Preparation and Properties of RDX@FOX-7 Composites by Microfluidic Technology
Abstract
:1. Introduction
2. Experimental Details
2.1. Raw Materials
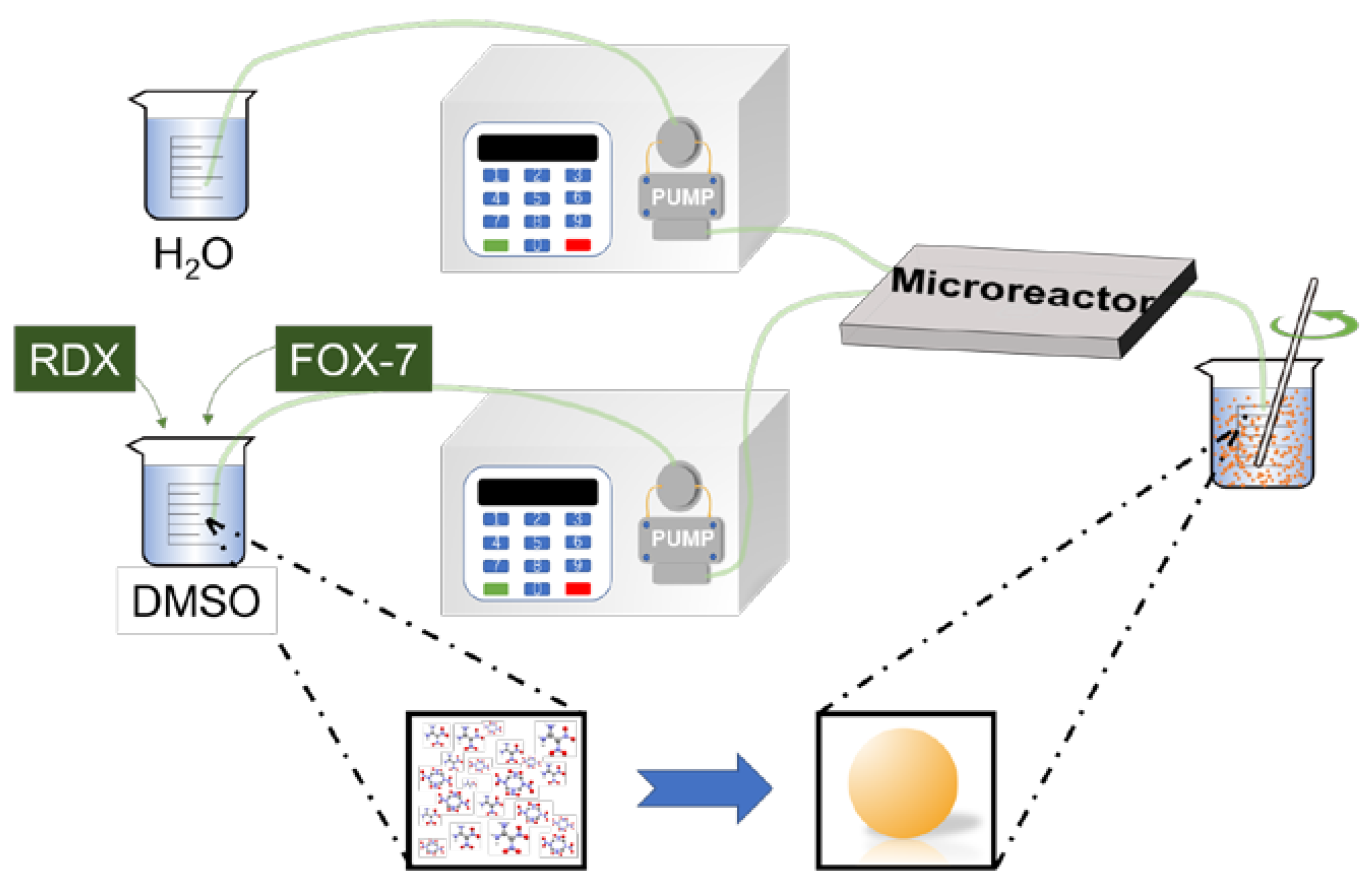
2.2. Preparation of RDX@FOX-7 Composite
2.3. Characterization Methods
3. Results and Discussion
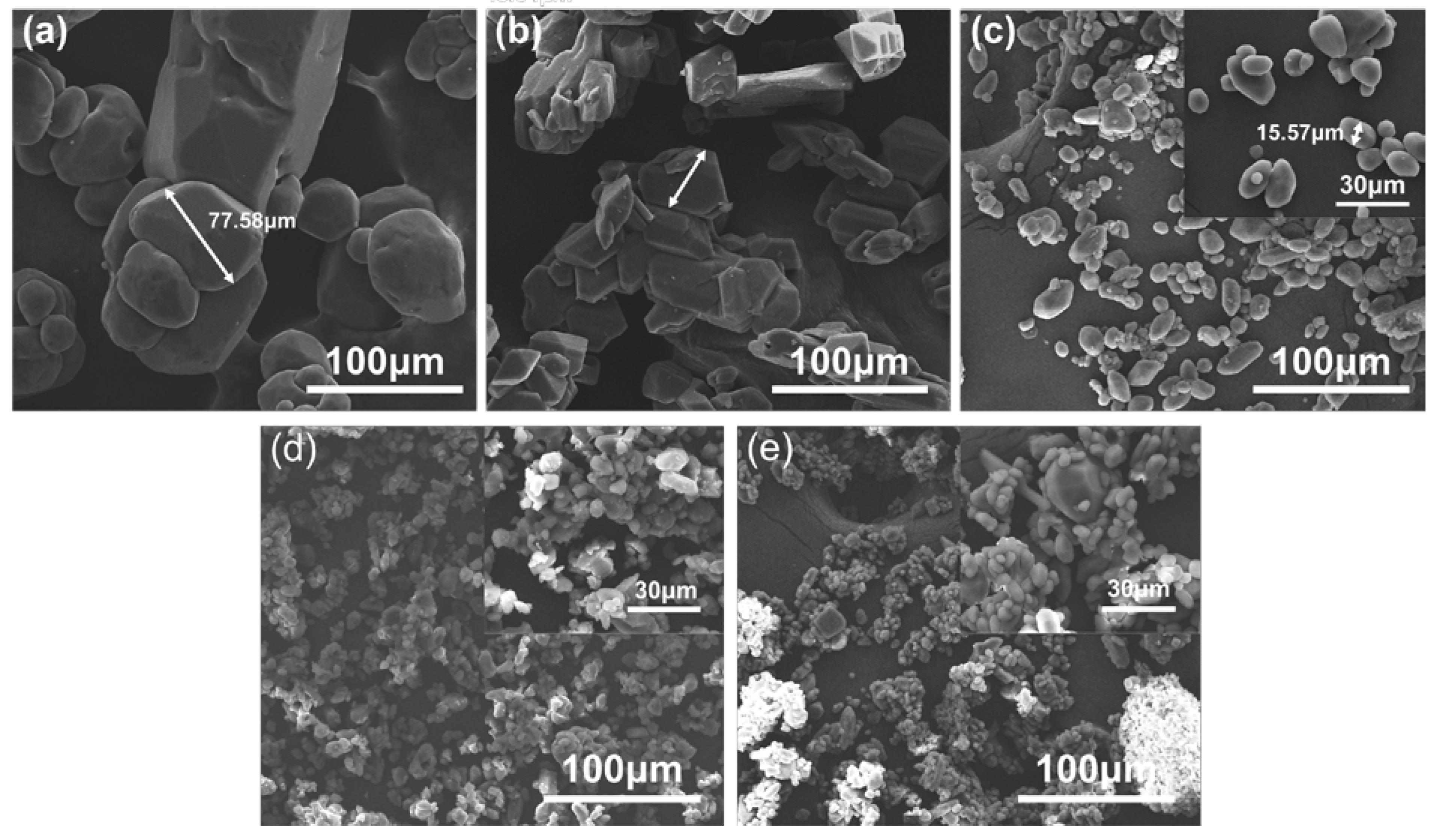
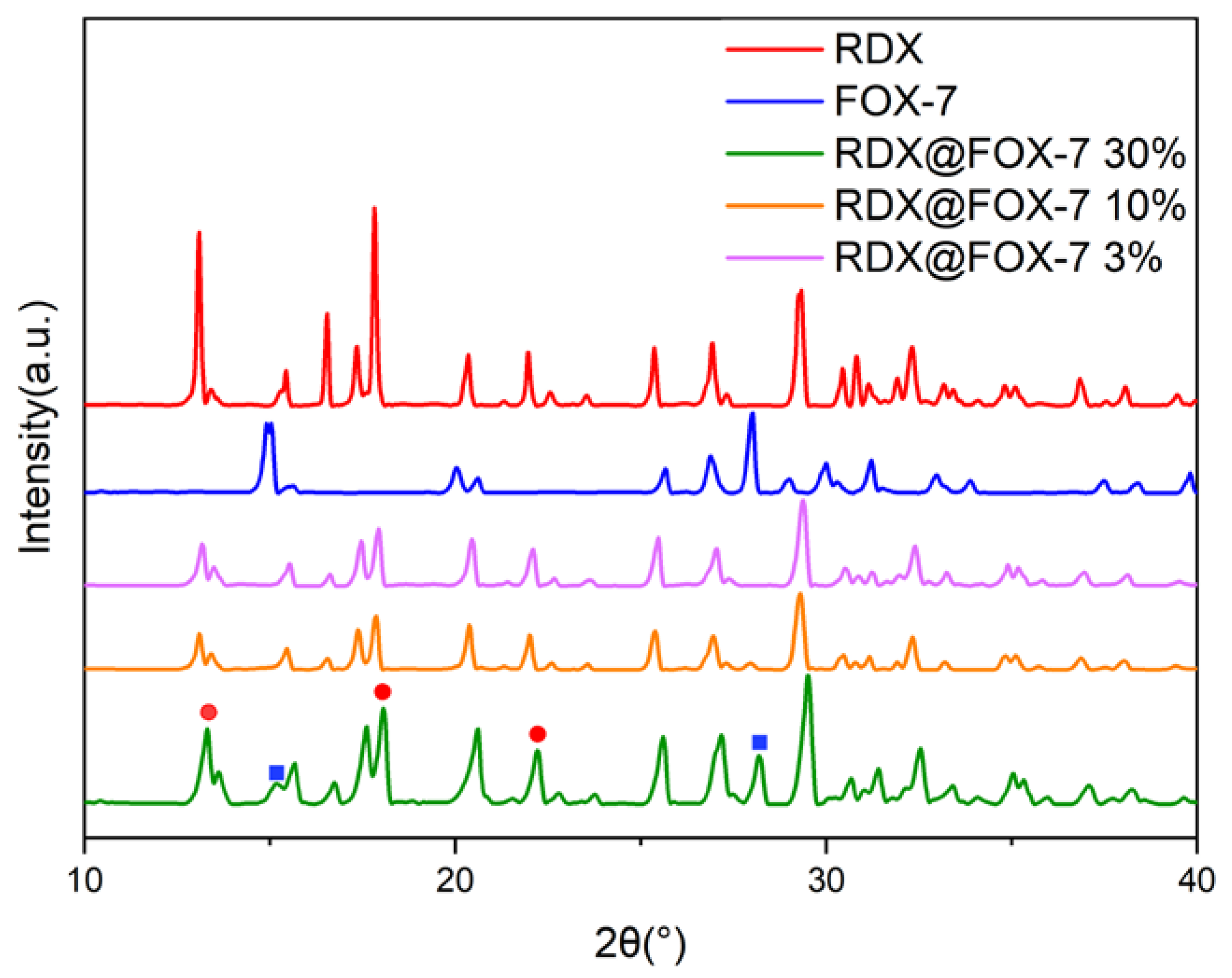
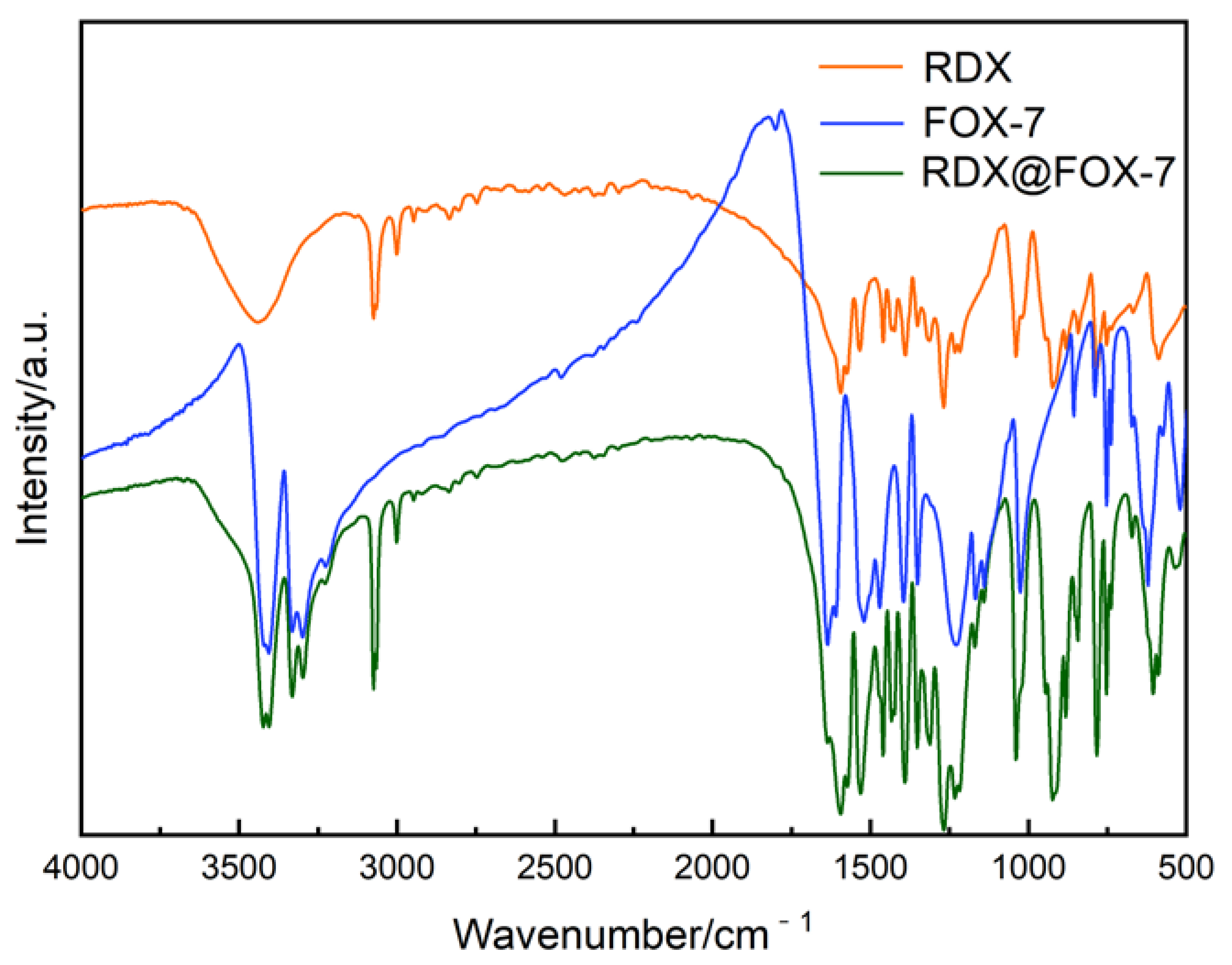
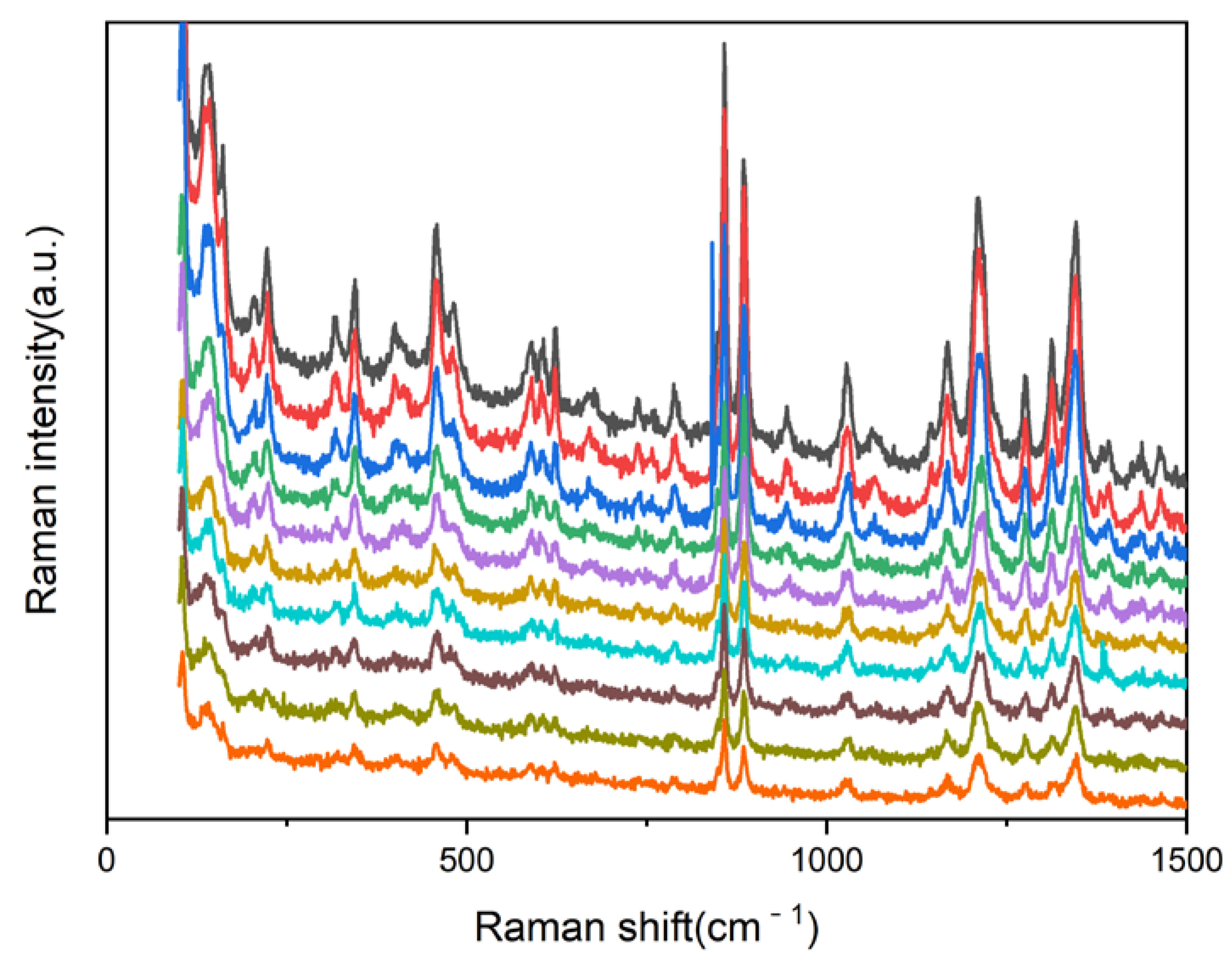
3.1. Morphological and Structure Analysis
3.2. Thermal Behavior of RDX@FOX-7 Composite
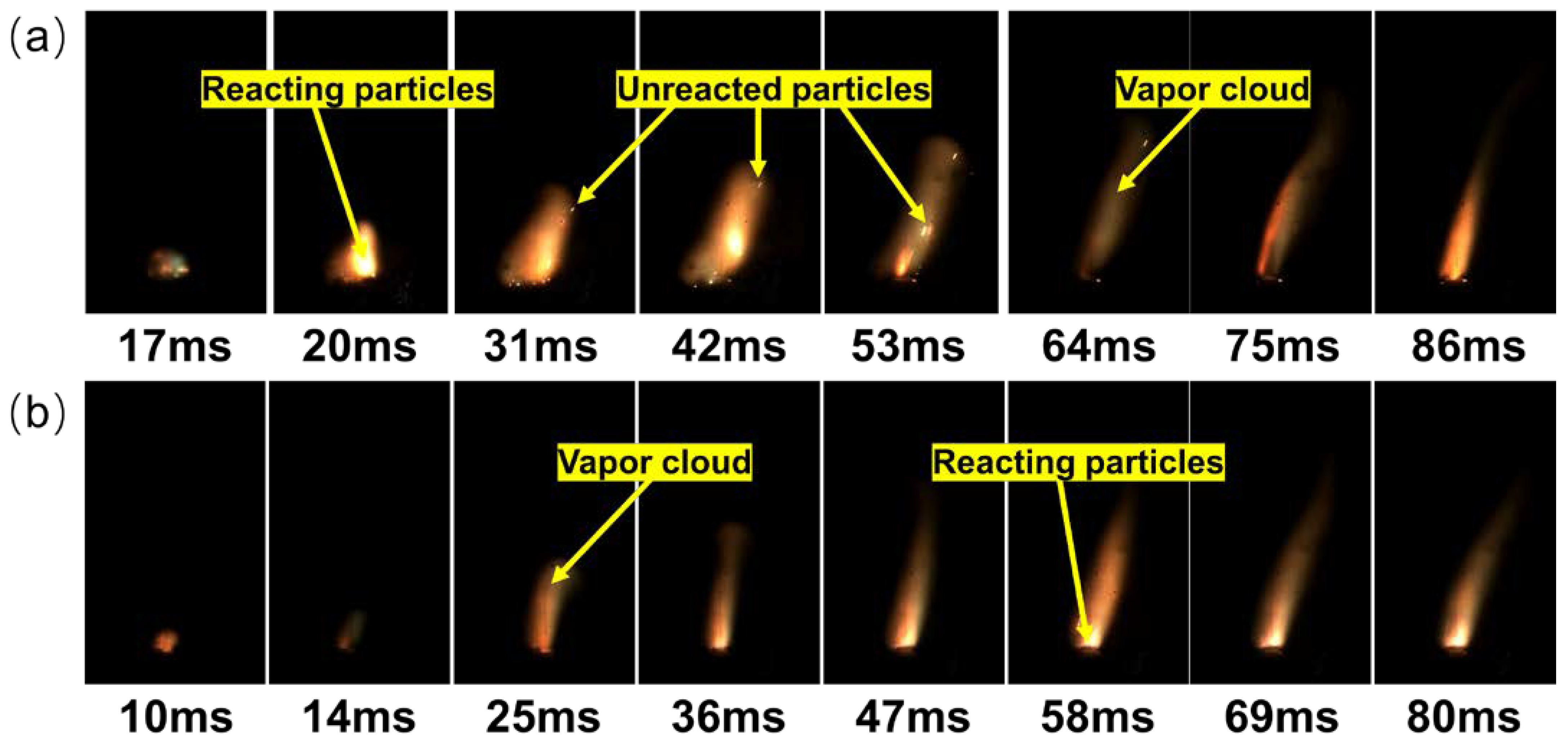
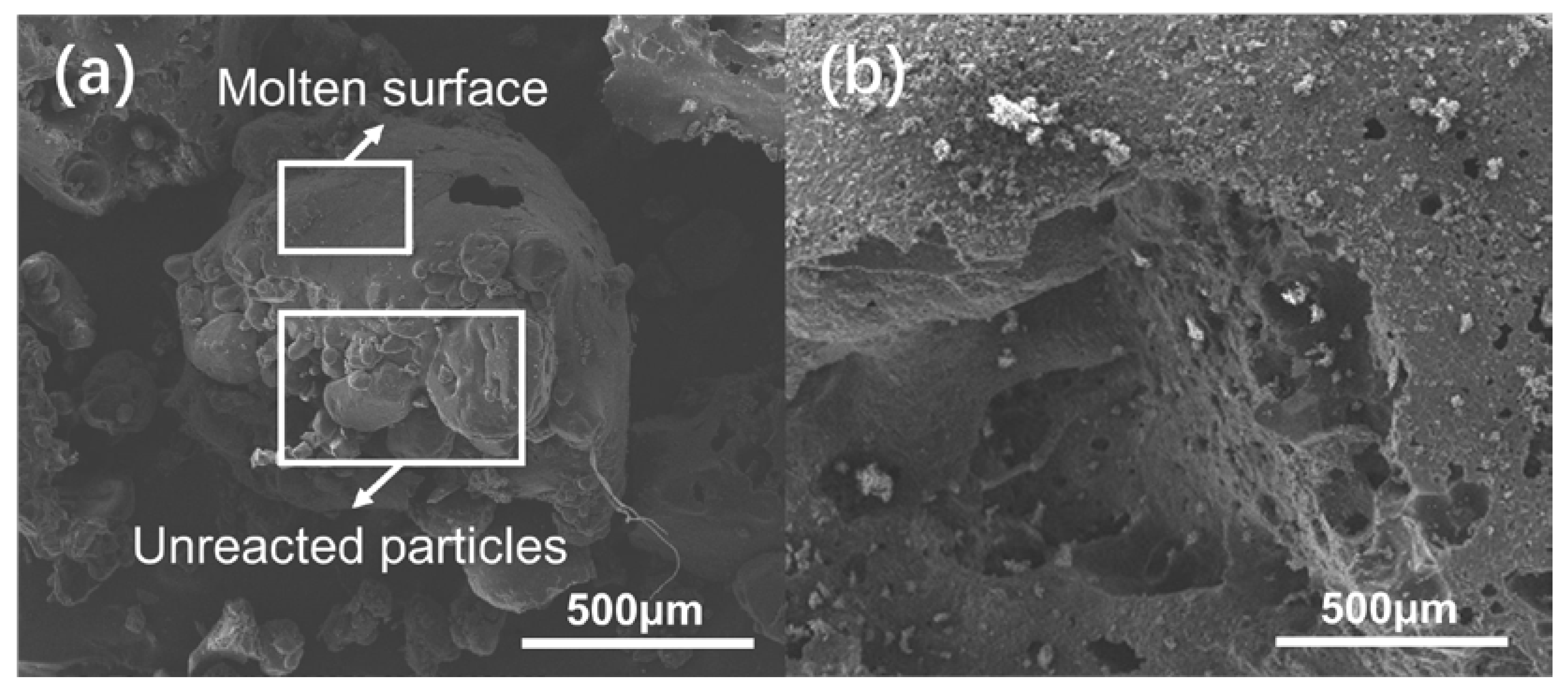
3.3. Ignition and Combustion Properties of RDX@FOX-7 Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, X.; Hou, C.; Wang, J.; Tan, Y.; Zhang, Y.; Li, C. Research Progress on the Desensitization Technology of Nitramine Explosives. Chin. J. Explos. Propellants 2018, 41, 326–333. [Google Scholar]
- Liu, J.; Jiang, W.; Yang, Q.; Song, J.; Hao, G.-Z.; Li, F.-S. Study of nano-nitramine explosives: Preparation, sensitivity and application. Def. Technol. 2014, 10, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Sikder, A.; Sikder, N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard. Mater. 2004, 112, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Hu, Y.; Xu, L.; Liu, X.; Ma, Y.; Fu, M.; Wang, J.; Xu, J. Preparation and Molecular Dynamics Simulation of RDX/MUF Nanocomposite Energetic Microspheres with Reduced Sensitivity. Processes 2019, 7, 692. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Deng, J.; Liang, X.; Wang, J.; Li, H.; Guo, F.; Li, Y.; Yan, T.; Wang, J. Comparison of the effects of several binders on the combination properties of cyclotrimethylene trinitramine (RDX). Sci. Technol. Energ. Mater. 2021, 82, 29–37. [Google Scholar]
- Jia, X.; Cao, Q.; Guo, W.; Li, C.; Shen, J.; Geng, X.; Wang, J.; Hou, C. Synthesis, thermolysis, and solid spherical of RDX/PMMA energetic composite materials. J. Mater. Sci. Mater. Electron. 2019, 30, 20166–20173. [Google Scholar] [CrossRef]
- Li, N.; Xiao, L.; Jian, X.; Xu, F.; Zhou, W. Coating of RDX with GAP-based energetic polyurethane elastomer. J. Solid Rocket Technol. 2012, 35, 212–215. [Google Scholar]
- Zhang, J.; Zhang, J.; Wang, B.; Liu, S.; Zhang, T.; Zhou, D. Technology of Ultra-fine RDX Coating with Rapid Expansion of Supercritical Solution Method. Energ. Mater. 2011, 19, 147–151. [Google Scholar]
- Shi, X.; Wang, J.; Li, X.; Wang, J. Preparation and characterization of RDX-based composite energetic microspheres. Chin. J. Energ. Mater. 2015, 23, 428–432. [Google Scholar]
- Guo, C.; Jia, X.; Geng, X.; Wang, J.; Hou, C.; Tan, Y. Effect of high temperature water vapor on morphology and thermal decomposition properties of RDX/PMMA. J. Solid Rocket Technol. 2018, 41, 338–342. [Google Scholar]
- Klaumünzer, M.; Pessina, F.; Spitzer, D. Indicating Inconsistency of Desensitizing High Explosives against Impact through Recrystallization at the Nanoscale. J. Energ. Mater. 2017, 35, 375–384. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Song, C.; Zhang, X.; Yang, W.; Wang, J. Effect of binder on properties of Cl-20/Fox-7 based PBX. Chin. J. Explos. Propellant 2020, 43, 51–56. [Google Scholar]
- Lal, S.; Staples, R.J.; Jean’ne, M.S. FOX-7 based nitrogen rich green energetic salts: Synthesis, characterization, propulsive and detonation performance. Chem. Eng. J. 2023, 452, 139600. [Google Scholar] [CrossRef]
- Fangjian, S.; Ting, W.; Yinhua, M.; Meiheng, L. Theoretical study on several important decomposition paths of FOX-7 and its derivatives. Comput. Theor. Chem. 2022, 1217, 113895. [Google Scholar]
- Nazin, G.M.; Dubikhin, V.V.; Kazakov, A.I.; Nabatova, A.V.; Krisyuk, B.E.; Volkova, N.N.; Shastin, A.V. Kinetics of the Decomposition of 1,1-Diamino-2,2-Dinitroethylene (FOX-7). Part 4: Comparison of the Decomposition Reactions of FOX-7 and Its Diazacyclic Derivatives. Russ. J. Phys. Chem. B 2022, 16, 308–315. [Google Scholar] [CrossRef]
- Gao, H.F.; Zhang, S.H.; Ren, F.D.; Liu, F.; Gou, R.J.; Ding, X. Theoretical insight into the co-crystal explosive of 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane (CL-20)/1,1-diamino-2,2-dinitroethylene (FOX-7). Comput. Mater. Sci. 2015, 107, 33–41. [Google Scholar] [CrossRef]
- Wei, Y.; Ren, F.; Shi, W.; Zhao, Q. Theoretical insight into the influences of molecular ratios on stabilities and mechanical properties, solvent effect of HMX/FOX-7 cocrystal explosive. J. Energ. Mater. 2016, 34, 426–439. [Google Scholar] [CrossRef]
- Tian, X.; Huang, Y.; Wang, X.; Xu, H.; Li, W.; Zhao, K.; Yang, H.; Li, Y. Influence of Mixture Ratio of FOX-7 and RDX on Slow Cook-off and Shock Sensitivity of Pressed Explosives. Explos. Mater. 2019, 48, 38–41. [Google Scholar]
- Zhou, C.; Chang, P.; Hu, L.; Li, X.; Xiong, C.; Wang, B. Preparation, Characterization and Properties of FOX-7/RDX Composite Explosive. Chin. J. Explos. Propellant 2020, 43, 669–673+680. [Google Scholar]
- Zhou, N.; Zhu, P.; Rong, Y.; Xia, H.; Shen, R.; Ye, Y.; Lv, S. Microfluidic Synthesis of Size-Controlled and Morphologically Homogeneous Lead Trinitroresorcinate Produced by Segmented Flow. Propellants Explos. Pyrotech. 2016, 41, 899–905. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, S.; Jiang, H.; Xu, S.; Zhao, F.; Shen, R.; Ye, Y.; Zhu, P. Multi-size control of homogeneous explosives by coaxial microfluidics. React. Chem. Eng. 2021, 6, 2354–2363. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Pan, J.; Fang, Q. Research progress of microfluidic technology in synthesis of micro/nano materials. Acta Chim. Sinica 2021, 79, 809–819. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, B.; Wang, M.; Liu, S.; Xie, Z.; An, C.; Wang, J. Accurate and efficient droplet microfluidic strategy for controlling the morphology of energetic microspheres. J. Energ. Mater. 2021, 1–18. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, P.; Zhang, Q.; Shi, J.; Wang, K.; Shen, R. Microcrystalline PETN Prepared Using Microfluidic Recrystallization Platform and Its Performance Characterization. Propellants Explos. Pyrotech. 2021, 46, 1097–1106. [Google Scholar] [CrossRef]
- Yu, J.; Xu, S.Y.; Jiang, H.Y.; Zhao, F.Q. Application and Development Trend of Microfluidic Technology in Preparation of Energetic Materials. Acta Pyrodynamites 2021, 6, 2354–2363. [Google Scholar]
- Wu, J.W.; Xia, H.M.; Zhang, Y.Y.; Zhao, S.F.; Zhu, P.; Wang, Z.P. An efficient micromixer combining oscillatory flow and divergent circular chambers. Microsyst. Technol. 2018, 25, 2741–2750. [Google Scholar] [CrossRef]
- Han, R.; Chen, J.; Zhang, F.; Wang, Y.; Zhang, L.; Lu, F.; Wang, H.; Chu, E. Fabrication of microspherical Hexanitrostilbene (HNS) with droplet microfluidic technology. Powder Technol. 2021, 379, 184–190. [Google Scholar] [CrossRef]
- Yazhi, C.; Qian, W.; Hui, R.; Tao, Y. Preparation and Characterization of nAl@PVDF@CL⁃20 Composite Energetic Particles Assembled via Microfluidic Method. Chin. J. Energ. Mater. 2022, 30, 1–9. [Google Scholar]
- Ruishan, H.; Fang, Z.; Feipeng, L.; Yanlan, W.; Lei, Z.; Jianhua, C.; Rui, Z.; Haifu, W.; Enyi, C. Preparation and Modification Technology of Lead Azide Primary Explosive Based on Microfluidics. Chin. J. Energ. Mater. 2022, 30, 451–458. [Google Scholar]
- Yipeng, F.; Jinyu, S.; Peng, Z.; Ruiqi, S.; Bin, Y.; Anmin, Y.; Enyi, C. Microscale Continuous Flow Preparation and Characterization of Ultra Zr@NC. Chin. J. Energ. Mater. 2022, 30, 417–423. [Google Scholar]
- Jinqiang, Z.; Kai, L.; Yunyan, G.; Rui, Z.; Jiahui, S.; Bidong, W.; Chongwei, A.; Jingyu, W. Preparation of TATB-based PBX Composite Microspheres by Droplet Microfluidic Technology. Chin. J. Energ. Mater. 2022, 30, 439–445. [Google Scholar]
- Li, L.; Ling, H.; Tao, J.; Pei, C.; Duan, X. Microchannel-confined crystallization: Shape-controlled continuous preparation of a high-quality CL-20/HMX cocrystal. Cryst. Eng. Comm. 2022, 24, 1523–1528. [Google Scholar] [CrossRef]
- Li, L.; Yin, T.; Wu, B.; Duan, X.; Pei, C. Preparation of CL-20/HMX Co-crystal by Microchannel Crystallization Based on Solvent/non-solvent Method. Chin. J. Energ. Mater. 2021, 29, 62–69. [Google Scholar]

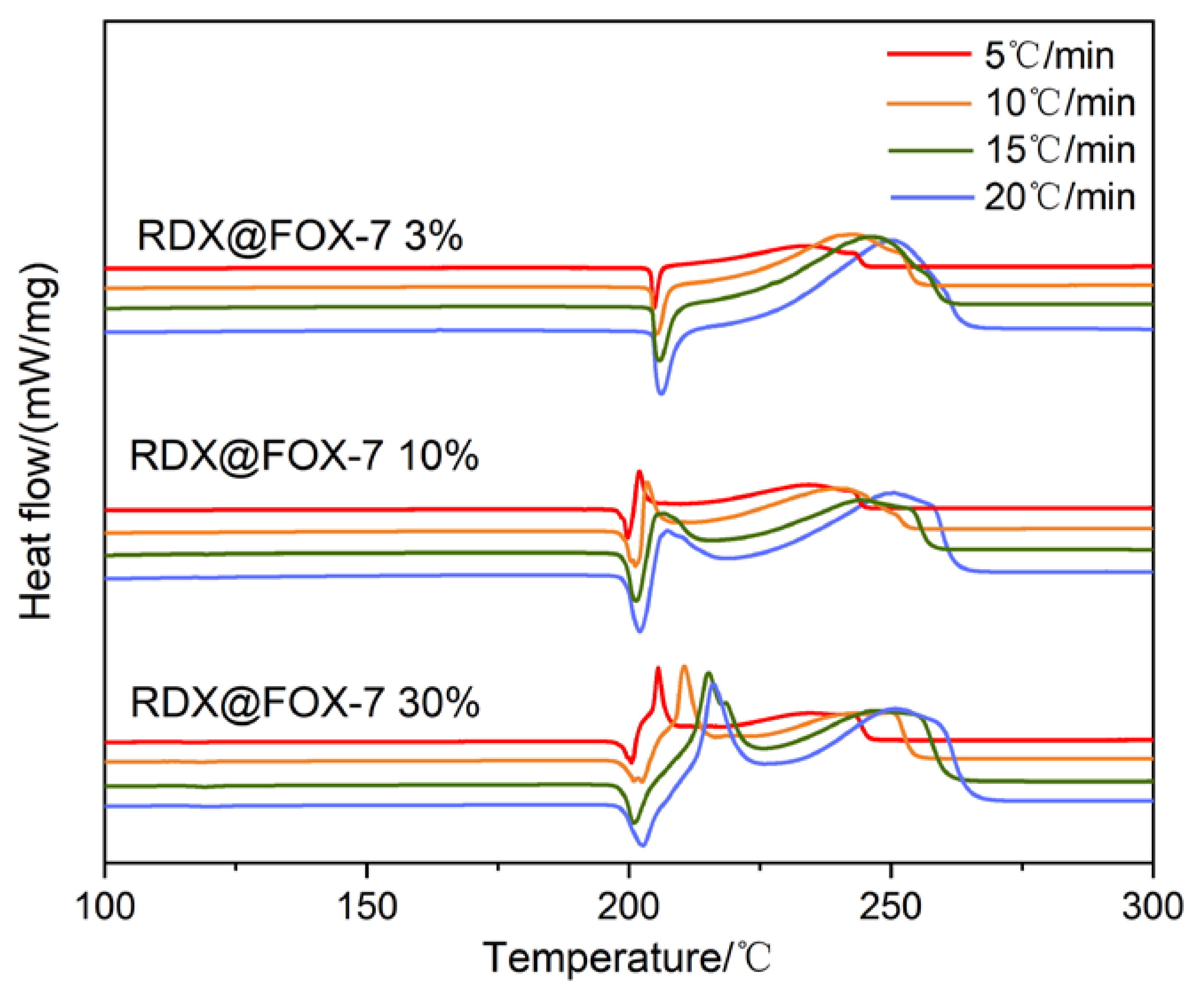


| Sample | Tp/°C | EO kJ/mol | EK kJ/mol | EF kJ/mol | ES kJ/mol | |||
|---|---|---|---|---|---|---|---|---|
| 5 °C/min | 10 °C/min | 15 °C/min | 20 °C/min | |||||
| RDX@FOX-7 3% | 233.7 | 242.8 | 245.7 | 250.1 | 180.43 | 181.19 | 186.51 | 181.38 |
| RDX@FOX-7 10% | 234.2 | 239.7 | 244.3 | 250.5 | 178.36 | 179.00 | 185.40 | 179.19 |
| RDX@FOX-7 30% | 230.8 | 240.1 | 248.4 | 251.7 | 132.37 | 134.01 | 138.20 | 132.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Jiang, H.; Xu, S.; Li, H.; Wang, Y.; Yao, E.; Pei, Q.; Li, M.; Zhang, Y.; Zhao, F. Preparation and Properties of RDX@FOX-7 Composites by Microfluidic Technology. Crystals 2023, 13, 167. https://doi.org/10.3390/cryst13020167
Yu J, Jiang H, Xu S, Li H, Wang Y, Yao E, Pei Q, Li M, Zhang Y, Zhao F. Preparation and Properties of RDX@FOX-7 Composites by Microfluidic Technology. Crystals. 2023; 13(2):167. https://doi.org/10.3390/cryst13020167
Chicago/Turabian StyleYu, Jin, Hanyu Jiang, Siyu Xu, Heng Li, Yiping Wang, Ergang Yao, Qing Pei, Meng Li, Yang Zhang, and Fengqi Zhao. 2023. "Preparation and Properties of RDX@FOX-7 Composites by Microfluidic Technology" Crystals 13, no. 2: 167. https://doi.org/10.3390/cryst13020167
APA StyleYu, J., Jiang, H., Xu, S., Li, H., Wang, Y., Yao, E., Pei, Q., Li, M., Zhang, Y., & Zhao, F. (2023). Preparation and Properties of RDX@FOX-7 Composites by Microfluidic Technology. Crystals, 13(2), 167. https://doi.org/10.3390/cryst13020167








