Building Manganese Halide Hybrid Materials with 0D, 1D, and 2D Dimensionalities
Abstract
:1. Introduction
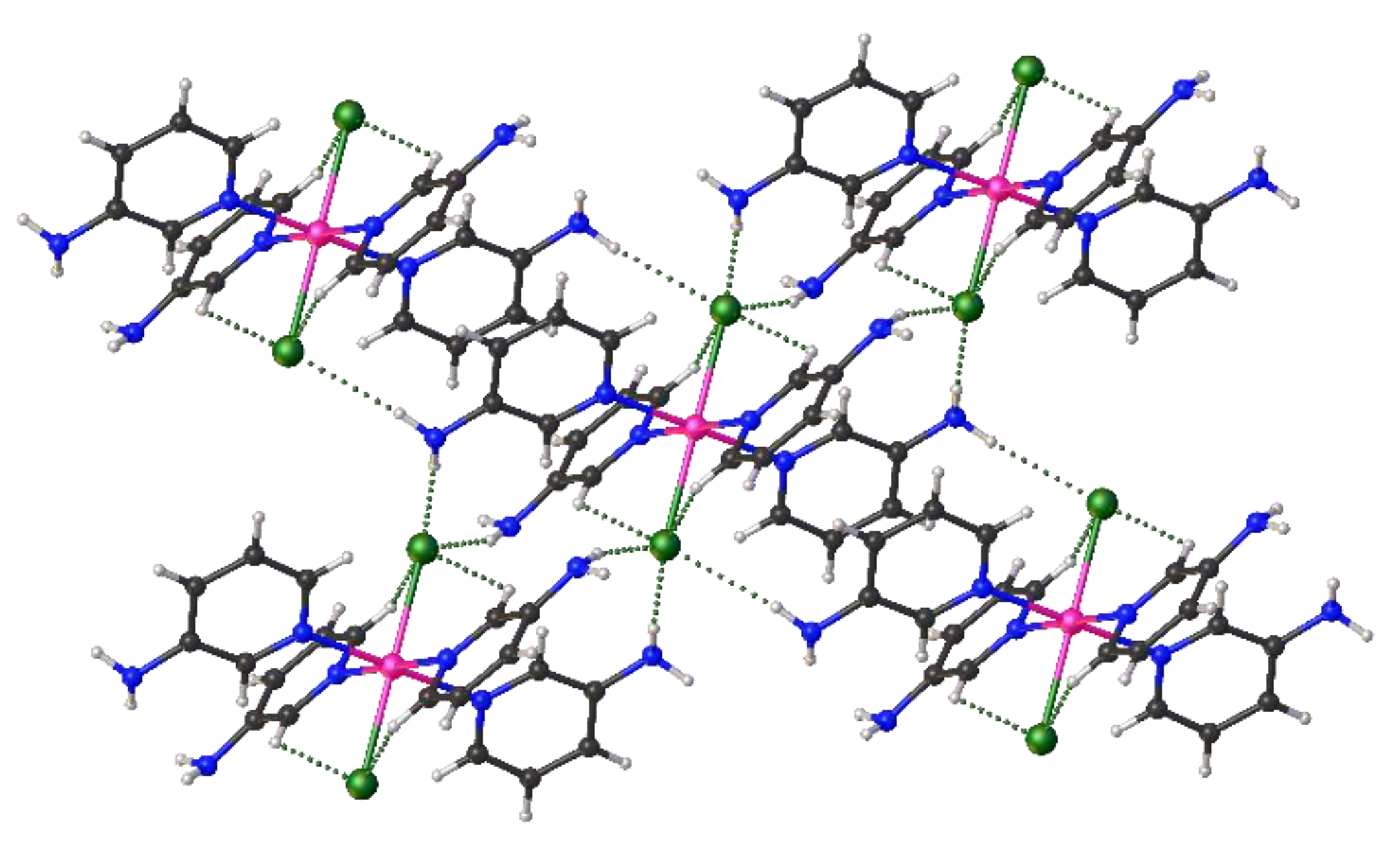
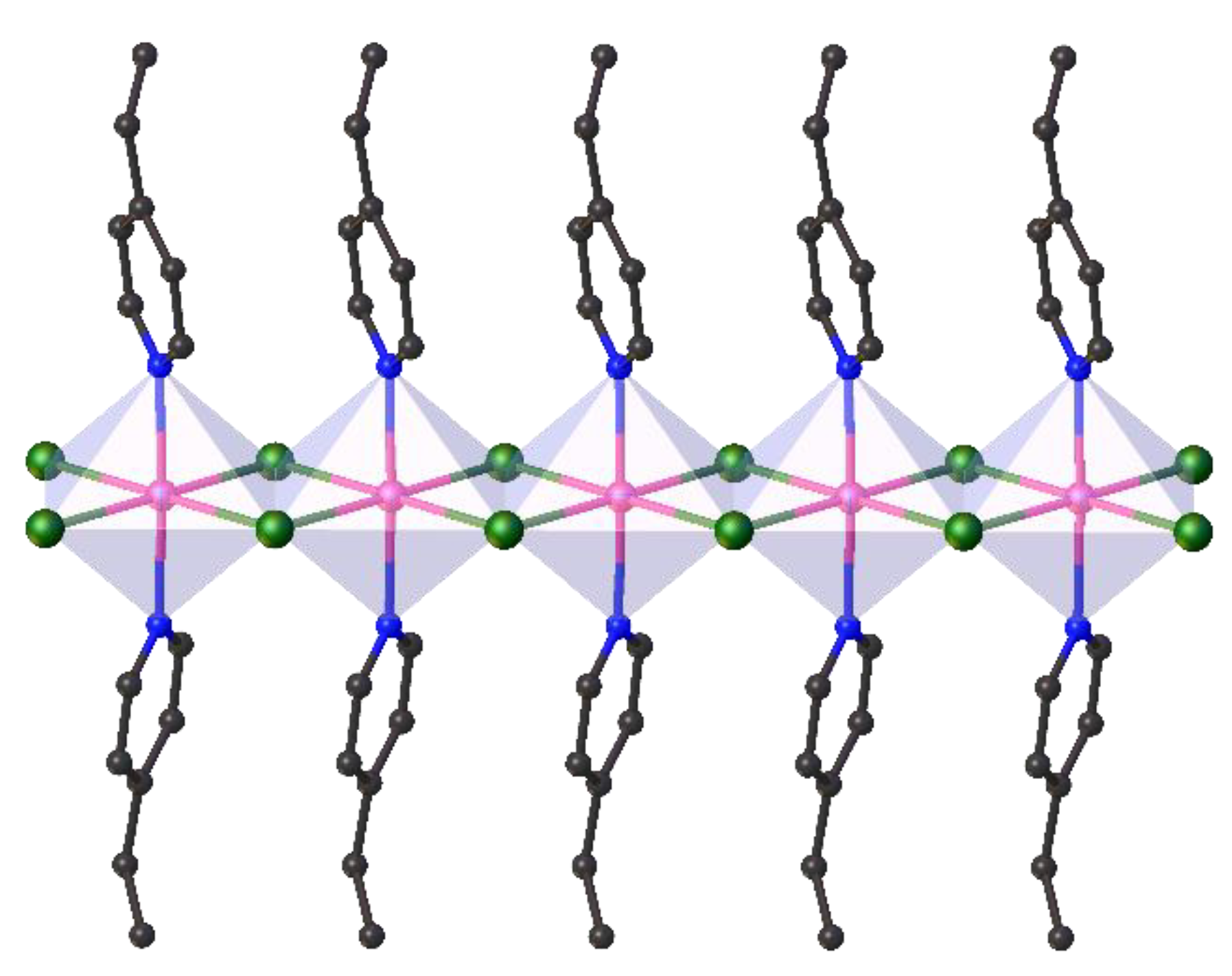
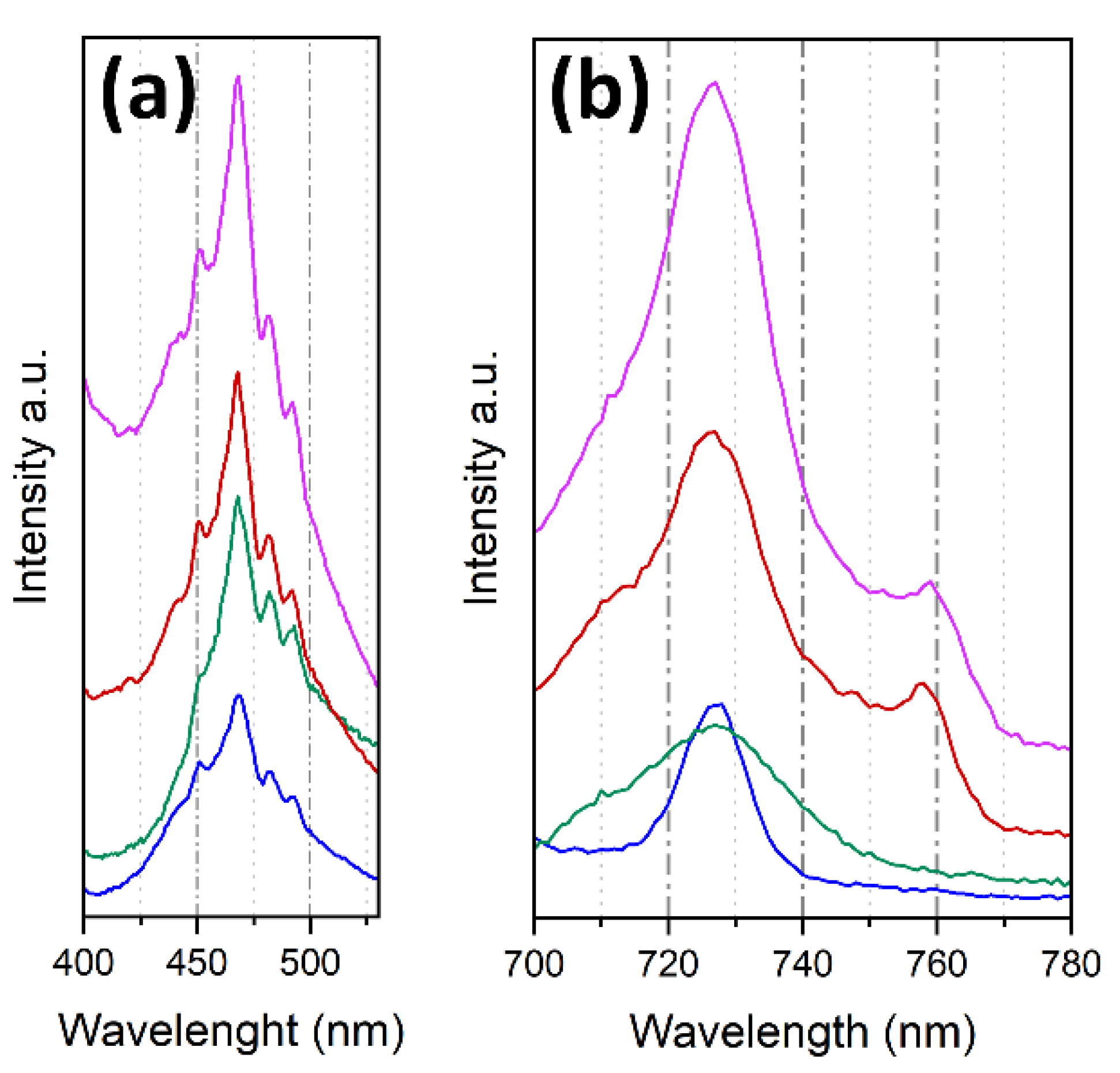
2. Results and Discussion
3. Conclusions
4. Methods and Materials
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roy, P.; Ghosh, A.; Barclay, F.; Khare, A.; Cuce, E. Perovskite Solar Cells: A Review of the Recent Advances. Coatings 2022, 12, 1089. [Google Scholar] [CrossRef]
- Lekesi, L.P.; Koao, L.F.; Motloung, S.V.; Motaung, T.E.; Malevu, T. Developments on Perovskite Solar Cells (PSCs): A Critical Review. Appl. Sci. 2022, 12, 672. [Google Scholar] [CrossRef]
- Pu, Y.; Su, H.; Liu, C.; Guo, M.; Liu, L.; Fu, H. A Review on Buried Interface of Perovskite Solar Cells. Energies 2023, 16, 5015. [Google Scholar] [CrossRef]
- Lye, Y.-E.; Chan, K.-Y.; Ng, Z.-N. A Review on the Progress, Challenges, and Performances of Tin-Based Perovskite Solar Cells. Nanomaterials 2023, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Abiram, G.; Thanihaichelvan, M.; Ravirajan, P.; Velauthapillai, D. Review on Perovskite Semiconductor Field-Effect Transistors and Their Applications. Nanomaterials 2022, 12, 2396. [Google Scholar] [CrossRef] [PubMed]
- Paulus, F.; Tyznik, C.; Jurchescu, O.D.; Vaynzof, Y. Switched-On: Progress, Challenges, and Opportunities in Metal Halide Perovskite Transistors. Adv. Funct. Mater. 2021, 31, 2101029. [Google Scholar] [CrossRef]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Gao, P.; Cheng, S.; Liu, J.; Li, J.; Guo, Y.; Deng, Z.; Qin, T.; Wang, A. Facile Synthesis of Highly Emissive All-Inorganic Manganese Bromide Compounds with Perovskite-Related Structures for White LEDs. Molecules 2022, 27, 8259. [Google Scholar] [CrossRef]
- Thien, G.S.H.; Ab Rahman, M.; Yap, B.K.; Tan, N.M.L.; He, Z.; Low, P.-L.; Devaraj, N.K.; Ahmad Osman, A.F.; Sin, Y.-K.; Chan, K.-Y. Recent Advances in Halide Perovskite Resistive Switching Memory Devices: A Transformation from Lead-Based to Lead-Free Perovskites. ACS Omega 2022, 7, 39472–39481. [Google Scholar] [CrossRef]
- Thien, G.S.; Chan, K.-Y.; Marlinda, A.R. The Role of Polymers in Halide Perovskite Resistive Switching Devices. Polymers 2023, 15, 1067. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Ge, C.; Tang, B.; Lin, H.; Zhang, X.; Huang, Y.; Zhu, Q.; Hu, H. Lead-Free Perovskite Single Crystals: A Brief Review. Crystals 2021, 11, 1329. [Google Scholar] [CrossRef]
- Umar, A.; Sadanand Singh, P.K.; Dwivedi, D.K.; Algadi, H.; Ibrahim, A.A.; Alhammai, M.A.M.; Baskoutas, S. High Power-Conversion Efficiency of Lead-Free Perovskite Solar Cells: A Theoretical Investigation. Micromachines 2022, 13, 2201. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, P.; Comba, P.; Dittmar, B.; Ndiaye, D.; Tóth, É.; Velmurugan, G.; Wadepohl, H. Exceptional Manganese(II) Stability and Manganese(II)/Zinc(II) Selectivity with Rigid Polydentate Ligands. Angew. Chem. Int. Ed. 2022, 61, e202115580. [Google Scholar] [CrossRef] [PubMed]
- Dube, K.S.; Harrop, T.C. Structure and properties of an eight-coordinate Mn(ii) complex that demonstrates a high water relaxivity. Dalton Trans. 2011, 40, 7496–7498. [Google Scholar] [CrossRef] [PubMed]
- Baldeau, S.M.; Slinn, C.H.; Krebs, B.; Rompel, A. Five manganese(II) complexes with seven- or eight-coordinated Mn(II), revealing different coordination modes for the nitrato ligands. Inorg. Chim. Acta 2004, 357, 3295–3303. [Google Scholar] [CrossRef]
- Morad, V.; Cherniukh, I.; Pöttschacher, L.; Shynkarenko, Y.; Yakunin, S.; Kovalenko, M.V. Manganese(II) in Tetrahedral Halide Environment: Factors Governing Bright Green Luminescence. Chem. Mater 2019, 31, 10161–10169. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-J.; Sun, C.-Z.; Xiao, H.; Wu, Y.; Chen, Z.-N. Green-Light-Emitting Diodes based on Tetrabromide Manganese(II) Complex through Solution Process. Adv. Mater. 2017, 29, 1605739. [Google Scholar] [CrossRef]
- Sen, A.; Swain, D.; Guru Row, T.N.; Sundaresan, A. Unprecedented 30 K hysteresis across switchable dielectric and magnetic properties in a bright luminescent organic–inorganic halide (CH6N3)2MnCl4. J. Mater. Chem. C 2019, 7, 4838–4845. [Google Scholar] [CrossRef]
- Vinogradova, K.A.; Shekhovtsov, N.A.; Berezin, A.S.; Sukhikh, T.S.; Krivopalov, V.P.; Nikolaenkova, E.B.; Plokhikh, I.V.; Bushuev, M.B. A near-infra-red emitting manganese(II) complex with a pyrimidine-based ligand. Inorg. Chem. Commun. 2019, 100, 11–15. [Google Scholar] [CrossRef]
- Stefańska, D. Effect of Organic Cation on Optical Properties of [A]Mn(H2POO)3 Hybrid Perovskites. Molecules 2022, 27, 8953. [Google Scholar] [CrossRef]
- Long, G.J.; Clarke, P.J. Crystal and molecular structures of trans-tetrakis(pyridine)dichloroiron(II), -nickel(II), and -cobalt(II) and trans-tetrakis(pyridine)dichloroiron(II) monohydrate. Inorg. Chem. 1978, 17, 1394–1401. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Chen, K.-J.; Schoedel, A.; Wojtas, L.; Perry Iv, J.J.; Zaworotko, M.J. Network diversity through two-step crystal engineering of a decorated 6-connected primary molecular building block. CrystEngComm 2016, 18, 8578–8581. [Google Scholar] [CrossRef]
- Heine, M.; Fink, L.; Schmidt, M.U. 3-Cyanopyridine as a bridging and terminal ligand in coordination polymers. CrystEngComm 2018, 20, 7556–7566. [Google Scholar] [CrossRef]
- He, X.; Lu, C.-Z.; Yu, Y.-Q.; Chen, S.-M.; Wu, X.-Y.; Liu, J.-H. trans-Tetrakis(3-aminopyridine)dichlorocadmium(II). Acta Crystallogr. Sect. E 2004, 60, m1639–m1640. [Google Scholar] [CrossRef]
- Csöregh, I.; Kenessey, G.; Wadsten, T.; Liptay, G.; Carson, B.R. Pyridine type complexes of transition-metal halides XI. Structural, thermal and spectroscopic studies of aminopyridine complexes of cobalt(II) halides. Z. Für Krist. Cryst. Mater. 2000, 215, 547–552. [Google Scholar] [CrossRef]
- Cain, B.R.; Freeman, J.M.; Henshall, T. Characteristic vibrations of the NH2 group. Can. J. Chem. 1969, 47, 2947. [Google Scholar] [CrossRef]
- Su, C.-W.; Wu, C.-P.; Chen, J.-D.; Liou, L.-S.; Wang, J.-C. Synthesis and structural characterization of two chain complexes of Mn(II) containing 2-aminopyridinium. Inorg. Chem. Commun. 2002, 5, 215–219. [Google Scholar] [CrossRef]
- Brede, F.A.; Mühlbach, F.; Sextl, G.; Müller-Buschbaum, K. Mechanochemical and thermal formation of 1H-benzotriazole coordination polymers and complexes of 3d-transition metals with intriguing dielectric properties. Dalton Trans. 2016, 45, 10609–10619. [Google Scholar] [CrossRef]
- Lee, L.M.; Elder, P.J.W.; Dube, P.A.; Greedan, J.E.; Jenkins, H.A.; Britten, J.F.; Vargas-Baca, I. The size of the metal ion controls the structures of the coordination polymers of benzo-2,1,3-selenadiazole. CrystEngComm 2013, 15, 7434–7437. [Google Scholar] [CrossRef]


| Abbreviation | [MnCl2(3AP)4] | [MnBr2(3AP)4] | [MnCl33AP]− [3APH]+ | {MnCl2(4EtP)2}n | {MnBr2(4EtP)2}n |
|---|---|---|---|---|---|
| Empirical formula | C20H24Cl2MnN8 | C20H24Br2MnN8 | C44H44Cl12Cu2Mn4N28O2 | C14H18Cl2MnN2 | C14H18Br2MnN2 |
| Formula weight | 502.31 | 591.23 | 350.53 | 340.14 | 429.06 |
| Temperature (K) | 100 | 100 | 100 | 100 | 100 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | Pī | Pī | P21/c | C2/c | C2/c |
| a (Å) | 7.974(5) | 8.154(1) | 11.749(3) | 24.06(3) | 23.671(2) |
| b (Å) | 8.823(6) | 8.856(1) | 10.478(3) | 3.875(5) | 3.9513(4) |
| c (Å) | 10.072(6) | 10.134(1) | 14.676(4) | 17.91(2) | 18.167(2) |
| α (°) | 97.60(2) | 98.018(4) | 90 | 90 | 90 |
| β (°) | 91.84(2) | 91.186(3) | 107.711(7) | 114.11(4) | 112.722(2) |
| γ (°) | 113.93(2) | 114.446(3) | 90 | 90 | 90 |
| Volume (Å3) | 639.0(7) | 657.2(2) | 1721.1(8) | 1524(3) | 1567.3(3) |
| Z | 1 | 1 | 4 | 4 | 4 |
| ρcalc (g/mL) | 1.305 | 1.494 | 1.353 | 1.483 | 1.818 |
| μ (mm−1) | 0.748 | 3.564 | 1.221 | 1.204 | 5.929 |
| F(000) | 259 | 295 | 708 | 700 | 844 |
| Data collection range (°) | 4.1 to 53.9 | 4 to 58 | 3.6 to 53.5 | 3.7 to 50.6 | 3.7 to 51.3 |
| Reflections collected | 7961 | 16035 | 19781 | 7526 | 1483 |
| Independent reflections | 2773 | 3446 | 3648 | 1378 | 1483 |
| Goof on F2 | 1.086 | 1.041 | 1.067 | 1.050 | 1.085 |
| R1 [I ≥ 2σ (I)] | 0.0243 | 0.0212 | 0.0269 | 0.0292 | 0.0296 |
| wR2 all reflections | 0.0685 | 0.0539 | 0.0717 | 0.0578 | 0.0685 |
| H-atom treatment | Mixed | Mixed | Mixed | Constrained | Constrained |
| D-H⸳⸳⸳A | D-H Distance (Å) | H⸳⸳⸳A Distance (Å) | D-A Distance (Å) | D-H⸳⸳⸳A Angle (°) |
|---|---|---|---|---|
| [MnII(3AP)4Cl2] | ||||
| N3-H3A⸳⸳⸳Cl1 | 0.892(13) | 2.639(14) | 3.523(3) | 171.5(15) |
| N3-H3B⸳⸳⸳Cl1 | 0.878(14) | 2.522(14) | 3.397(2) | 175.0(16) |
| N4-H4A⸳⸳⸳Cl1 | 0.887(15) | 2.549(16) | 3.414(2) | 165(2) |
| [MnII(3AP)4Br2] | ||||
| N3-H3A⸳⸳⸳Br1 | 0.845(15) | 2.803(17) | 3.616(2) | 162(2) |
| N3-H3B⸳⸳⸳Br1 | 0.849(15) | 2.661(16) | 3.507(2) | 175(2) |
| N4-H4A⸳⸳⸳Br1 | 0.857(16) | 2.672(18) | 3.509(2) | 166(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peoble, A.; Gallegos, K.; Ozide, M.O.; Castañeda, R. Building Manganese Halide Hybrid Materials with 0D, 1D, and 2D Dimensionalities. Crystals 2023, 13, 1634. https://doi.org/10.3390/cryst13121634
Peoble A, Gallegos K, Ozide MO, Castañeda R. Building Manganese Halide Hybrid Materials with 0D, 1D, and 2D Dimensionalities. Crystals. 2023; 13(12):1634. https://doi.org/10.3390/cryst13121634
Chicago/Turabian StylePeoble, Anna, Kandee Gallegos, Michael O. Ozide, and Raúl Castañeda. 2023. "Building Manganese Halide Hybrid Materials with 0D, 1D, and 2D Dimensionalities" Crystals 13, no. 12: 1634. https://doi.org/10.3390/cryst13121634





