O-Vacancy-Rich ε-MnO2 Synthesized at Hydrophobic Interface: An Efficient Fenton-like Catalyst for Removing Ciprofloxacin from Water
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Chemicals
2.2. Synthesis of ε-MnO2 Catalysts
2.3. Characterizations
2.4. Degradation Procedure and Analytical Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, Z.; Jiao, W.; Shao, F.; Song, H.; Zhang, H.; Zhou, Q.; Li, A. Fabrication and characterization of amphiphilic magnetic water purification materials for efficient PPCPs removal. Chem. Eng. J. 2019, 360, 511–518. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Wu, X.; Siddique, M.S.; Su, Z.; Liu, M.; Yu, W. Reducing ROS generation and accelerating the photocatalytic degradation rate of PPCPs at neutral pH by doping Fe-N-C to g-C3N4. Appl. Catal. B 2022, 301, 120790. [Google Scholar] [CrossRef]
- Dang, V.D.; Adorna, J.; Annadurai, T.; Bui, T.A.N.; Tran, H.L.; Lin, L.; Doong, R. Indirect Z-scheme nitrogen-doped carbon dot decorated Bi2MoO6/g-C3N4 photocatalyst for enhanced visible-light-driven degradation of ciprofloxacin. Chem. Eng. J. 2021, 422, 130103. [Google Scholar] [CrossRef]
- Chen, M.; Niu, H.; Niu, C.; Guo, H.; Liang, S.; Yang, Y. Metal-organic framework-derived CuCo/carbon as an efficient magnetic heterogeneous catalyst for persulfate activation and ciprofloxacin degradation. J. Hazard. Mater. 2022, 424, 127196. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, X.; Yang, X.; Wang, P.; Li, S.; Lu, Z.; Chen, H. Oxygen vacancy boosted photocatalytic decomposition of ciprofloxacin over Bi2MoO6: Oxygen vacancy engineering, biotoxicity evaluation and mechanism study. J. Hazard. Mater. 2019, 364, 691–699. [Google Scholar] [CrossRef]
- Ding, J.; Dai, Z.; Qin, F.; Zhao, H.; Zhao, S.; Chen, R. Z-scheme BiO1−xBr/Bi2O2CO3 photocatalyst with rich oxygen vacancy as electron mediator for highly efficient degradation of antibiotics. Appl. Catal. B 2017, 205, 281–291. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Pang, S.; Jiang, J.; Yang, Y.; Ma, J.; Yang, Y.; Duan, J.; Guo, Q. Oxidation of fluoroquinolone antibiotics by peroxymonosulfate without activation: Kinetics, products, and antibacterial deactivation. Water Res. 2018, 145, 210–219. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Z.; Zheng, Z.; Xu, H.; Wang, H.; Hu, K.; Yan, K. Remarkably enhanced sulfate radical-based photo-fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnOx microspheres. Chem. Eng. J. 2020, 379, 122340. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient fenton-like catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Oh, W.; Dong, Z.; Lim, T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, R.; Li, X.; Wei, Y.; Feng, L. A bifunctional β-MnO2 mesh for expeditious and ambient degradation of dyes in activation of peroxymonosulfate (PMS) and simultaneous oil removal from water. J. Colloid Interface Sci. 2020, 579, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Milh, H.; Yu, X.; Cabooter, D.; Dewil, R. Degradation of ciprofloxacin using UV-based advanced removal processes: Comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci. Total Environ. 2021, 764, 144510. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Choi, J.; Kim, M.; Kim, M.S.; Lee, D.; Bang, W.H.; Yun, E.; Lee, H.; Lee, J.; Lee, C.; et al. Chloride-mediated enhancement in heat-induced activation of peroxymonosulfate: New reaction pathways for oxidizing radical production. Environ. Sci. Technol. 2021, 55, 5382–5392. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Ma, Y.; Song, Y.; Li, T.; Dong, S. Tetracycline hydrochloride degradation over manganese cobaltate (MnCo2O4) modified ultrathin graphitic carbon nitride (g-C3N4) nanosheet through the highly efficient activation of peroxymonosulfate under visible light irradiation. J. Colloid Interface Sci. 2021, 600, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, C.; Gao, M.; Han, X.; Zhang, Y.; Zhang, W.; Li, W. Mn−O covalency governs the intrinsic activity of Co-Mn spinel oxides for boosted peroxymonosulfate activation. Angew. Chem. Int. Ed. 2020, 60, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Singewald, K.; Liu, C.; Saxena, S.; Zhang, H. Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem. Eng. J. 2019, 370, 906–915. [Google Scholar] [CrossRef]
- Tao, P.; Shao, M.; Song, C.; Li, C.; Yin, Y.; Wu, S.; Cheng, M.; Cui, Z. Morphologically controlled synthesis of porous Mn2O3 microspheres and their catalytic applications on the degradation of methylene blue. Desalin. Water Treat. 2015, 57, 7079–7084. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, M.; Ma, X.; Wu, S.; Ge, M.; Yu, X. Insights into the transformations of Mn species for peroxymonosulfate activation by tuning the Mn3O4 shapes. Chem. Eng. J. 2021, 404, 127097. [Google Scholar] [CrossRef]
- Lin, M.; Chen, Z. A facile one-step synthesized epsilon-MnO2 nanoflowers for effective removal of lead ions from wastewater. Chemosphere 2020, 250, 126329. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Cao, H.; Xie, Y.; Xiao, J.; Chen, Y.; Ghazi, Z.A. Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol. Chemosphere 2017, 168, 1457–1466. [Google Scholar] [CrossRef]
- Wang, H.; Yin, F.; Chen, B.; Li, G. Synthesis of an ε-MnO2/metal–organic-framework composite and its electrocatalysis towards oxygen reduction reaction in an alkaline electrolyte. J. Mater. Chem. A 2015, 3, 16168–16176. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Li, N.; Wang, S.; Yu, J.; Li, X. Efficient degradation of ciprofloxacin by magnetic γ-Fe2O3-MnO2 with oxygen vacancy in visible-light/peroxymonosulfate system. Chemosphere 2021, 276, 130257. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Q.; Hong, J.; Dong, Z.; Wang, J. Degradation of bisphenol A by persulfate activation via oxygen vacancy-rich CoFe2O4–x. Chemosphere 2019, 221, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zheng, Z.; Wang, H.; Chen, Y.; Luo, C.; Liang, D.; Hu, B.; Qiu, R.; Yan, K. 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl. Catal. B 2020, 277, 119171. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, T.; Wang, H.; Zhang, H.; Sun, Y.; Chen, L.; Song, S.; Li, L.; Shi, H. Oxygen-defective MnO2-x rattle-type microspheres mediated singlet oxygen oxidation of organics by peroxymonosulfate activation. Chem. Eng. J. 2020, 394, 124458. [Google Scholar] [CrossRef]
- Hong, W.; Shao, M.; Zhu, T.; Wang, H.; Sun, Y.; Shen, F.; Li, X. To promote ozone catalytic decomposition by fabricating manganese vacancies in ε-MnO2 catalyst via selective dissolution of Mn-Li precursors. Appl. Catal. B 2020, 274, 119088. [Google Scholar] [CrossRef]
- Xiao, Y.; Huo, W.; Yin, S.; Jiang, D.; Zhang, Y.; Zhang, Z.; Liu, X.; Dong, F.; Wang, J.; Li, G.; et al. One-step hydrothermal synthesis of Cu-doped MnO2 coated diatomite for degradation of methylene blue in Fenton-like system. J. Colloid Interface Sci. 2019, 556, 466–475. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, X.; Nie, Y.; Yang, C.; Wang, Y. Enhanced peroxymonosulfate activation for phenol degradation over MnO2 at pH 3.5–9.0 via Cu(II) substitution. J. Hazard. Mater. 2018, 360, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Yang, Y.; Hoffmann, M.R. Activation of peroxymonosulfate by oxygen vacancies-enriched cobalt-doped black TiO2 nanotubes for the removal of organic pollutants. Environ. Sci. Technol. 2019, 53, 6972–6980. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; An, F.; Zhu, C.; Zhou, D. Efficient transformation of DDT with peroxymonosulfate activation by different crystallographic MnO2. Sci. Total Environ. 2021, 759, 142864. [Google Scholar] [CrossRef]
- Xie, M.; Tang, J.; Kong, L.; Lu, W.; Natarajan, V.; Zhu, F.; Zhan, J. Cobalt doped g-C3N4 activation of peroxymonosulfate for monochlorophenols degradation. Chem. Eng. J. 2019, 360, 1213–1222. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Liu, Y.; Kim, S.H.; Dionysiou, D.D. Facile preparation of porous Mn/Fe3O4 cubes as peroxymonosulfate activating catalyst for effective bisphenol A degradation. Chem. Eng. J. 2019, 376, 119193. [Google Scholar] [CrossRef]
- Wang, L.; Lan, X.; Peng, W.; Wang, Z. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124436. [Google Scholar] [CrossRef]
- Pan, F.; Ji, H.; Du, P.; Huang, T.; Wang, C.; Liu, W. Insights into catalytic activation of peroxymonosulfate for carbamazepine degradation by MnO2 nanoparticles in-situ anchored titanate nanotubes: Mechanism, ecotoxicity and DFT study. J. Hazard. Mater. 2021, 402, 123779. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Wang, J.; Li, W. Fe@C carbonized resin for peroxymonosulfate activation and bisphenol S degradation. Environ. Pollut. 2019, 252, 1042–1050. [Google Scholar] [CrossRef]

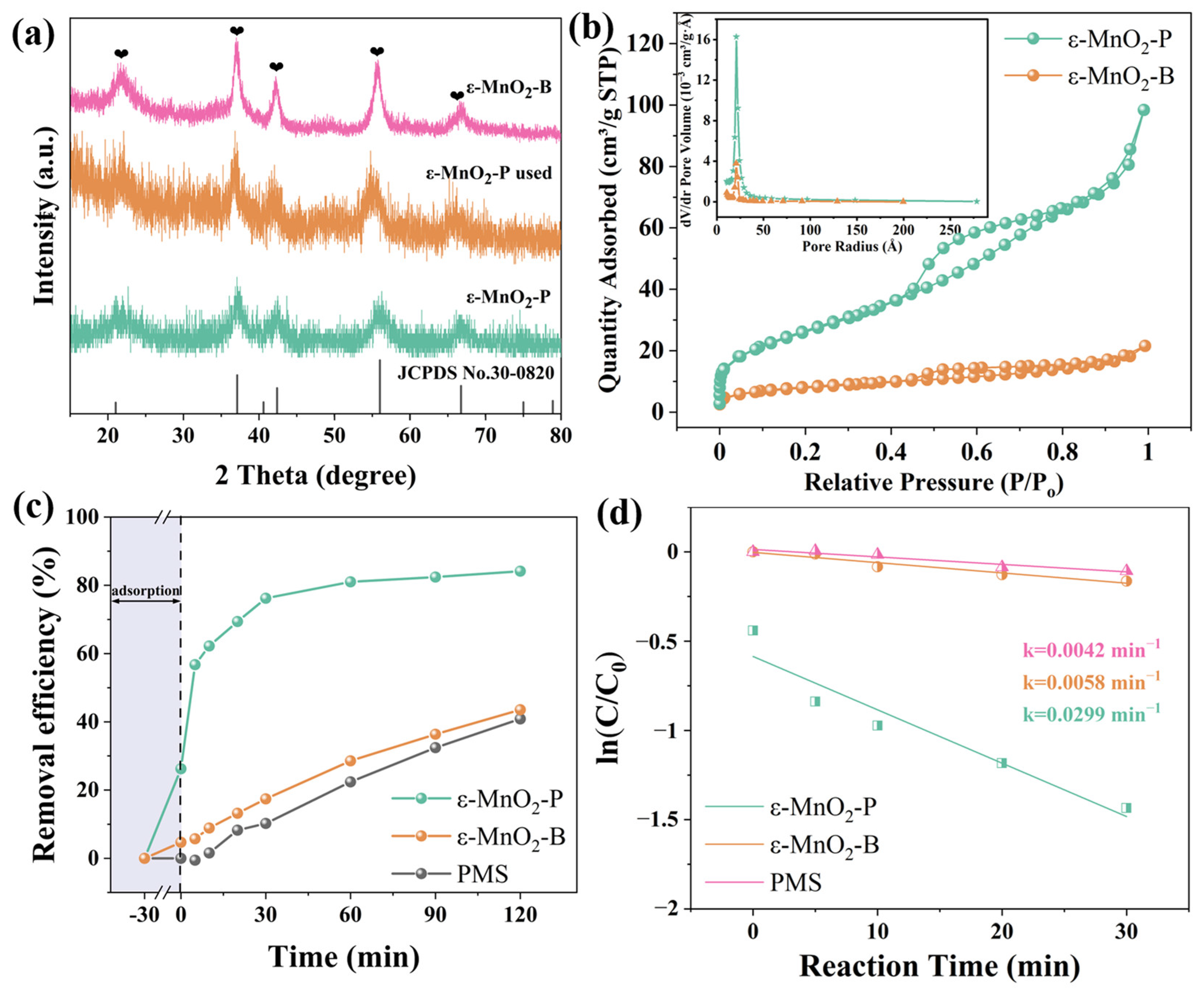
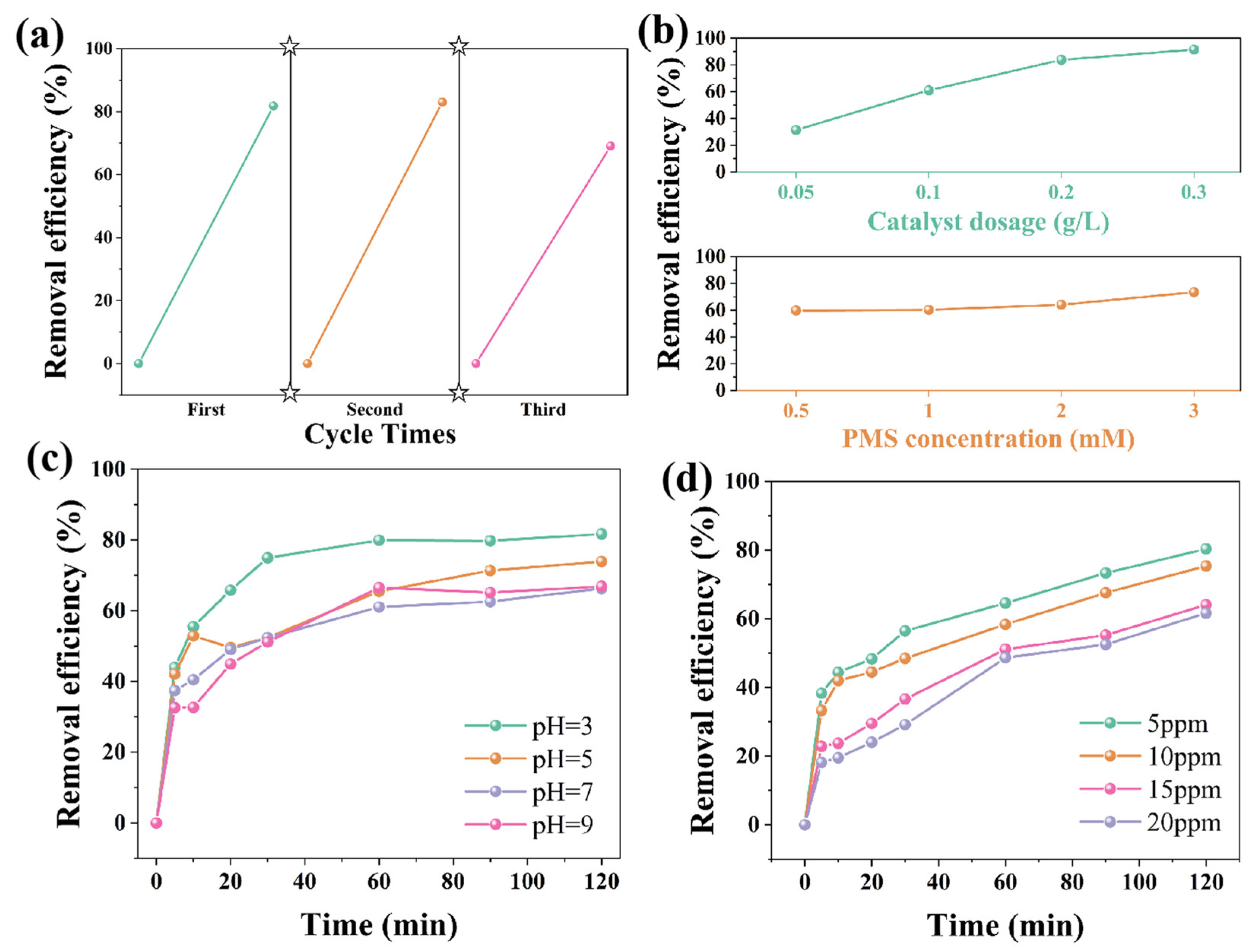
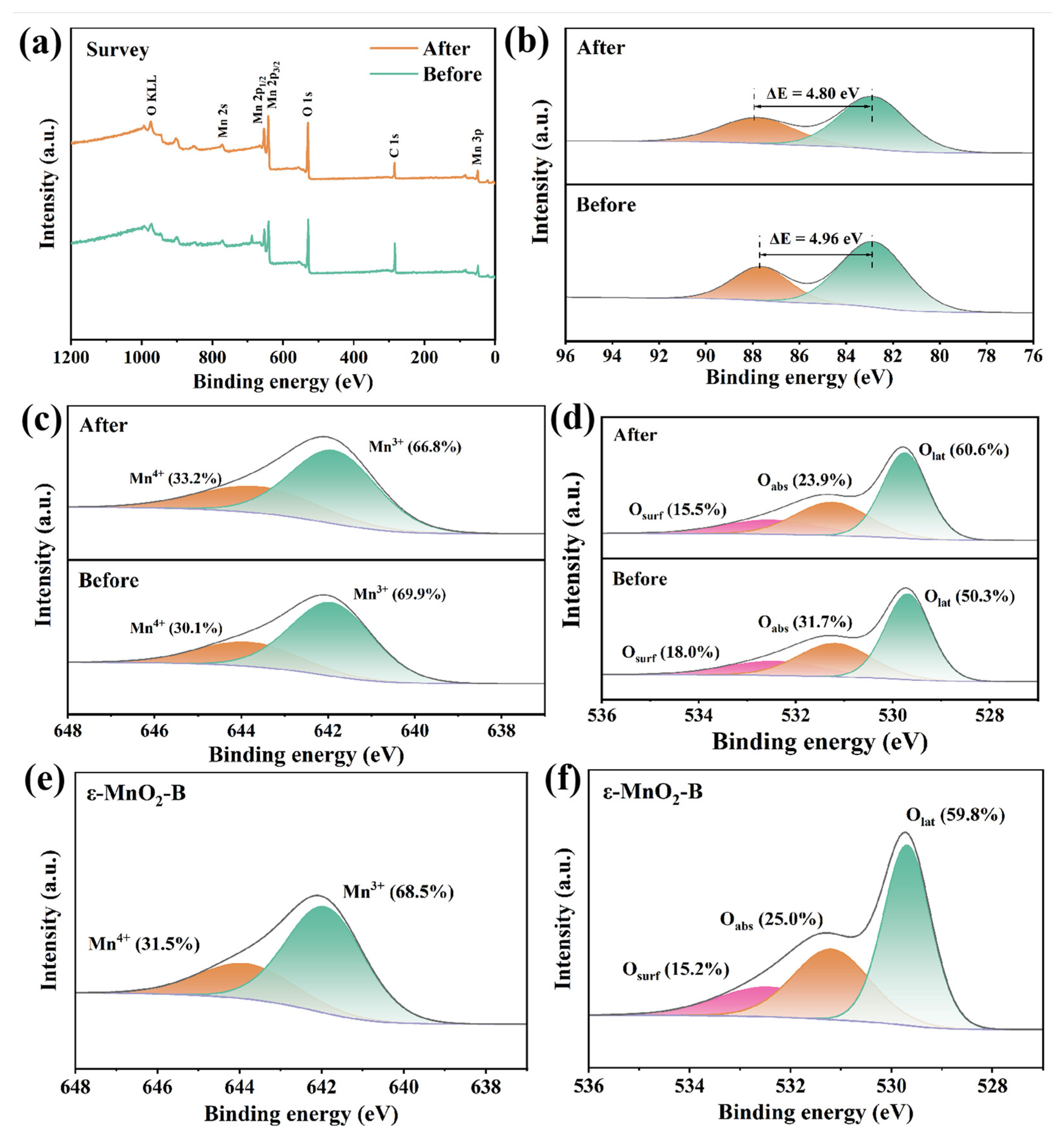
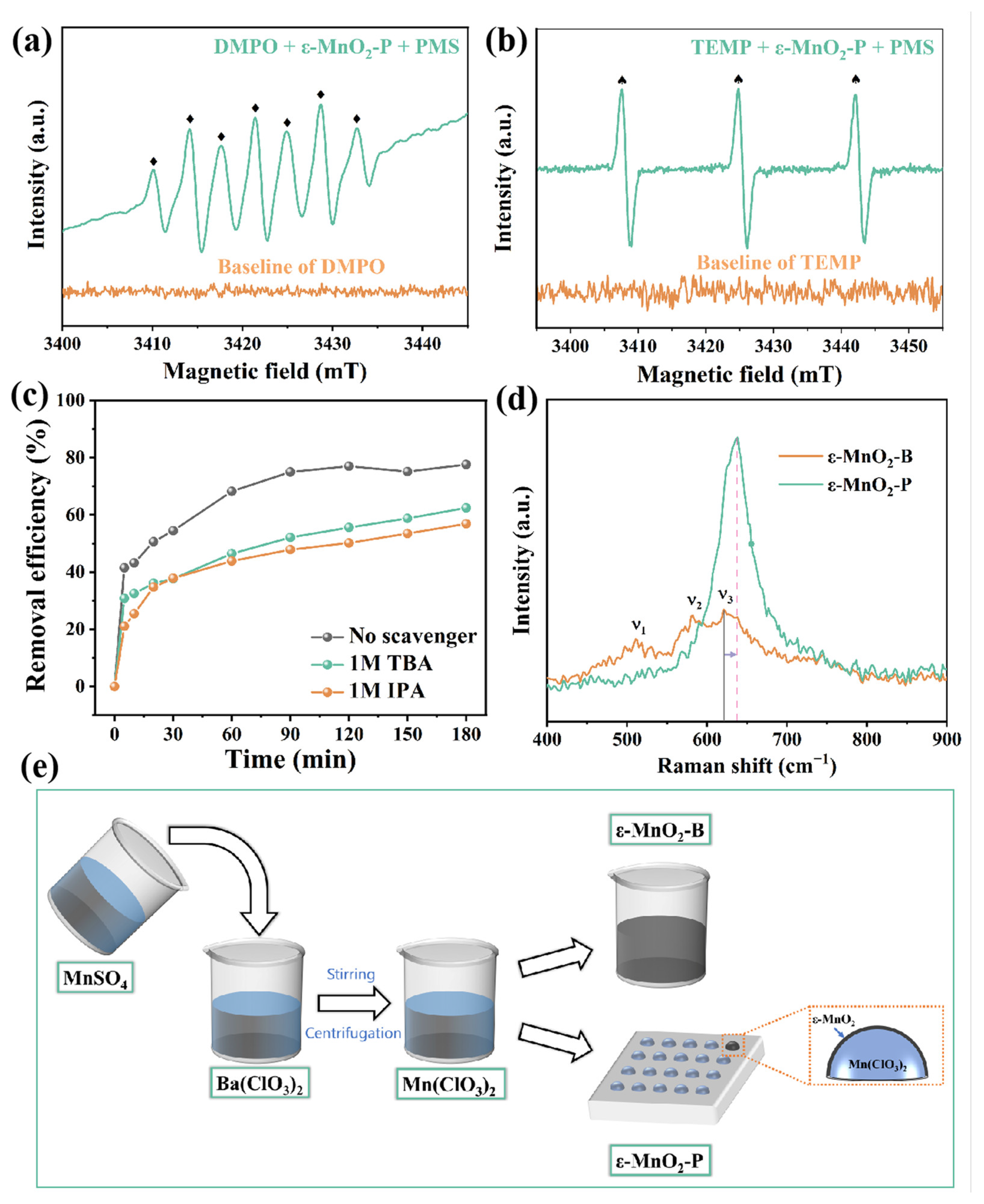

| Sample | Reaction Condition | Adsorption (within 30 min) | Removal Efficiency (within 120 min) |
|---|---|---|---|
| ε-MnO2-P + PMS | Dried with a hydrophobic interface | 26.2% | 84.1% |
| ε-MnO2-B + PMS | Dried with a hydrophilic interface | 4.68% | 43.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chi, Y.; Wu, X.; Lin, C.; Lin, T.; Gao, M.; Zhao, C.; Sa, B. O-Vacancy-Rich ε-MnO2 Synthesized at Hydrophobic Interface: An Efficient Fenton-like Catalyst for Removing Ciprofloxacin from Water. Crystals 2023, 13, 1664. https://doi.org/10.3390/cryst13121664
Chen Y, Chi Y, Wu X, Lin C, Lin T, Gao M, Zhao C, Sa B. O-Vacancy-Rich ε-MnO2 Synthesized at Hydrophobic Interface: An Efficient Fenton-like Catalyst for Removing Ciprofloxacin from Water. Crystals. 2023; 13(12):1664. https://doi.org/10.3390/cryst13121664
Chicago/Turabian StyleChen, Yulong, Yuan Chi, Xiao Wu, Cong Lin, Tengfei Lin, Min Gao, Chunlin Zhao, and Baisheng Sa. 2023. "O-Vacancy-Rich ε-MnO2 Synthesized at Hydrophobic Interface: An Efficient Fenton-like Catalyst for Removing Ciprofloxacin from Water" Crystals 13, no. 12: 1664. https://doi.org/10.3390/cryst13121664





