Abstract
In this study, we investigate the effects of chlorine doping on the structural, electronic, and thermoelectric properties of Bi2O2Se by employing density functional theory combined with semiclassical Boltzmann transport theory. It is shown that chlorine doping has significant effects on the electronic structure and thermoelectric properties of Bi2O2Se. As chlorine is incorporated into the selenium sites in Bi2O2Se, additional electrons are acquired, thereby inducing metallic properties in chlorine-doped Bi2O2Se. Meanwhile, Cl doping leads to an increase in the electrical conductivity of Bi2O2Se at room temperature by 25 times (from 358.59 to 9390 ), and the power factor is enhanced by a factor of 2.12 (from 4.04 mW/mK2 to 12.59 mW/mK2). This study demonstrates that chlorine doping is an effective method to modify the physical properties of Bi2O2Se.
1. Introduction
In recent years, thermoelectric (TE) materials have received extensive attention because of their environmentally friendly and sustainable characteristics. Thermoelectric materials can directly convert heat into electricity, thus reducing carbon dioxide and greenhouse gas emissions [1,2,3,4,5,6]. The energy conversion efficiency of thermoelectric materials can be evaluated using the ZT () value [7,8], where , S, T, and are the electrical conductivity, Seebeck coefficient, absolute temperature, thermal conductivity, and power factor, respectively [9,10,11,12]. Excellent thermoelectric materials should have a high Seebeck coefficient, high electrical conductivity, and low thermal conductivity [5]. However, these parameters are strongly coupled; e.g., the electrical conductivity is proportional to the thermal conductivity, while the carrier concentration is inversely proportional to the Seebeck coefficient. Thus, it is difficult to further improve the ZT value of TE materials.
Bismuth oxyselenide (Bi2O2Se) is a potential thermoelectric material due to its low thermal conductivity; however, its ZT value (0.05~0.5) is lower than that of other thermoelectric materials (~1) [13,14,15,16,17,18]. In the past decades, many techniques have been employed to improve the ZT value of Bi2O2Se. Experimental studies performed by Zhan et al. have shown that Bi defects significantly influence the thermoelectric performance of Bi2O2Se. Compared to the ZT value of 0.05 for pure Bi2O2Se, Bi1.9O2Se exhibits a 130% improvement in ZT value, reaching 0.12 at 773 K [19]. Guo et al. investigated the effect of Nb doping on the ZT value of Bi2O2Se using ball milling and hot-pressing sintering. They found that Nb-doped Bi2O2Se has a ZT value of 0.195 at 823 K, which is 325% higher than the value of 0.045 for pure Bi2O2Se [20]. Hong et al. studied the influence of Ce4+ doping on the thermoelectric properties of Bi2O2Se employing spark plasma sintering and found that Ce4+-doped Bi2O2Se has a ZT value of 0.26 at 773 K, which is 1.27 times higher than the value of 0.11 for pure Bi2O2Se [21]. Song et al. studied the influence of Ti doping on the thermoelectric properties of Bi2O2Se and found that the ZT value of Ti-doped Bi2O2Se is 0.56 at 773 K, which is 20% higher than the ZT value of 0.47 for the original Bi2O2Se [22]. Chen et al. investigated the effect of Sb doping on the thermoelectric properties of Bi2O2Se and found that the ZT value of Sb-doped Bi2O2Se reaches 0.59 at 773 K, which is 80% higher than that of 0.33 for the original Bi2O2Se [23]. Pan et al. synthesized polycrystalline Bi2O2−xSxSe (x = 0, 0.01, 0.02, and 0.03) via a high-temperature solid-state reaction and demonstrated that the substitution of a small amount of S for O can effectively improve the thermoelectric performance of Bi2O2Se. The doped Bi2O2Se exhibits a ZT value of 0.29, which is 3.2 times higher than that of 0.09 for pristine Bi2O2Se at 793 K [24]. Fu et al. synthesized Bi2−xZrxO2Se (x = 0, 0.02, 0.04, 0.06) via a combination of high-energy ball milling and cold pressing. It was found that Zr doping has a significant impact on the thermoelectric performance of Bi2O2Se. The peak ZT value of the Bi1.96Zr0.04O2Se sample is approximately 0.27, which is 2.4 times higher than that of the undoped sample (~0.11) [25]. Song et al. obtained Bi2O2Se with Bi2Te2.7Se0.3 through a liquid-assisted shear exfoliation-reassembly (LASE-R) process and found a significant improvement in its thermoelectric performance. At 772 K, the addition of 0.3 mol% Bi2Te2.7Se0.3 results in a ZT value of 0.7, several times higher than the pristine sample (~0.1) [26]. Despite the above studies, the ZT value of Bi2O2Se remains low and requires further improvement.
In recent years, chlorine (Cl) has been widely used to improve the thermoelectric properties of materials [27,28,29]. Tan et al. experimentally studied the effect of Cl doping on the thermoelectric performance of AgBi3S5. They found that the ZT value of Cl-doped AgBi3S5 reaches 1 at 800 K, which is five times that of the original AgBi3S5 (0.2) [28]. Zhang et al. systematically studied the effect of Cl doping on the thermoelectric properties of AgPb18SbSe20 using experimental methods. It was shown that the ZT value of Cl-doped AgPb18SbSe20 is 1.3 at 873 K, which is 766% higher than that of the original AgPb18SbSe20 (0.15) [29]. Furthermore, Wang et al. carried out experimental investigations of the effect of Cl doping on the thermoelectric properties of BiSbSe3 and reported that the ZT value of Cl-doped BiSbSe3 is 1.0 at 800 K, which is five times higher than that of the original BiSbSe3 (0.2) [27]. These investigations demonstrate that Cl doping is an effective method to improve the thermoelectric properties of materials.
In this work, the effects of Cl doping on the structure, electronic, and thermoelectric properties of Bi2O2Se are investigated by combining the DFT method and semi-classical Boltzmann transport theory. The relaxation time , electrical conductivity , Seebeck coefficient S, power factor (PF), thermal conductivity , and dimensionless figure of merit ZT are provided. The results show that Cl doping can introduce additional electrons, causing Bi2O2Se to exhibit metallic character, thus enhancing the electrical conductivity and power factor. Therefore, this study provides a theoretical basis for experimental observation and reveals the potential mechanism of Cl-doping-induced improvement in the thermoelectric properties of Bi2O2Se.
2. Computational Details
The density functional theory (DFT) method in the Vienna Ab initio Simulation Package (VASP) code (Vienna, Austria) [30] is used to perform geometrical optimization and electronic structure calculations. The projector augmented-wave approach (PAW) [31] pseudopotential is used to describe the interaction between electrons and ions. In addition, the exchange-correlation potentials among electrons are described by the Perdew–Burke–Ernzerhof (PBE) functional within the generalized gradient approximation (GGA) [32] and the Heyd–Scuseria–Ernzerhof (HSE06) hybrid functional [33]. In this work, the Monkhorst–Pack scheme [34] with a 6 × 6 × 6 k-point sampling for the Brillouin zone is used for structural optimization and electronic structure calculations. The cut-off energy of the plane waves is set as 500 eV. The electronic transport coefficients are calculated by semi-classical Boltzmann theory using the BOLTZTRAP code [35] and the Brillouin zone is sampled with an 8 × 8 × 8 k-point grid. The lattice thermal conductivity is calculated by using the Slack equation [36], and the energy and force convergence criteria are set to be 1 × 10−5 eV/atom and 1 × 10−2 eV/Å, respectively. For chemical element doping, a 2 × 2 × 1 supercell containing 40 atoms is used to build the structural model for Bi2O2Se0.875Cl0.125, with Cl dopants substituting for the Se atoms. It should be pointed out that the doping concentration in this work is 1.31 × 1021 e/cm3, which may exceed the saturation limit. The dissolution limit can be raised by tuning experimental conditions, such as the experimental temperature and powder size of the samples [37,38,39]. On the other hand, in the literature, Liu et al. have demonstrated that the carrier concentration of Ge-doped Bi2O2Se can reach 1021–1022 cm−3 by controlling experimental conditions [40]. Therefore, the doping concentration of 1.31 × 1021 e/cm3 can probably be achieved experimentally.
3. Results and Discussions
3.1. Structural Properties of Pristine and Cl-Doped Bi2O2Se
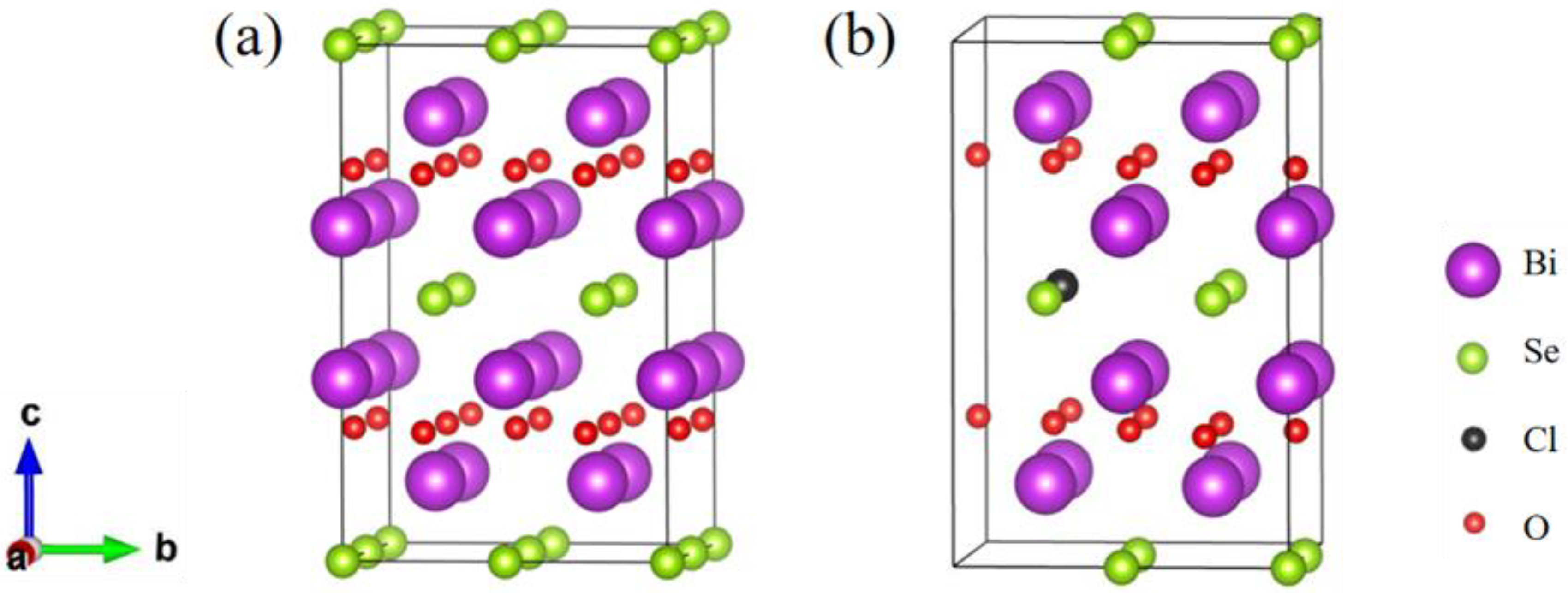
The bent [Bi2O2]2+ layers and [Se]2− layers are alternately stacked along the c-axis through weak electrostatic interactions to form Bi2O2Se [41], in which the [Bi2O2]2+ layer is an insulating layer while the [Se]2− layer is a conducting layer. Bulk Bi2O2Se has a tetragonal I4/mmm (No. 139) crystal structure, and its unit cell contains ten atoms. The optimized geometrical structures of Bi2O2Se and Bi2O2Se0.875Cl0.125 are shown in Figure 1, and both structures contain 40 atoms. Table 1 shows the lattice constants, volume, and bond lengths of Bi2O2Se and Bi2O2Se0.875Cl0.125 calculated by us and from other literature sources [42,43]. The lattice constants of Bi2O2Se are a0 = b0 = 3.917 Å and c0 = 12.357 Å, which are in good agreement with experimental results (a0 = b0 = 3.88 Å and c0 = 12.16 Å) [42] as well as calculated results (a0 = b0 = 3.90 Å and c0 = 12.39 Å) [43] reported in the literature. Additionally, the calculated <Bi-O> and <Bi-Se> bond lengths of Bi2O2Se are 2.337 Å and 3.331 Å, respectively, which agree well with the calculated values of 2.312 Å and 3.272 Å reported by Wu et al. [44]. Compared with Bi2O2Se, the calculated lattice constants a0 and b0 (3.946 Å) of Bi2O2Se0.875Cl0.125 increased by 0.74%, respectively, while the c0 (12.287 Å) decreased by 0.57%, resulting in a 0.76% increase in volume. The calculated <Bi-O> bond length (2.339 Å) of Bi2O2Se0.875Cl0.125 increased by 0.09%, and <Bi-Se> bond length (3.336 Å) increased by 0.76%. As Cl substitutes for Se in Bi2O2Se, despite the smaller ionic radii of Cl (1 Å) compared to Se (1.15 Å), the stronger electronegativity (3.16) of the Cl atom than Se (2.55) results in the attraction of electrons from its neighboring atoms. Consequently, the interactions between Bi atoms and O as well as Se atoms are weakened, as indicated by the increased bond lengths of <Bi-O> and <Bi-Se> by 0.09% and 0.76%, respectively (see Table 1). This probably eventually leads to the volume expansion of 0.9% caused by Cl doping in Bi2O2Se. Considering that Cl and Se atoms have 7 and 6 valence electrons, respectively, when Cl is used to substitute for Se, it is likely that one additional electron is introduced.
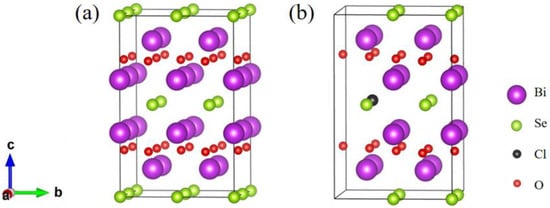
Figure 1.
The structure of (a) Bi2O2Se and (b) Bi2O2Se0.875Cl0.125. The purple, green, black, and red spheres denote Bi, Se, Cl, and O atoms, respectively.

Table 1.
Comparison of lattice constants a0 and c0 (Å), volume (Å3), and bond length (Å) of Bi2O2Se and Bi2O2Se0.875Cl0.125 with experimental and other theoretical results.
3.2. The Influence of Cl Doping on the Electronic Structure of Bi2O2Se
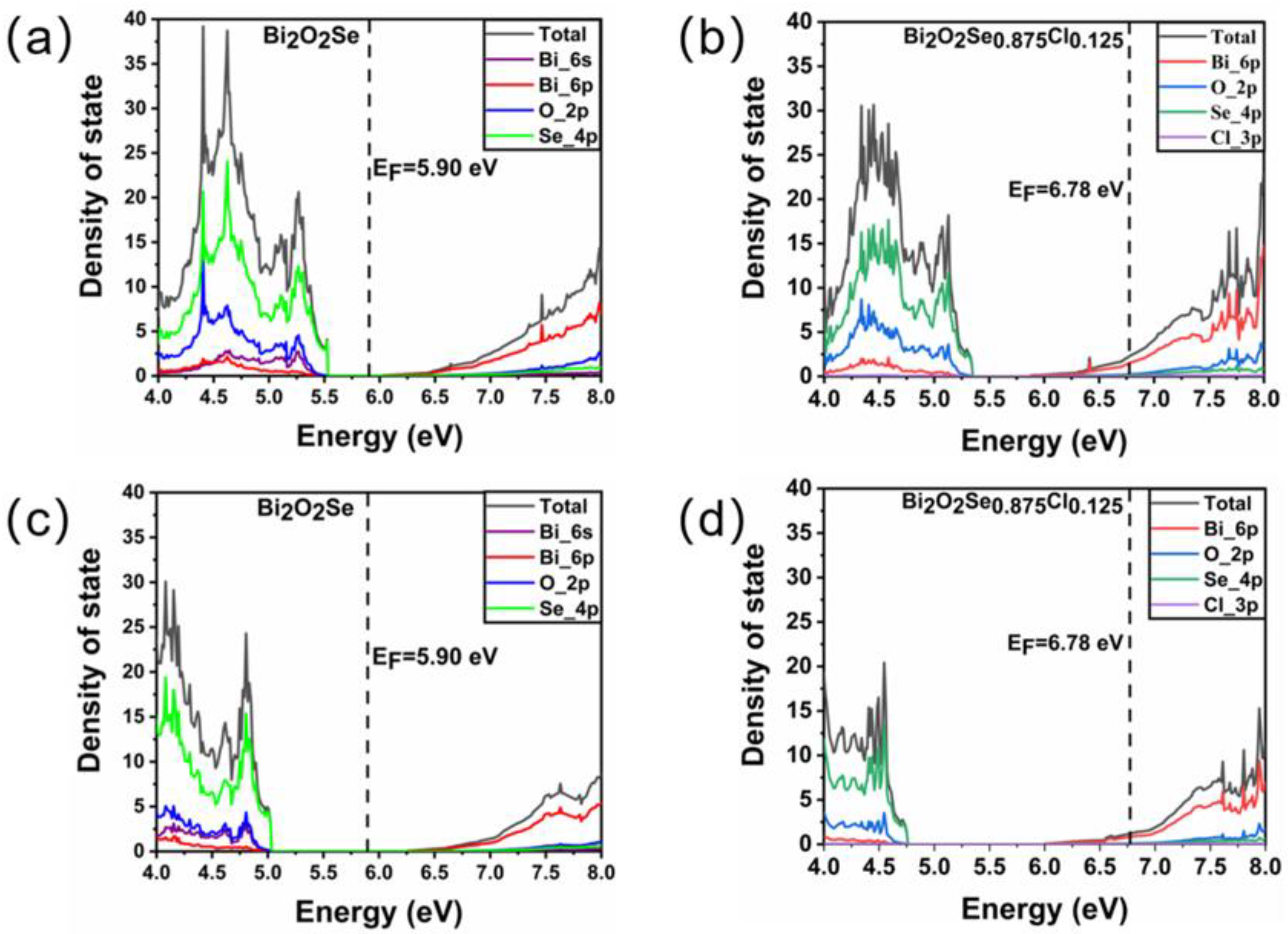
The density of state (DOS) distribution of Bi2O2Se before and after Cl doping is first studied using standard and hybrid DFT methods. Figure 2a,b show the total and projected density of state distribution of Bi2O2Se and Bi2O2Se0.875Cl0.125 around the Fermi level obtained by the standard DFT method. For Bi2O2Se, the valence bands from 4 to 5.53 eV are mainly composed of Se 4p orbitals hybridized with O 2p and Bi 6s orbitals, while the conduction bands from 6.02 to 8 eV are mainly composed of Bi 6p and O 2p orbitals. The bandgap between the maximum value of the valence band and the minimum value of the conduction band is 0.49 eV, which is similar to the theoretical values of 0.41 eV [45], 0.43 eV [46], and 0.472 eV [47]. Figure 2b shows the DOS distribution of Bi2O2Se0.875Cl0.125, revealing that many electrons are distributed at the Fermi level. These features indicate that Bi2O2Se0.875Cl0.125 has metallic properties that differ from the results reported by Tan et al. [48]. This is mainly due to the fact that our doping concentration of 2.5% is far greater than the solubility limit of 1.5% in the experimental literature.
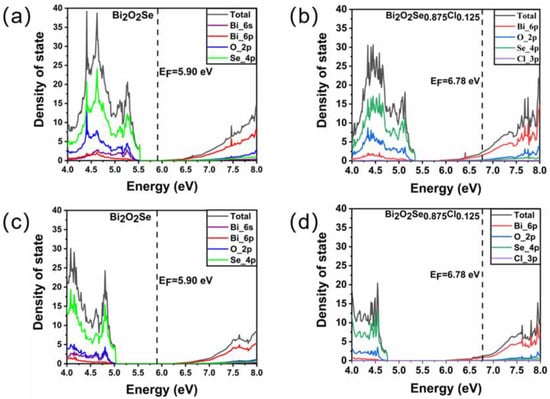
Figure 2.
The total and projected density of state distribution for Bi2O2Se and Bi2O2Se0.875Cl0.125 obtained by (a,b) standard DFT method and (c,d) hybrid DFT method. The EF denotes the Fermi energy.
As we know, the standard DFT method severely underestimates the bandgap of materials. In order to obtain a more accurate bandgap, we use the hybrid DFT method to further calculate the DOS distribution of Bi2O2Se before and after Cl doping. Figure 2c,d show the DOS distribution of Bi2O2Se before and after Cl doping, respectively. For Bi2O2Se, the calculated band gap between the maximum of the valence band and the minimum of the conduction band is 1.05 eV, which is similar to other calculated hybrid DFT results of 0.9 eV [49], 0.99 eV [50] and 1.01 eV [51]. For Bi2O2Se0.875Cl0.125, it can be seen that as compared with the standard DFT method, the hybrid DFT method obtains a more delocalized electron distribution, and a certain number of electrons still distribute on the Fermi level, i.e., the doped Bi2O2Se exhibits metallic properties.
3.3. Thermal Transport Properties of Bi2O2Se and Bi2O2Se0.875Cl0.125
3.3.1. Relaxation Time and Electrical Conductivity of Pure and Cl-Doped Bi2O2Se
When calculating thermoelectric properties such as the Seebeck coefficient, electrical conductivity, and thermal conductivity, two variables, i.e., carrier concentration and relaxation time, must be determined first, both of which are functions of temperature. Considering the phenomenon that Cl doping results in metallic behavior in Bi2O2Se similar to that of W doping [52], we refer to the carrier concentration of W-doped Bi2O2Se for our calculation. Therefore, the carrier concentration of pure and Cl-doped Bi2O2Se is n = 1.36 × 1019 cm−3 and n = 1.56 × 1019 cm−3, respectively. The relaxation time for Bi2O2Se at 300 K can be obtained with the following formula [53]:
where m* is the effective mass and is the carrier mobility. Employing m* = 0.33 m0 and 0 at 300 K, as reported by Gao et al. [52], we obtain τ = 1.5 × 10−14 s at 300 K for Bi2O2Se, which is comparable with the experimental result of 1.2 × 10−14 s reported by Pan et al. [54]. As for Bi2O2Se0.875Cl0.125, referring to the relaxation time of W-doped Bi2O2Se (1.9 × 10−14 s) [52] and other metals (0.31 × 10−14 s~2.2 × 10−14 s) [55], an approximate relaxation time τ of 1 × 10−14 s at 300 K is employed.
The relaxation time for Bi2O2Se and Bi2O2Se0.875Cl0.125 at different temperatures can be calculated by the following equation [56]:
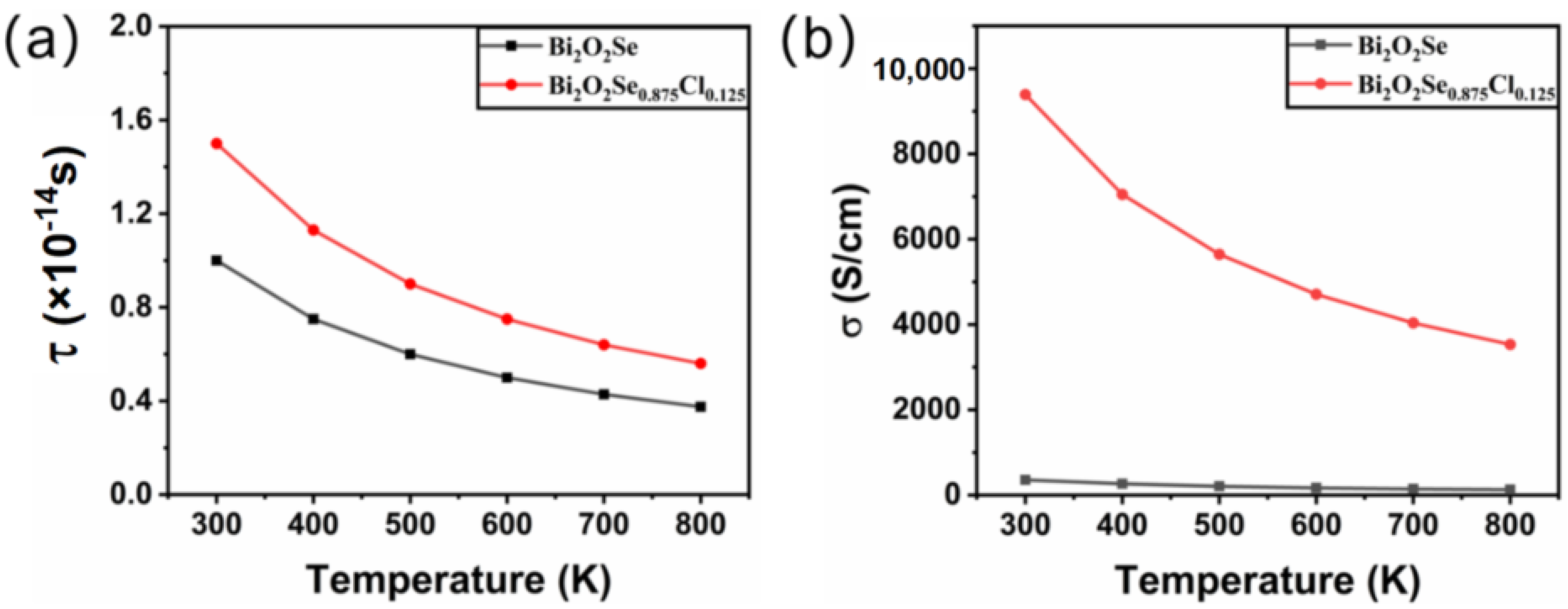
where n is the carrier concentration, T is the temperature, and C is a constant. According to the experimental literature [52], carrier concentrations are fixed at 1.36 × 1019 for Bi2O2Se and 1.56 × 1019 for Bi2O2Se0.875Cl0.125, which are also employed in other investigations [23,56,57,58,59]. Taking the relaxation time calculated at 300 K into Equation (2), the constants C are determined to be 1.78 × 10−10 for Bi2O2Se and 8.21 × 10−10 for Bi2O2Se0.875Cl0.125. Then, by substituting the constants C and n into Equation (2), the relaxation time at different temperatures is obtained. The calculated results are plotted in Figure 3a. It is shown that the relaxation time is dependent on the temperature and becomes smaller with increasing temperature.
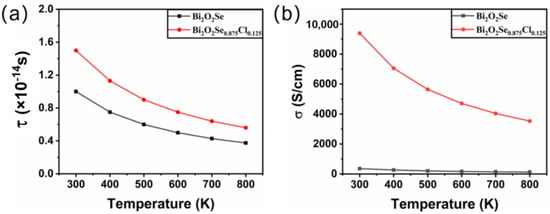
Figure 3.
The calculated (a) relaxation time and (b) electrical conductivity for Bi2O2Se and Bi2O2Se0.875Cl0.125 as a function of temperature.
Based on the determined relaxation time and carrier concentration, we further calculate the electrical conductivity of Bi2O2Se before and after Cl doping. Figure 3b shows the temperature-dependent electrical conductivity of Bi2O2Se and Bi2O2Se0.875Cl0.125. For Bi2O2Se, the calculated electrical conductivity decreases with increasing temperature, which is consistent with the experimental literature [52]. However, our results differ from those of Rurova et al. [60], due to the reason that the carrier concentration is fixed in our calculations while dependent on the temperature in the work of Rurova et al. As for Bi2O2Se0.875Cl0.125, the electrical conductivity is as high as 9390 at 300 K, which is 26 times larger than the value of 358.5 for Bi2O2Se. Experimentally, the Bi2O2Se sample (= 175 S/cm) was prepared by the exfoliation technique [52], which may produce defects and influence its physical properties [61]. In addition, the dissolution limit of Cl in Bi2O2Se reported in the literature is 1.5% [48], while the dopant concentration considered in this work is much higher, i.e., 2.5%. Although the dopant concentration considered in this work is higher than the experimental dissolution limit, the dissolution limit can be raised by techniques such as tuning the experimental temperature and powder size of the samples [37,38,39]. Therefore, the presented results will provide a theoretical reference for further related experimental research.
3.3.2. Seebeck Coefficient and Power Factor of Bi2O2Se and Bi2O2Se0.875Cl0.125
The Seebeck coefficient S is an essential parameter for predicting the thermoelectric performance of materials. It is mainly determined by the thermal electromotive force () and the temperature difference () and can be expressed as [62]:
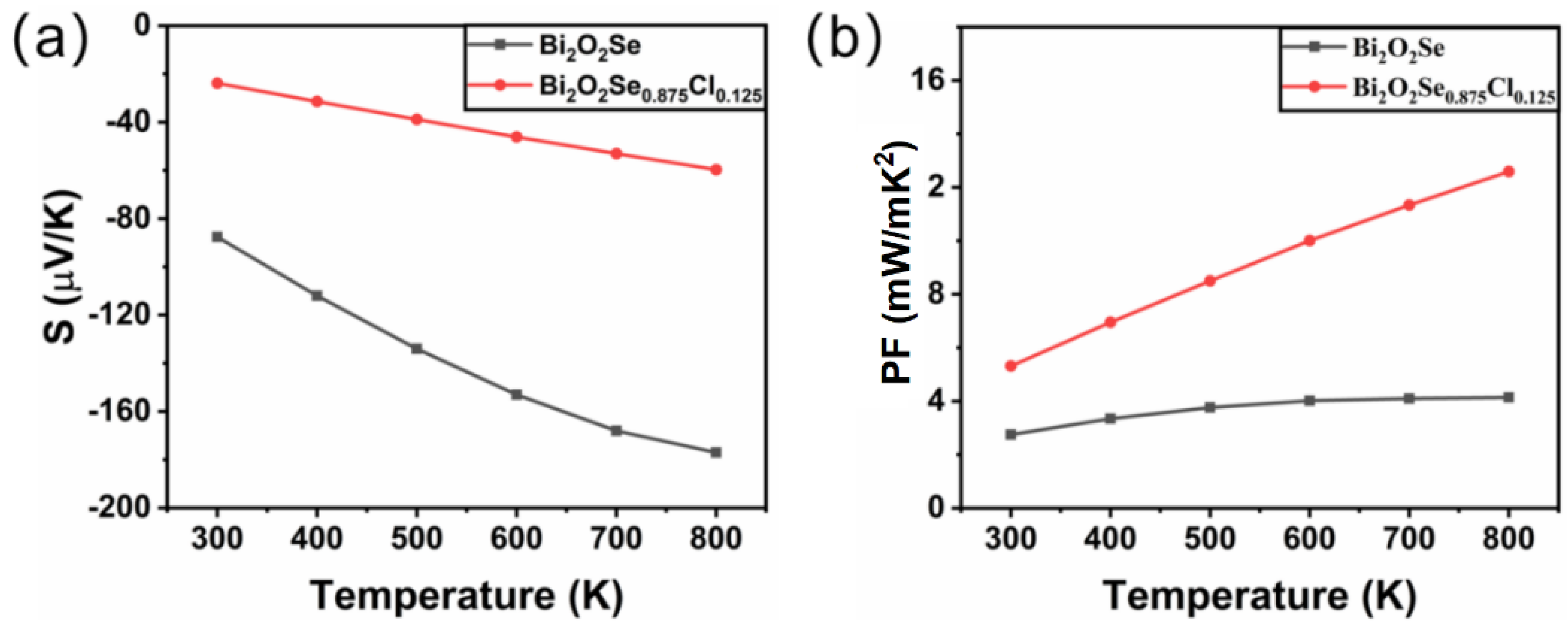
Figure 4a presents the variation of the Seebeck coefficient S with temperature for Bi2O2Se and Bi2O2Se0.875Cl0.125. It is shown that the Seebeck coefficients S of Bi2O2Se and Bi2O2Se0.875Cl0.125 are both negative, indicating that electrons are the dominant charge carriers. Due to the increase in free electron concentration, Cl doping leads to a significant decrease in the absolute value of the Seebeck coefficient S of Bi2O2Se. In addition, as the temperature increases, the absolute Seebeck coefficient S increases for both Bi2O2Se and Bi2O2Se0.875Cl0.125. For Bi2O2Se, the absolute Seebeck coefficient S increases from 87.6 μV/K at room temperature to 177 μV/K at 800 K; for Bi2O2Se0.875Cl0.125, the absolute Seebeck coefficient S increases from 23.8 μV/K at room temperature to 59.7 μV/K at 800 K.
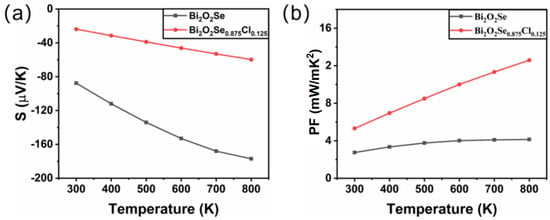
Figure 4.
The calculated (a) Seebeck coefficient and (b) power factor for Bi2O2Se and Bi2O2Se0.875Cl0.125 as a function of temperature.
Based on the calculated electrical conductivity and Seebeck coefficient S, we further calculate the power factor PF of Bi2O2Se before and after Cl doping using the following formula:
Figure 4b shows the temperature-dependent power factors (PFs) of Bi2O2Se and Bi2O2Se0.875Cl0.125. It is evident that the PF values for Bi2O2Se and Bi2O2Se0.875Cl0.125 increase with increasing temperature. In addition, Cl doping significantly enhances the PF of Bi2O2Se. For example, at a temperature of 800 K, the power factor PF of Bi2O2Se0.875Cl0.125 is 12.59 mW/m K2, which is 3.04 times that of pure Bi2O2Se (4.14 mW/m K2).
3.3.3. Thermal Conductivity and Figure of Merit ZT of Pure and Cl-Doped Bi2O2Se
The thermal conductivity of a crystal is determined by the combined effects of electronic thermal conductivity and lattice thermal conductivity, i.e.,
The Wiedemann–Franz law [35,63,64] can be applied to calculate the electronic thermal conductivity of materials, i.e.,
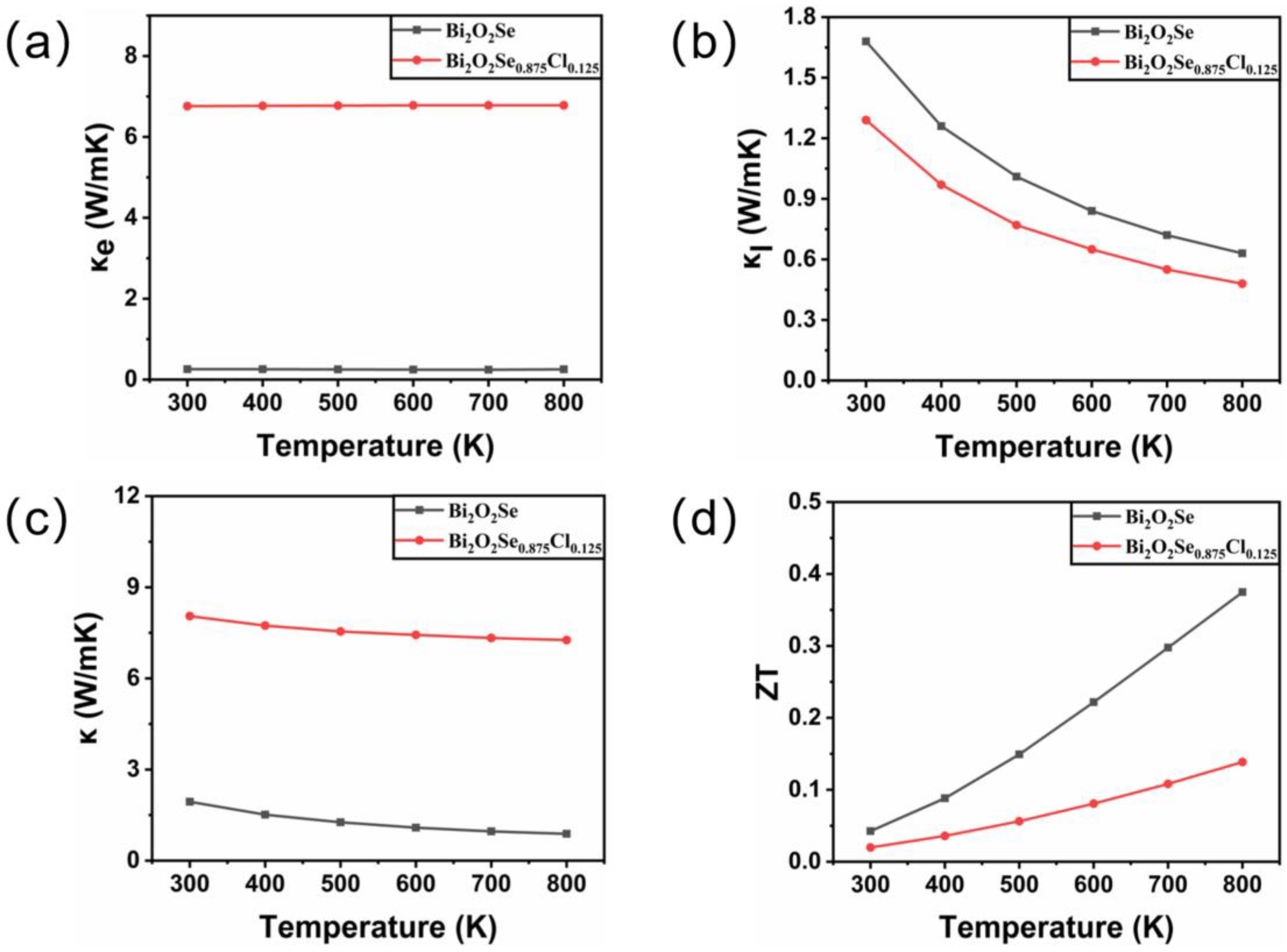
Here, L represents the Lorenz constant (2.4 × 10−8 ). The temperature-dependent behavior of electronic thermal conductivity is shown in Figure 5a. It is observed that the electronic thermal conductivity of Bi2O2Se and Bi2O2Se0.875Cl0.125 increases with increasing temperature. Moreover, the electronic thermal conductivity of Bi2O2Se0.875Cl0.125 is significantly higher than that of Bi2O2Se. For instance, at a temperature of 800 K, the calculated value of Bi2O2Se0.875Cl0.125 is 6.78 W/mK, which is 27.12 times larger than that of Bi2O2Se (0.25 W/mK). This can be attributed to the remarkable improvement in the electronic conductivity of Bi2O2Se caused by Cl doping.
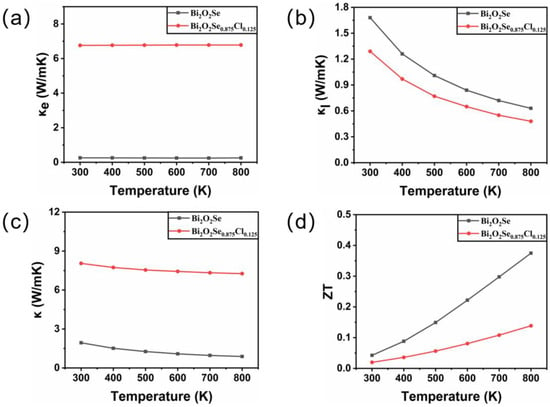
Figure 5.
The calculated (a) electronic thermal conductivity κe, (b) lattice thermal conductivity κl, (c) total thermal conductivity κ, and (d) figure of merit ZT for Bi2O2Se and Bi2O2Se0.875Cl0.125 as a function of temperature.
The Slack equation [36] can be used to calculate the lattice thermal conductivity :
The Slack model has been demonstrated to provide lattice thermal conductivity results that agree well with experimental measurements and has been extensively used in the calculation of lattice thermal conductivity for materials [49,65,66,67]. Here, A is a constant, and the calculation formula is given by:
In this equation, represents the average atomic mass, is the volume of each atom, γ is the Grüneisen parameter, Θ is the Debye temperature, and T is the absolute temperature. Figure 5b shows the temperature-dependent behavior of the lattice thermal conductivity . It is observed that the lattice thermal conductivity of both Bi2O2Se and Bi2O2Se0.875Cl0.125 decreases with increasing temperature. For Bi2O2Se, the calculated lattice thermal conductivity at 300 K is 1.68 W/mK, which agrees well with the experimental data of κ = 1.8 W/mK and other calculated results of κ = 1.2 W/mK [51]. Furthermore, Cl doping reduces the lattice thermal conductivity of Bi2O2Se. For example, the calculated lattice thermal conductivity of Bi2O2Se0.875Cl0.125 at 300 K is 1.29 W/mK, which is 23.21% smaller than that of Bi2O2Se (1.68 W/mK). This is mainly due to the low elastic constants of Bi2O2Se0.875Cl0.125. The reduced lattice thermal conductivity will be beneficial for suppressing heat transfer and minimizing thermal energy loss.
Combining the calculated electronic thermal conductivity and lattice thermal conductivity into Equation (5), the thermal conductivity of Bi2O2Se before and after Cl doping can be obtained, as depicted in Figure 5c. It is shown that Cl doping results in an increase in the thermal conductivity of Bi2O2Se, due to a substantial increase in the electronic thermal conductivity .
The ZT value of Bi2O2Se before and after Cl doping can be obtained by the following formula [7]:
as shown in Figure 5d. At 300 K, the calculated ZT value for Bi2O2Se is 0.04, which is in good agreement with the reported values of 0.05 by Gao et al. [52] and 0.045 by Song et al. [22]. Furthermore, the ZT values of both Bi2O2Se and Bi2O2Se0.875Cl0.125 increase with increasing temperature. However, the ZT value of Bi2O2Se0.875Cl0.125 is lower than that of Bi2O2Se due to the introduction of Cl dopants. This is mainly due to the decrease in the Seebeck coefficient caused by the introduction of Cl dopants. The presented results thus suggest that Cl doping is not beneficial for improving the thermoelectric properties of Bi2O2Se.
4. Conclusions
In summary, the effects of Cl doping on the structural, electronic, and thermoelectric properties of Bi2O2Se are investigated by combining the DFT method and semi-classical Boltzmann theory. The results indicate that Cl doping leads to a 0.76% volume expansion of Bi2O2Se and a significant shift of the Fermi level into the conduction band, resulting in metallic properties. Additionally, Cl doping can introduce extra electrons, resulting in a higher electrical conductivity (σ), a lower absolute Seebeck coefficient (S), and a higher power factor (PF). These findings suggest that Cl doping can optimize the thermoelectric properties of Bi2O2Se. However, the introduction of Cl dopants in Bi2O2Se results in a significant increase in the absolute value of the Seebeck coefficient, leading to a notable decrease in its ZT value. In conclusion, Cl doping can affect the thermoelectric properties of Bi2O2Se significantly, providing insights for further related experimental and theoretical studies.
Author Contributions
Conceptualization, B.L. and H.X.; methodology, H.X., M.L., H.Q. and X.Z.; software, H.X.; validation, B.L., M.L. and H.Q.; formal analysis, B.L. and M.L.; investigation, B.L.; resources, X.Z., H.X. and L.Q.; data curation, B.L.; writing—original draft preparation, B.L.; writing—review and editing, H.X. and L.Q.; visualization, B.L.; supervision, H.Q., M.L. and H.X.; project administration, B.L.; funding acquisition, H.X. and L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
Haiyan Xiao was supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U1930120). Liang Qiao acknowledges the support from the National Natural Science Foundation of China (Grant No. 52072059). All calculations in this paper were carried out at the Hefei Advanced Computing Center.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Liu, W.; Jie, Q.; Kim, H.S.; Ren, Z. Current progress and future challenges in thermoelectric power generation: From materials to devices. Acta Mater. 2015, 87, 357–376. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, C.; Xie, Y. Design of Highly Efficient Thermoelectric Materials: Tailoring Reciprocal-Space Properties by Real-Space Modification. Adv. Mater. 2018, 30, e1802000. [Google Scholar] [CrossRef] [PubMed]
- Boukai, A.I.; Bunimovich, Y.; Tahir-Kheli, J.; Yu, J.K.; Goddard, W.A., 3rd; Heath, J.R. Silicon nanowires as efficient thermoelectric materials. Nature 2008, 451, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.; Xiao, H.; Gong, H.; Liu, Z.; Zu, X.; Qiao, L. Optimizing the thermoelectric transport properties of Bi2O2Se monolayer via biaxial strain. Phys. Chem. Chem. Phys. 2019, 21, 15097–15105. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of Thermoelectric Efficiency in PbTe by Distortion of the Electronic Density of States. Science 2008, 321, 554–557. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, L.D.; Kanatzidis, M.G. Rationally Designing High-Performance Bulk Thermoelectric Materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Chen, R.; Delgado, R.D.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced thermoelectric performance of rough silicon nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef]
- Lu, W.; Ji, Z.; Pfeiffer, L.; West, K.W.; Rimberg, A.J. Real-time detection of electron tunnelling in a quantum dot. Nature 2003, 423, 422–425. [Google Scholar] [CrossRef]
- Heikes, R.; Ure, R., Jr. Thermo Electricity: Science and Engineering Intersciences; Interscience Publishers: New York, NY, USA, 1961; Volume 285, p. 40. [Google Scholar]
- Gelbstein, Y.; Dashevsky, Z.; Dariel, M.P. High performance n-type PbTe-based materials for thermoelectric applications. Phy. B 2005, 363, 196–205. [Google Scholar] [CrossRef]
- Fleischmann, H.; Luy, H.; Rupprecht, J. Neuere Untersuchungen an halbleitenden IV VI-1 V VI2-Mischkristallen. Zeitschri’ Für Naturforschung A 1963, 18, 646–649. [Google Scholar] [CrossRef]
- Wood, C. Materials for thermoelectric energy conversion. Rep. Prog. Phys. 1988, 51, 459–539. [Google Scholar] [CrossRef]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPb(m)SbTe(2+m): Bulk thermoelectric materials with high figure of merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Rosi, F.; Hockings, E.; Lindenblad, N. Semiconducting materials for thermoelectric power generation. RCA Rev. 1961, 22, 82–121. [Google Scholar]
- Zhan, B.; Liu, Y.; Tan, X.; Lan, J.l.; Lin, Y.h.; Nan, C.W.; Zhou, X.D. Enhanced Thermoelectric Properties of Bi2O2Se Ceramics by Bi Deficiencies. J. Am. Ceram. Soc. 2015, 98, 2465–2469. [Google Scholar] [CrossRef]
- Li, Y.; Huo, H.; Huang, H.; Guo, K.; Yang, X.; Xing, J.; Luo, J.; Rao, G.-H.; Zhao, J.-T. Optimization of electrical and thermal transport properties of layered Bi2O2Se via Nb doping. J. Mater. Sci 2021, 56, 12732–12739. [Google Scholar] [CrossRef]
- Hong, H.Y.; Kim, D.H.; Won, S.O.; Park, K. Enhancement of the thermoelectric performance of n−type Bi2O2Se by Ce4+ doping. J. Mater. Res. Technol. 2021, 15, 4161–4172. [Google Scholar] [CrossRef]
- Song, C.; Song, Y.; Pan, L.; Chen, C.; Zong, P.; Wang, Y. Thermoelectric properties of Bi2TiO2Se with the shear exfoliation-restacking process. J. Alloys Compd. 2022, 892, 162147. [Google Scholar] [CrossRef]
- Yang, N.; Pan, L.; Chen, C.; Wang, Y. Effects of Sb-doping on the electron-phonon transport properties of Bi2O2Se. J. Alloys Compd. 2021, 858, 157748. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, Z.; Yang, N.; Xing, W.; Zhang, J.; Liu, Y.; Chen, C.; Li, D.; Wang, Y. Effects of sulfur substitution for oxygen on the thermoelectric properties of Bi2O2Se. J. Eur. Ceram. Soc. 2020, 40, 5543–5548. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, J.-L.; Dong, S.-T.; Yu, M.-C.; Zhao, L.; Wang, L.; Yao, S.-H. Effects of Zr substitution on structure and thermoelectric properties of Bi2O2Se. J. Mater. Res. Thechnol. 2022, 21, 640–647. [Google Scholar] [CrossRef]
- Song, C.; Zhou, H.; Gu, Y.; Pan, L.; Chen, C.; Wang, Y. Enhanced thermoelectric properties of Bi2O2Se by Bi2Te2.7Se0.3 addition. J. Alloys Compd. 2023, 930, 167439. [Google Scholar] [CrossRef]
- Wang, S.; Su, L.; Qiu, Y.; Xiao, Y.; Zhao, L.-D. Enhanced thermoelectric performance in Cl-doped BiSbSe3 with optimal carrier concentration and effective mass. J. Mater. Sci. Technol. 2021, 70, 67–72. [Google Scholar] [CrossRef]
- Tan, G.; Hao, S.; Zhao, J.; Wolverton, C.; Kanatzidis, M.G. High thermoelectric performance in electron-doped AgBi3S5 with ultralow thermal conductivity. J. Am. Chem. Soc. 2017, 139, 6467–6473. [Google Scholar] [CrossRef]
- Zhang, Q.; Lan, Y.; Yang, S.; Cao, F.; Yao, M.; Opeil, C.; Broido, D.; Chen, G.; Ren, Z. Increased thermoelectric performance by Cl doping in nanostructured AgPb18SbSe20−xClx. Nano Energy 2013, 2, 1121–1127. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmu¨ller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, S.; Xiao, H.Y.; Singh, D.J.; Zhang, K.H.L.; Liu, Z.J.; Zu, X.T.; Li, S. Orbital controlled band gap engineering of tetragonal BiFeO3 for optoelectronic applications. J. Mater. Chem. C 2018, 6, 1239–1247. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Singh, D.J.; BoltzTra, P. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 2006, 175, 67–71. [Google Scholar] [CrossRef]
- Morelli, D.T.; Slack, G.A. High Lattice Thermal Conductivity of Solids; Springer: New York, NY, USA, 2006; pp. 37–68. [Google Scholar]
- Serier, H.; Gaudon, M.; Ménétrier, M. Al-doped ZnO powdered materials: Al solubility limit and IR absorption properties. Solid State Sci. 2009, 11, 1192–1197. [Google Scholar] [CrossRef]
- Martínez, V.d.P.; Aguilar, C.; Marín, J.; Ordoñez, S.; Castro, F. Mechanical alloying of Cu–Mo powder mixtures and thermodynamic study of solubility. Mater. Lett. 2007, 61, 929–933. [Google Scholar] [CrossRef]
- Zhou, E.; Suryanarayana, C.; Froes, F.S. Effect of premilling elemental powders on solid solubility extension of magnesium in titanium by mechanical alloying. Mater. Lett. 1995, 23, 27–31. [Google Scholar] [CrossRef]
- Liu, R.; Lan, J.-L.; Tan, X.; Liu, Y.-C.; Ren, G.-K.; Liu, C.; Zhou, Z.-F.; Nan, C.-W.; Lin, Y.-H. Carrier concentration optimization for thermoelectric performance enhancement in n-type Bi2O2Se. J. Eur. Ceram. Soc. 2018, 38, 2742–2746. [Google Scholar] [CrossRef]
- Wu, J.; Tan, C.; Tan, Z.; Liu, Y.; Yin, J.; Dang, W.; Wang, M.; Peng, H. Controlled Synthesis of High-Mobility Atomically Thin Bismuth Oxyselenide Crystals. Nano Lett. 2017, 17, 3021–3026. [Google Scholar] [CrossRef]
- Boiler, I. Die Kristallstruktur von Bi2O2Se. Monatsh. Chem. 1973, 104, 916–919. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, P.; Qin, M.; Lou, Z.; Gong, L.; Xu, J.; Kong, J.; Yan, H.; Gao, F. Effect of La3+, Ag+ and Bi3+ doping on thermoelectric properties of SrTiO3: First-principles investigation. Ceram. Int. 2022, 48, 13803–13816. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Meng, M.; Chen, C.; Sun, Y.; Chen, Z.; Dang, W.; Tan, C.; Liu, Y.; Yin, J.; et al. High electron mobility and quantum oscillations in non-encapsulated ultrathin semiconducting Bi2O2Se. Nat. Commun. 2017, 12, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Han, J.; Xu, B.; Lin, Y.H. Thermoelectric power factor of doped Bi2O2Se: A computational study. Phys. Chem. Chem. Phys. 2020, 22, 27096–27104. [Google Scholar] [CrossRef]
- Li, J.Q.; Cheng, C.; Duan, M.Y. The electronic and optical properties of multi-layer Bi2O2X (X = S, Se, Te) by first-principles calculations. Appl. Surf. Sci. 2023, 618, 156541. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, B.; Yu, G.; Zhang, J.; Ma, S.; Yuan, S.; Sun, T.; Wang, Y. Electronic structure and thermoelectric properties of Bi2O2Se with GGA and TB-mBJ potentials. Jpn. J. Appl. Phys. 2019, 58, 015501. [Google Scholar] [CrossRef]
- Tan, X.; Lan, J.-l.; Ren, G.; Liu, Y.; Lin, Y.-H.; Nan, C.-W. Enhanced thermoelectric performance of n-type Bi2O2Se by Cl-doping at Se site. J. Am. Ceram. Soc. 2017, 100, 1494–1501. [Google Scholar] [CrossRef]
- Li, M.; Wang, N.; Jiang, M.; Xiao, H.; Zhang, H.; Liu, Z.; Zu, X.; Qiao, L. Improved thermoelectric performance of bilayer Bi2O2Se by the band convergence approach. J. Mater. Chem. C 2019, 7, 11029–11039. [Google Scholar] [CrossRef]
- Liu, J.; Tian, L.; Mou, Y.; Jia, W.; Zhang, L.; Liu, R. Electronic and mechanical property of high electron mobility semiconductor Bi2O2Se. J. Alloys Compd. 2018, 764, 674–678. [Google Scholar] [CrossRef]
- Zhu, X.L.; Liu, P.F.; Xie, G.; Wang, B.T. First-principles study of thermal transport properties in the two- and three-dimensional forms of Bi2O2Se. Phys. Chem. Chem. Phys. 2019, 21, 10931–10938. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Pan, L.; Chen, C.; Zong, P.; Wang, Y. Enhancing thermoelectric performance of Bi2O2Se by W-doping with the shear exfoliation-restacking process. Mater. Lett. 2022, 308, 131291. [Google Scholar] [CrossRef]
- Bardeen, J.; Shockley, W. Deformation Potentials and Mobilities in Non-Polar Crystals. Phys. Rev. 1950, 80, 72–80. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, J.; Chen, C.; Wang, Y. Enhanced thermoelectric properties of highly textured Bi2O2-Se1+ with liquid-phase mechanical exfoliation. Scr. Mater. 2020, 178, 376–381. [Google Scholar] [CrossRef]
- Smith, J.B.; Ehrenreich, H. Frequency dependence of the optical relaxation time in metals. Phys. Rev. B 1982, 25, 923–930. [Google Scholar] [CrossRef]
- Guo, D.; Hu, C.; Xi, Y.; Zhang, K. Strain Effects To Optimize Thermoelectric Properties of Doped Bi2O2Se via Tran–Blaha Modified Becke–Johnson Density Functional Theory. J. Phys. Chem. C 2013, 117, 21597–21602. [Google Scholar] [CrossRef]
- Sikam, P.; Moontragoon, P.; Ikonic, Z.; Kaewmaraya, T.; Thongbai, P. The study of structural, morphological and optical properties of (Al, Ga)-doped ZnO: DFT and experimental approaches. Appl. Surf. Sci. 2019, 480, 621–635. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, H.; Snyder, G.J. Band engineering of thermoelectric materials. Adv. Mater. 2012, 24, 6125–6135. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; He, D.; Feng, D.; Yin, M.; Qin, X.; He, J. Extraordinary Thermoelectric Performance Realized in n-Type PbTe through Multiphase Nanostructure Engineering. Adv. Mater. 2017, 29, 1703148. [Google Scholar] [CrossRef]
- Ruleova, P.; Drasar, C.; Lostak, P.; Li, C.P.; Ballikaya, S.; Uher, C. Thermoelectric properties of Bi2O2Se. Mater. Chem. Phys. 2010, 119, 299–302. [Google Scholar] [CrossRef]
- Islam, M.A.; Serles, P.; Kumral, B.; Demingos, P.G.; Qureshi, T.; Meiyazhagan, A.; Puthirath, A.B.; Abdullah, M.S.B.; Faysal, S.R.; Ajayan, P.M.; et al. Exfoliation Mechanisms of 2D Materials and Their Applications. Appl. Phys. Rev. 2022, 9, 041301. [Google Scholar] [CrossRef]
- Kim, M.; Park, D.; Kim, J. Enhancement of Bi2O2Se thermoelectric power factor via Nb doping. J. Alloys Compd. 2021, 851, 156905. [Google Scholar] [CrossRef]
- Stojanovic, N.; Maithripala, D.H.S.; Berg, J.M.; Holtz, M. Thermal conductivity in metallic nanostructures at high temperature: Electrons, phonons, and the Wiedemann-Franz law. Phys. Rev. B 2010, 82, 075418. [Google Scholar] [CrossRef]
- Wang, D.; He, W.; Chang, C.; Wang, G.; Wang, J.; Zhao, L.-D. Thermoelectric transport properties of rock-salt SnSe: First-principles investigation. J. Mater. Chem. C 2018, 6, 12016–12022. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, J. Approaching extremely low thermal conductivity by crystal structure engineering in Mg2Al4Si5O18. J. Mater. Res. 2015, 30, 3729–3739. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Tian, Z.; Wang, J.; Wang, J. Theoretical exploration of the abnormal trend in lattice thermal conductivity for monosilicates RE2SiO5 (RE = Dy, Ho, Er, Tm, Yb and Lu). J. Eur. Ceram. Soc. 2018, 38, 3539–3546. [Google Scholar] [CrossRef]
- Shindé, S.L.; Goela, J. (Eds.) High Thermal Conductivity Materials; Springer: New York, NY, USA, 2006; p. 91. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).