Investigation of Hafnium Oxide Containing Zirconium in the Scaled Region on the Surface of As-Cast Nickel-Based Single Crystal Superalloy Turbine Blades
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Microstructural Characterisation
3. Results
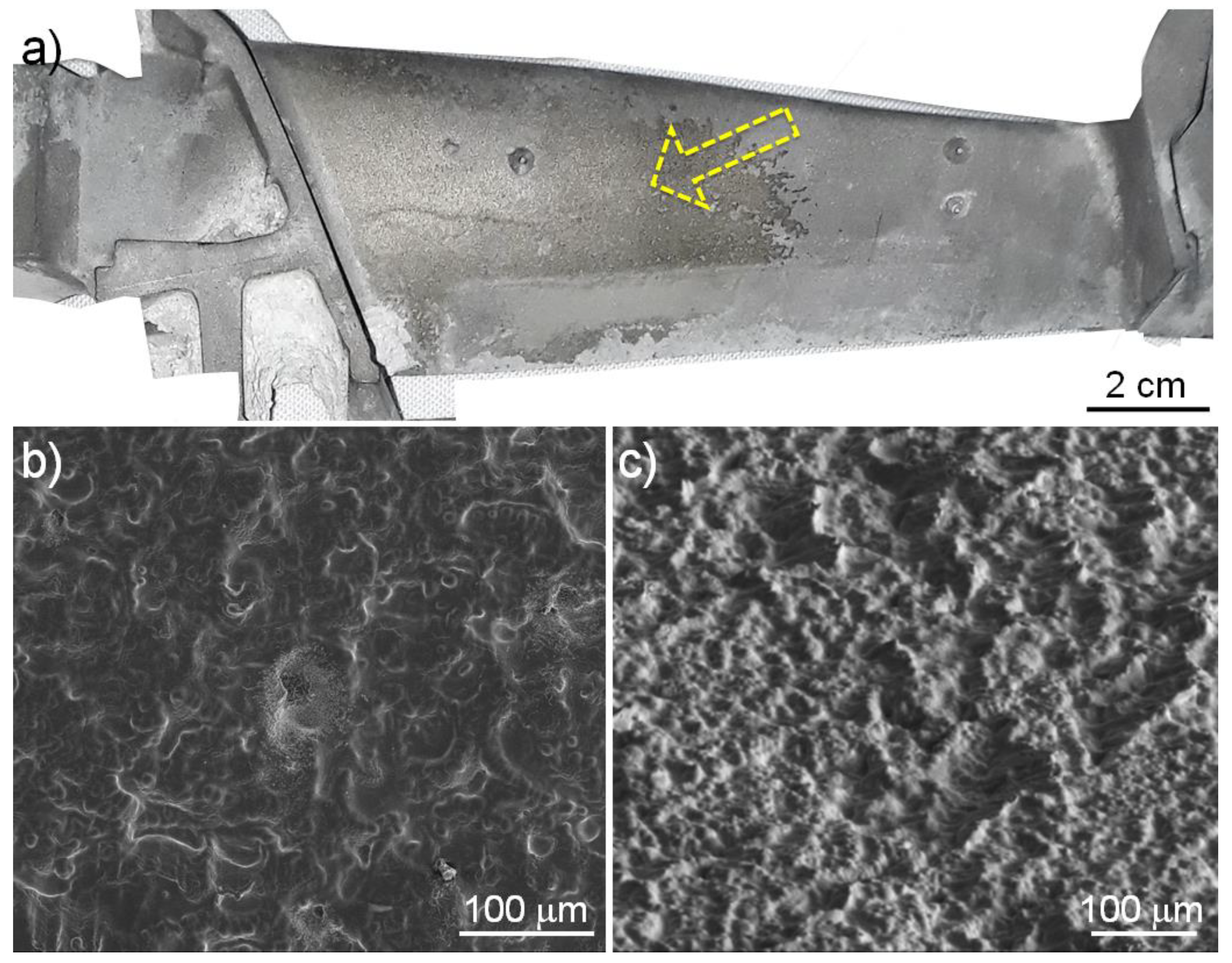
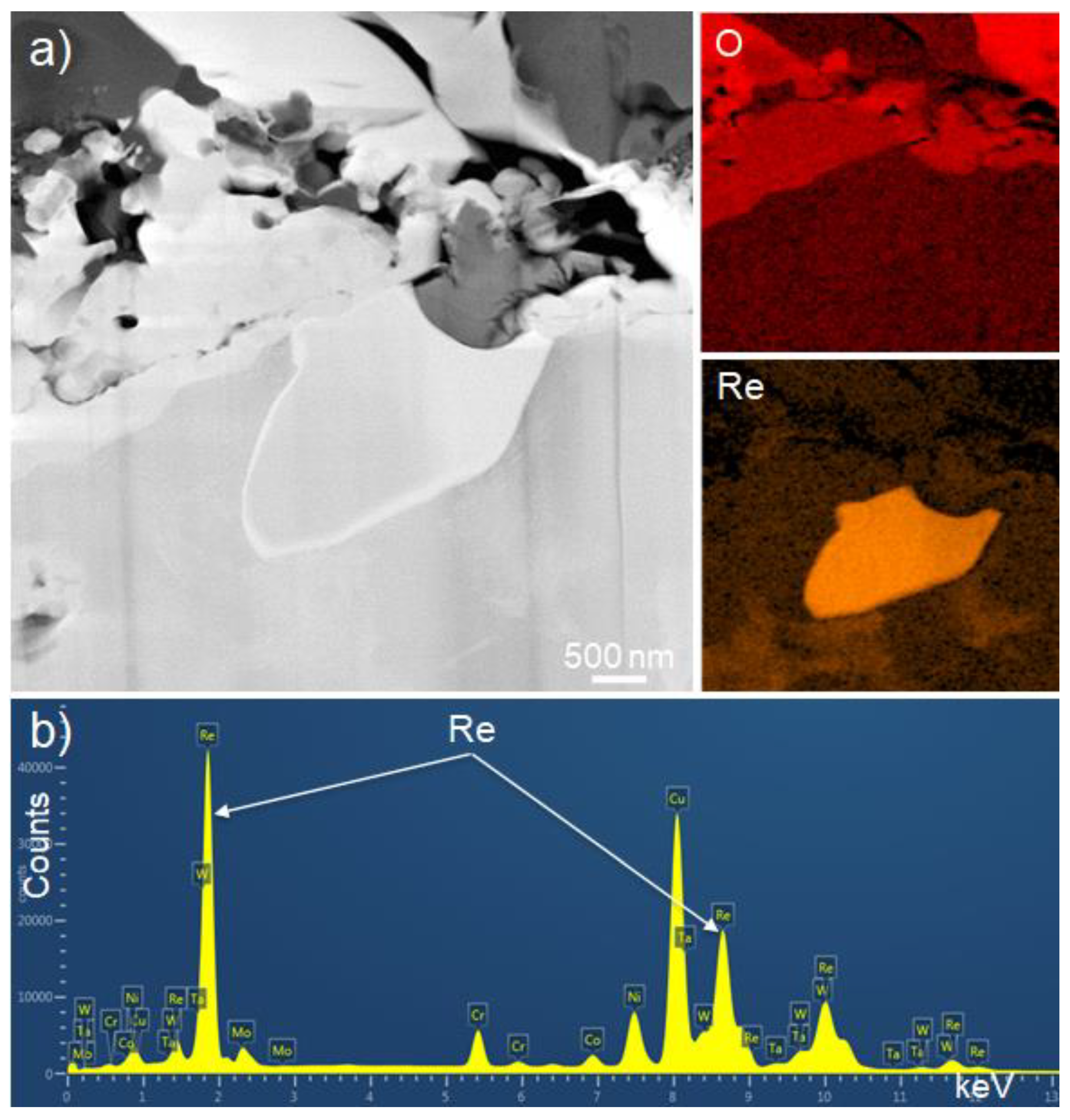
3.1. Formation of a Scaled Region and Detection of Particles Containing Hafnium
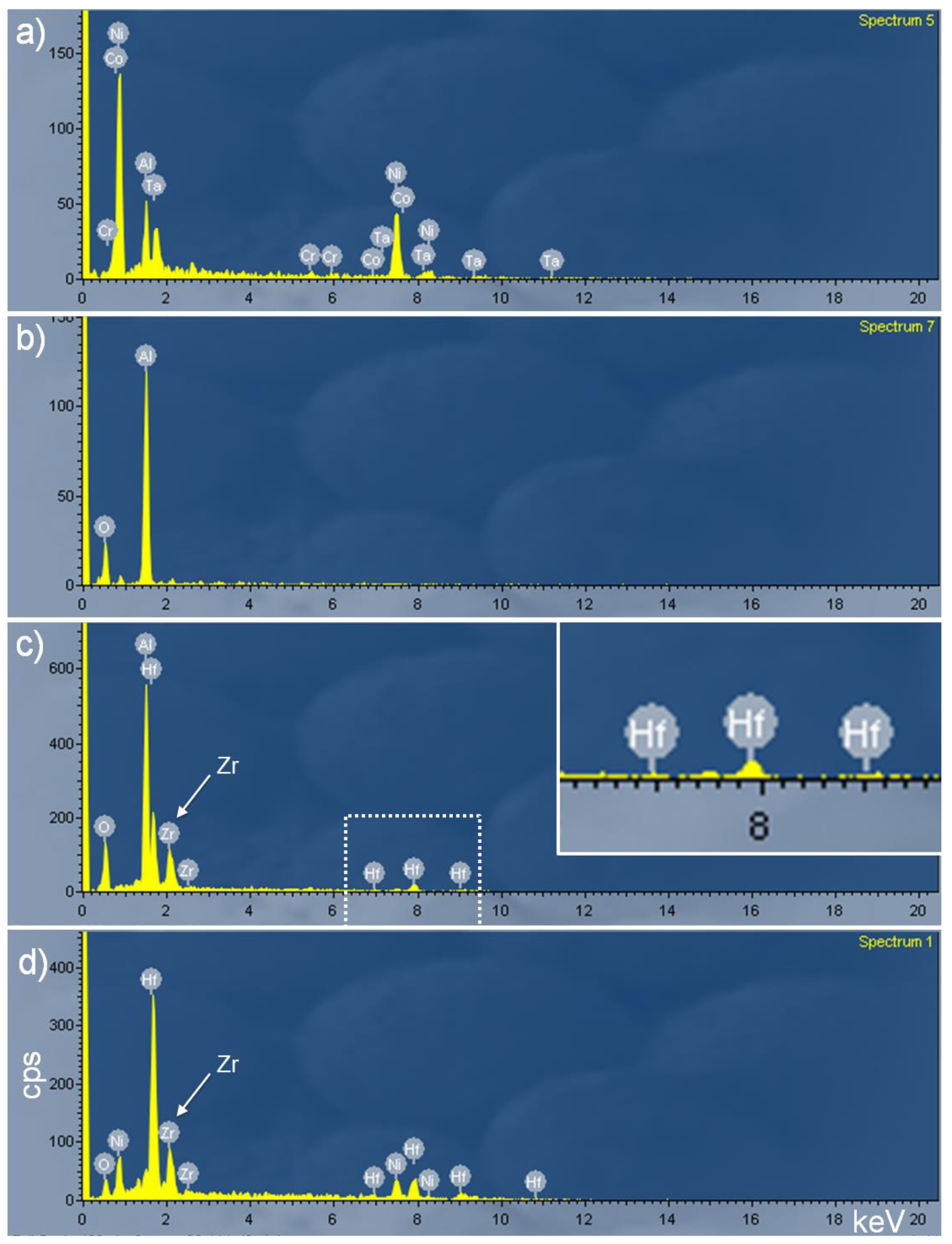
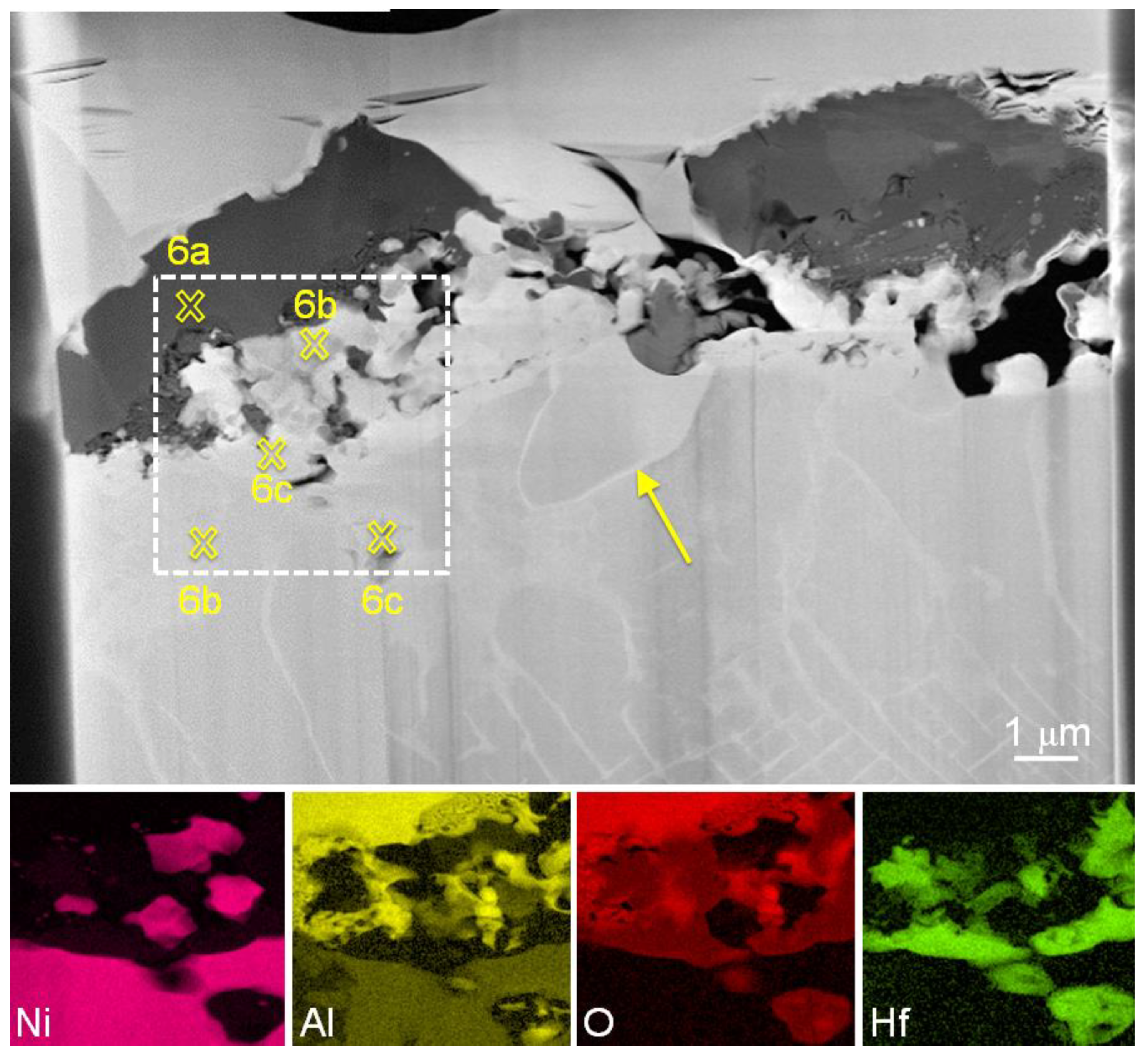
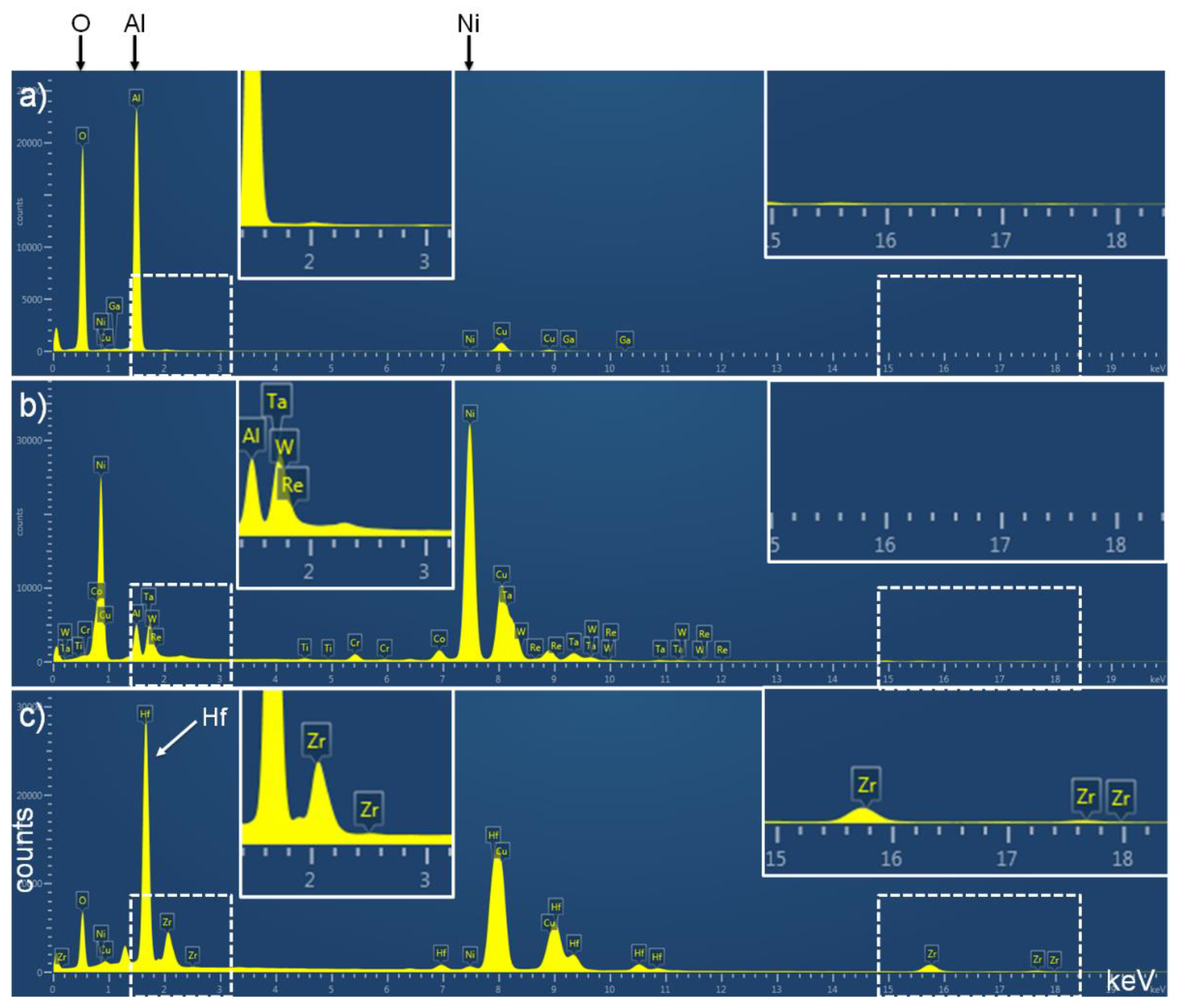
3.2. Detailed Analysis of Particles Containing Hafnium
4. Discussion
4.1. Formation of the Scaled Region
4.2. Formation of Hafnium Oxide Containing Zirconium on the Surface of a Scaled Region
- That the solubility of hafnium in γ and γ′ phases is low;
- That the concentration of hafnium is extremely low in the raw material;
- That the scaled region where the hafnium oxide is usually detected, solidifies late in the process.
4.2.1. Possibility of Formation of Hafnium Oxide by the Diffusion of Hafnium and Zirconium
4.2.2. Possibility of a Reaction of Hafnium with Mould Wall Materials
4.2.3. Formation of Hafnium Oxide Containing Zirconium
4.3. Meaning of the Detection of Hafnium Oxide Containing Zirconium on the Surface of a Scaled Region
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006; pp. 33–86. [Google Scholar]
- Geddes, B.; Leon, H.; Huang, X. Superalloys: Alloying and Performance; ASM International: Materials Park, OH, USA, 2010; pp. 1–109. [Google Scholar]
- Durand-Charre, M. The Microstructure of Superalloys; OPA: Amsterdam, The Netherlands, 1997; pp. 1–12. [Google Scholar]
- Bar-Cohen, Y. High Temperature Materials and Mechanisms; CRC Press: Boca Raton, FL, USA, 2014; pp. 110–117. [Google Scholar]
- Kotval, P.S.; Venables, J.D.; Calder, R.W. The role of hafnium in modifying the microstructure of cast nickel-base superalloys. Metall. Mater. Trans. B 1972, 3, 457–462. [Google Scholar] [CrossRef]
- Amouyal, Y.; Seidman, D.N. The role of hafnium in the formation of misoriented defects in Ni-based superalloys: An atom-probe tomographic study. Acta Mater. 2011, 59, 3321–3333. [Google Scholar] [CrossRef]
- Hou, J.S.; Guo, J.T.; Wu, Y.X.; Zhou, L.Z.; Ye, H.Q. Effect of hafnium on creep behavior of a corrosion resistant nickel base superalloy. Mater. Sci. Eng. A 2010, 527, 1548–1554. [Google Scholar] [CrossRef]
- Brewster, G.; D’Souza, N.; Ryder, K.S.; Simmonds, S.; Dong, H.B. Mechanism for Formation of Surface Scale during Directional Solidification of Ni-Base Superalloys. Metall. Mater. Trans. A 2012, 43, 1288–1302. [Google Scholar] [CrossRef]
- Panwisawas, C.; Mathur, H.; Gebelin, J.C.; Putman, D.; Rae, C.M.F.; Reed, R.C. Prediction of recrystallization in investment cast single-crystal superalloys. Acta Mater. 2013, 61, 51–66. [Google Scholar] [CrossRef]
- Mathur, H.N.; Panwisawas, C.; Jones, C.N.; Reed, R.C.; Rae, C.M.F. Nucleation of recrystallisation in castings of single crystal Ni-based superalloys. Acta Mater. 2017, 129, 112–123. [Google Scholar] [CrossRef]
- Brewster, G.; Dong, H.B.; Green, N.R.; D’Souza, N. Surface Segregation during Directional Solidification of Ni-Base Superalloys. Metall. Mater. Trans. B 2008, 39, 87–93. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The formation of aluminum oxide scales on high-temperature alloys. Oxid. Met. 1992, 38, 233–254. [Google Scholar] [CrossRef]
- Simmonds, S.; Souza, N.D.; Ryder, K.S.; Dong, H. Analysis of surface scale on the Ni-based superalloy CMSX-10N and proposed mechanism of formation. IOP Conf. Series: Mater. Sci. Eng. 2012, 27, 012038. [Google Scholar] [CrossRef]
- Ul-Hamid, A. TEM Study of Scale Microstructures Formed on Ni–10Cr and Ni–10Cr–5Al Alloys with and Without Y Addition. Oxid. Met. 2002, 58, 41–56. [Google Scholar] [CrossRef]
- Park, K.; Withey, P. Formation and Prevention of a Surface Defect on the Aerofoil of As-Cast Nickel-Based Single-Crystal Turbine Blades. Adv. Eng. Mater. 2021, 23, 2100302. [Google Scholar] [CrossRef]
- Park, K.; Withey, P. Formation of Secondary Phases in the Boundary between Surface Defect Grains and Matrix in Third Generation Nickel-Based Single Crystal Superalloy Turbine Blades. In Thermec 2018: 10th International Conference on Processing and Manufacturing of Advanced Materials; Shabadi, R., Ionescu, M., Jeandin, M., Richard, C., Chandra, T., Eds.; Trans Tech Publications: Bäch, Switzerland, 2018; Volume 941, pp. 766–771. [Google Scholar]
- Kim, K.; Watanabe, M.; Kawakita, J.; Kuroda, S. Grain refinement in a single titanium powder particle impacted at high velocity. Scripta Mater. 2008, 59, 768–771. [Google Scholar] [CrossRef]
- Kim, K. Formation of Fine Clusters in High-Temperature Oxidation of Molten Aluminum. Metall. Mater. Trans. A 2014, 45, 3650–3660. [Google Scholar] [CrossRef]
- Kim, K.; Watanabe, M.; Kuroda, S.; Kawano, N. Observation of high resolution microstructures in thermal sprayed coatings and single deposited splats using ion beam milling. Mater. Trans. 2011, 52, 439–446. [Google Scholar] [CrossRef]
- Kim, K.; Watanabe, M.; Kawakita, J.; Kuroda, S. Effects of Temperature of In-flight Particles on Bonding and Microstructure in Warm-Sprayed Titanium Deposits. J. Therm. Spray Tech. 2009, 18, 392–400. [Google Scholar] [CrossRef]
- Kim, K.; Withey, P. Formation of an Intermediate Layer Between Grains in Nickel-Based Superalloy Turbine Blades. Metall. Mater. Trans. A 2017, 48, 2932–2942. [Google Scholar] [CrossRef]
- JEOL-Energy Table for EDS Analysis. Available online: www.jeol.com (accessed on 28 September 2013).
- Warren, P.J.; Cerezo, A.; Smith, G.D.W. An atom probe study of the distribution of rhenium in a nickel-based superalloy. Mater. Sci. Eng. A 1998, 250, 88–92. [Google Scholar] [CrossRef]
- Erickson, G.L. The development and application of CMSX (R)-10. In Superalloys 1996; Kissinger, R.D., Deye, D.J., Anton, D.L., Cetel, A.D., Nathal, M.V., Pollock, T.M., Woodford, D.A., Eds.; Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1996; pp. 35–44. [Google Scholar]
- Kim, K.; Withey, P.; Griffiths, W.D. Detection of an Intermediate Layer Containing a Rhenium-Rich Particle at Grain Boundaries Formed Within Single Crystal Nickel-Based Superalloys. Metall. Mater. Trans. A 2015, 46, 1024–1029. [Google Scholar] [CrossRef]
- Kim, K.; Withey, P. Microstructural Investigation of the Formation and Development of Topologically Close-Packed Phases in a 3rd Generation Nickel-Base Single Crystal Superalloy. Adv. Eng. Mater. 2017, 19, 1700041. [Google Scholar] [CrossRef]
- Kim, K. FIB serial milling and lifting out of fine inclusions in an intensively melt sheared aluminum alloy. Mater. Lett. 2014, 117, 74–77. [Google Scholar] [CrossRef]
- Shimizu, R.; Ze-Jun, D. Monte Carlo modelling of electron-solid interactions. Rep. Prog. Phys. 1992, 55, 487–531. [Google Scholar] [CrossRef]
- Demers, H.; Poirier-Demers, N.; Couture, A.R.; Joly, D.; Guilmain, M.; de Jonge, N.; Drouin, D. Three-dimensional electron microscopy simulation with the CASINO Monte Carlo software. Scanning 2011, 33, 135–146. [Google Scholar] [CrossRef]
- Park, K.; Withey, P. Compositions of Gamma and Gamma Prime Phases in an As-Cast Nickel-Based Single Crystal Superalloy Turbine Blade. Crystals 2022, 12, 299. [Google Scholar] [CrossRef]
- Yvon, H.J. Table of X-ray Emission Lines. Available online: www.horiba.com (accessed on 28 September 2022).
- Kim, K.; Withey, P. Detection of Rhenium-Rich Particles at Grain Boundaries in Nickel-Base Superalloy Turbine Blades. Mater. Trans. 2016, 57, 1698–1706. [Google Scholar] [CrossRef]
- Kim, K.; Withey, P. General View of Rhenium-Rich Particles along Defect Grain Boundaries Formed in Nickel-Based Single-Crystal Superalloy Turbine Blades: Formation, Dissolution and Comparison with Other Phases. Crystals 2021, 11, 1201. [Google Scholar]
- Barin, I. Thermochemical Data of Pure Substances; Wiley-VCH: New York, NY, USA, 1995. [Google Scholar]
- Duval, J.D.; Risbud, S.H.; Shackelford, J.F. Mullite. In Ceramic and Glass Materials; Shackelford, J., Doremus, R.H., Robert, H., Eds.; Springer: New York, NY, USA, 2008; pp. 27–39. [Google Scholar]
- Janotti, A.; Krčmar, M.; Fu, C.L.; Reed, R.C. Solute Diffusion in Metals: Larger Atoms Can Move Faster. Phys. Rev. Lett. 2004, 92, 085901. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.T.; Wallace, W. Impurities and trace elements in nickel-base superalloys. Int. Met. Rev. 1976, 21, 1–24. [Google Scholar] [CrossRef]
- Shuwei, M.; Yunrong, Z. Diffusion behaviour of hafnium in Ni and Ni3Al. J. Mater. Sci. Lett. 1997, 16, 1761–1763. [Google Scholar] [CrossRef]
- Takeda, O.; Uda, T.; Okabe, T.H. Rare Earth, Titanium Group Metals, and Reactive Metals Production. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 995–1069. [Google Scholar]


| Element | Raw | Full Heat Treated | Base * | Hf Oxide | Al Oxide | Re-Rich | |
|---|---|---|---|---|---|---|---|
| γ | γ′ | ||||||
| Al | 13.6 | 6.3 | 15.6 | 8.6 | - | 33.9 | 2.2 |
| Ti | 0.1 | 0.0 | 0.1 | 0.3 | - | - | 0.1 |
| Cr | 1.8 | 4.1 | 1.0 | 1.7 | - | - | 12.5 |
| Co | 3.2 | 5.9 | 2.7 | 3.1 | - | - | 4.7 |
| Nb | 0.0 ** | 0.0 | 0.0 | 0.0 | - | - | 0.5 |
| Mo | 0.3 | 0.3 | 0.0 | 0.1 | - | - | 3.6 |
| Hf | 0.0 *** | 0.0 | 0.0 | 0.1 | 36.7 | - | 0.1 |
| Ta | 3.0 | 3.6 | 4.6 | 4.6 | - | - | 1.2 |
| W | 1.9 | 3.4 | 2.4 | 0.9 | - | - | 7.6 |
| Re | 2.4 | 5.9 | 0.0 | 0.4 | - | - | 43.6 |
| O | - | - | - | - | 52.5 | 66.0 | - |
| Zr | - | - | - | - | 9.7 | - | - |
| Ni | Bal. | Bal. | Bal. | Bal. | 1.1 | 0.1 | Bal. |
| Reactions | ΔGf (at 1800 K, kJ/mol) |
|---|---|
| −295.189 | |
| −366.144 | |
| −412.522 | |
| −225.242 | |
| −381.782 | |
| −337.389 | |
| −70.955 | |
| −30.740 | |
| −46.378 | |
| −117.333 | |
| −75.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Withey, P. Investigation of Hafnium Oxide Containing Zirconium in the Scaled Region on the Surface of As-Cast Nickel-Based Single Crystal Superalloy Turbine Blades. Crystals 2023, 13, 277. https://doi.org/10.3390/cryst13020277
Park K, Withey P. Investigation of Hafnium Oxide Containing Zirconium in the Scaled Region on the Surface of As-Cast Nickel-Based Single Crystal Superalloy Turbine Blades. Crystals. 2023; 13(2):277. https://doi.org/10.3390/cryst13020277
Chicago/Turabian StylePark, KeeHyun, and Paul Withey. 2023. "Investigation of Hafnium Oxide Containing Zirconium in the Scaled Region on the Surface of As-Cast Nickel-Based Single Crystal Superalloy Turbine Blades" Crystals 13, no. 2: 277. https://doi.org/10.3390/cryst13020277
APA StylePark, K., & Withey, P. (2023). Investigation of Hafnium Oxide Containing Zirconium in the Scaled Region on the Surface of As-Cast Nickel-Based Single Crystal Superalloy Turbine Blades. Crystals, 13(2), 277. https://doi.org/10.3390/cryst13020277










