (°) | 70.629(7) | 91.915(9) |
| β (°) | 74.519(6) | 94.146(9) |
| γ (°) | 72.199(7) | 115.268(11) |
| V (Å3) | 1607.8(2) | 1080.0(2) |
| Z | 2 | 2 |
| Calculated density (Mg m−3) | 1.259 | 1.275 |
| Absorption coefficient (mm−1) | 0.146 | 0.179 |
| F(000) | 644 | 440 |
| Crystal size (mm3) | 0.389 × 0.105 × 0.079 | 0.431 × 0.179 × 0.155 |
| Reflections collected | 15,540 | 9197 |
| Independent reflections | 7698 | 5143 |
| R(int) | 0.0451 | 0.0206 |
| Goodness-of-fit on F2 | 1.028 | 1.061 |
| R1 (I > 2σ(I)) | 0.0633 | 0.0517 |
| wR2 (I > 2σ(I)) | 0.1369 | 0.1189 |
| R1 (all data) | 0.1434 | 0.0785 |
| wR2 (all data) | 0.1817 | 0.1392 |
| Max/Min residual densities (e Å−3) | 0.17/–0.25 | 0.19/–0.24 |
| Molecule 1 | 1–2 | 1–DMF | lit. 1 [19] |
| Ring twist angle (°) | |||
| A/B | 3.38 (21) | 4.73(14) | 6.48 |
| B/C | 77.59 (11) | 83.30(6) | 84.41 |
| Max deviation from ring B plane/Å | 0.278(4) | 0.373(3) | 0.357 |
| Torsion angle (°) | |||
| C1–O1–C2–C7 | 0.88 (50) | 179.85(19) | 179.48 |
| C16–C15–O2–C18 | 7.69 (46) | 4.06(33) | 0.70 |
| C17–C12–C10–N2 | 17.47 (35) | 15.13(24) | 26.80 |
| Molecule 2 | 1–2 | lit. 2 [33] | lit. 3 [35] |
| Ring twist angle (°) | |||
| D/E | 5.14(3) | 4.29 | 57.58 |
| Torsion angle (°) | |||
| C19–O3–C20–C25 | 2.66 (46) | 170.65 | 178.66 |
| C35–O5–C32–C31 | 2.50 (47) | 170.57 | 174.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariuki, B.M.; Abdel-Wahab, B.F.; Bekheit, M.S.; El-Hiti, G.A. Intermolecular Interactions of 3,5-bis(4-Methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in a Cocrystal with 1,3-bis(4-Methoxyphenyl)prop-2-en-1-one and Dimethylformamide Solvate. Crystals 2022, 12, 663. https://doi.org/10.3390/cryst12050663
Kariuki BM, Abdel-Wahab BF, Bekheit MS, El-Hiti GA. Intermolecular Interactions of 3,5-bis(4-Methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in a Cocrystal with 1,3-bis(4-Methoxyphenyl)prop-2-en-1-one and Dimethylformamide Solvate. Crystals. 2022; 12(5):663. https://doi.org/10.3390/cryst12050663
Chicago/Turabian StyleKariuki, Benson M., Bakr F. Abdel-Wahab, Mohamed S. Bekheit, and Gamal A. El-Hiti. 2022. "Intermolecular Interactions of 3,5-bis(4-Methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in a Cocrystal with 1,3-bis(4-Methoxyphenyl)prop-2-en-1-one and Dimethylformamide Solvate" Crystals 12, no. 5: 663. https://doi.org/10.3390/cryst12050663







