Structural and Spectroscopic Effects of Li+ Substitution for Na+ in LixNa1−xCaLa0.5Er0.05Yb0.45(MoO4)3 Upconversion Scheelite-Type Phosphors
Abstract
:1. Introduction
2. Experimental Methods
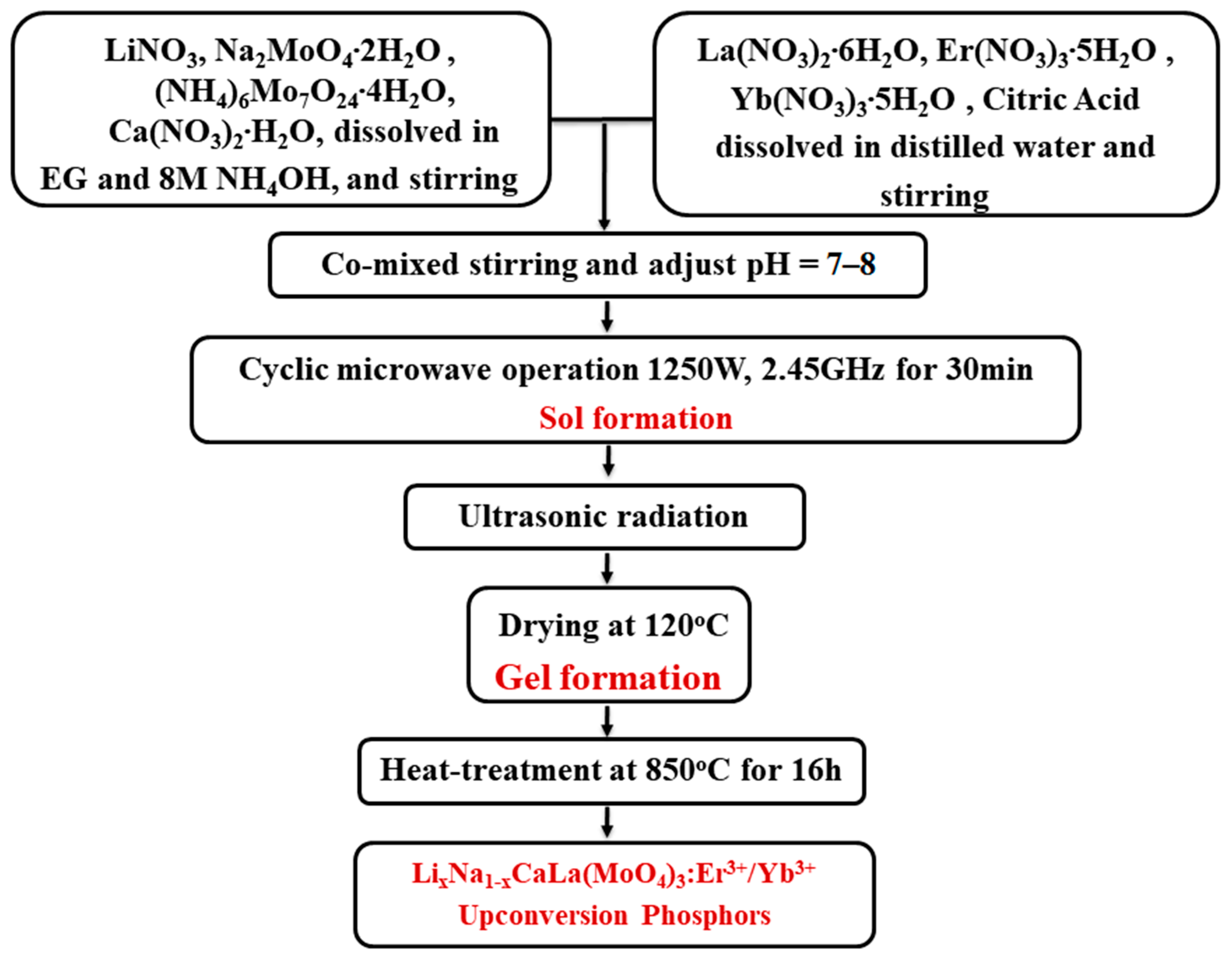
2.1. Preparation of Chemical Solutions and Phosphor Products
2.2. Characterization
2.3. Quantum-Chemical Calculations
3. Results and Discussion
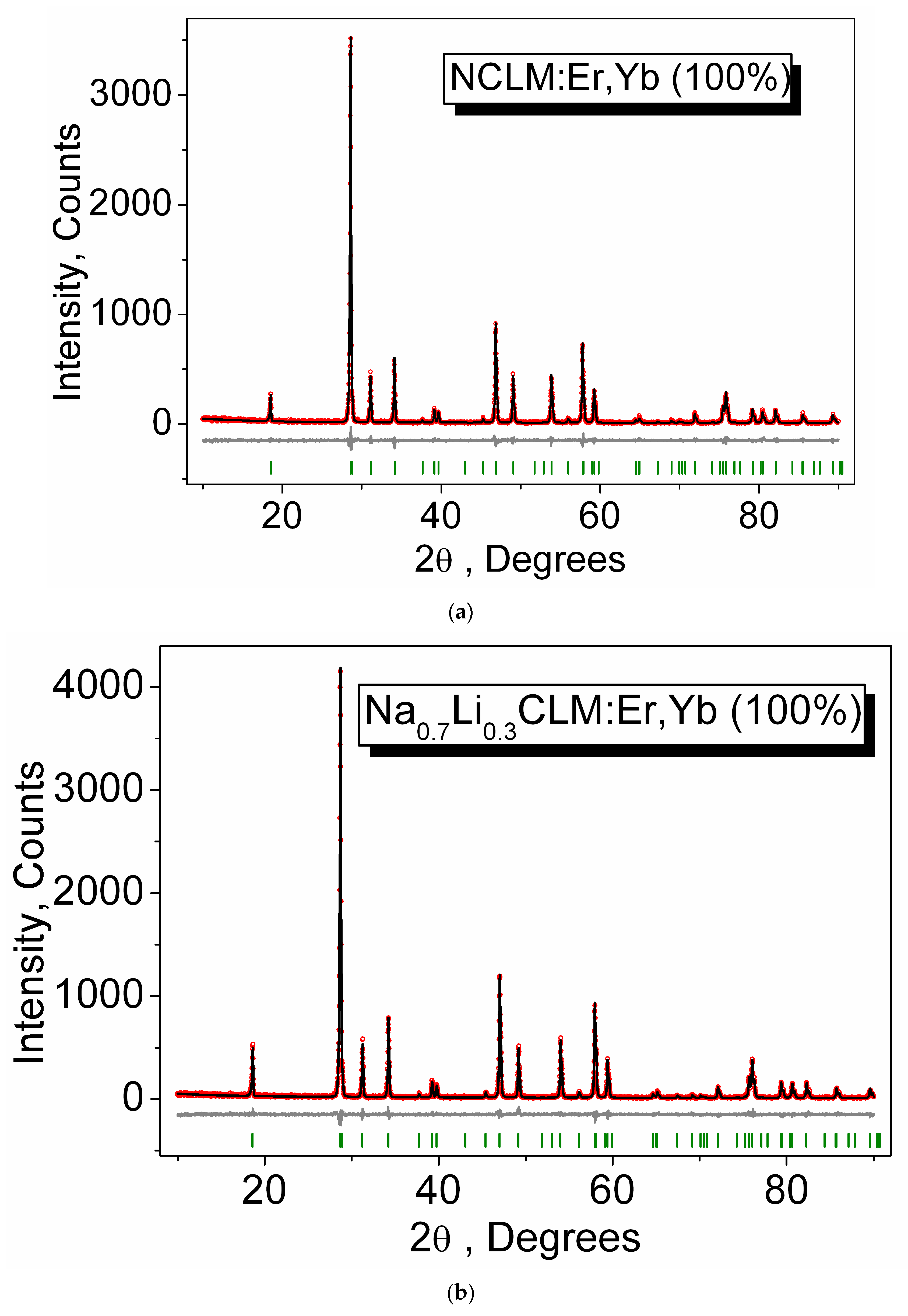
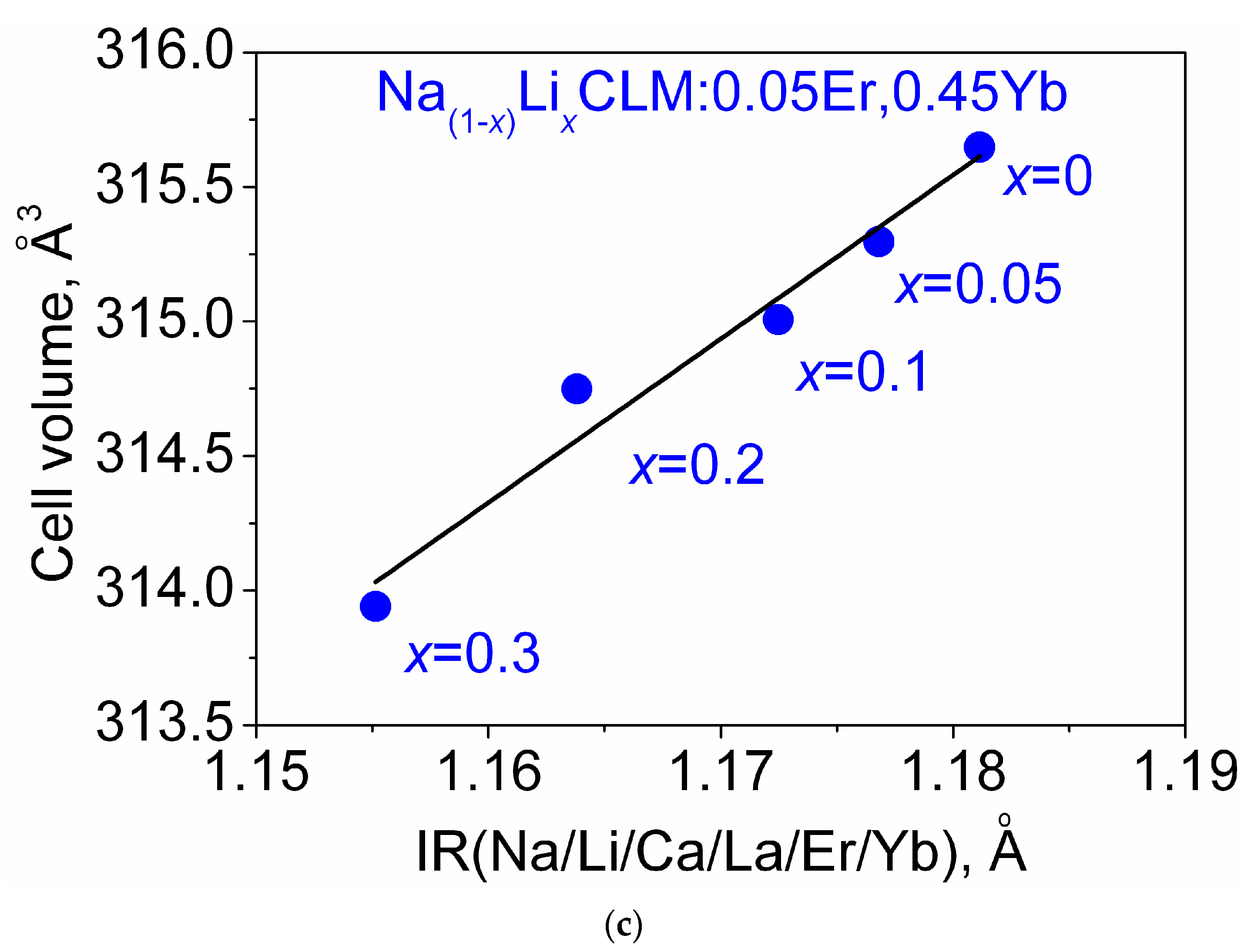
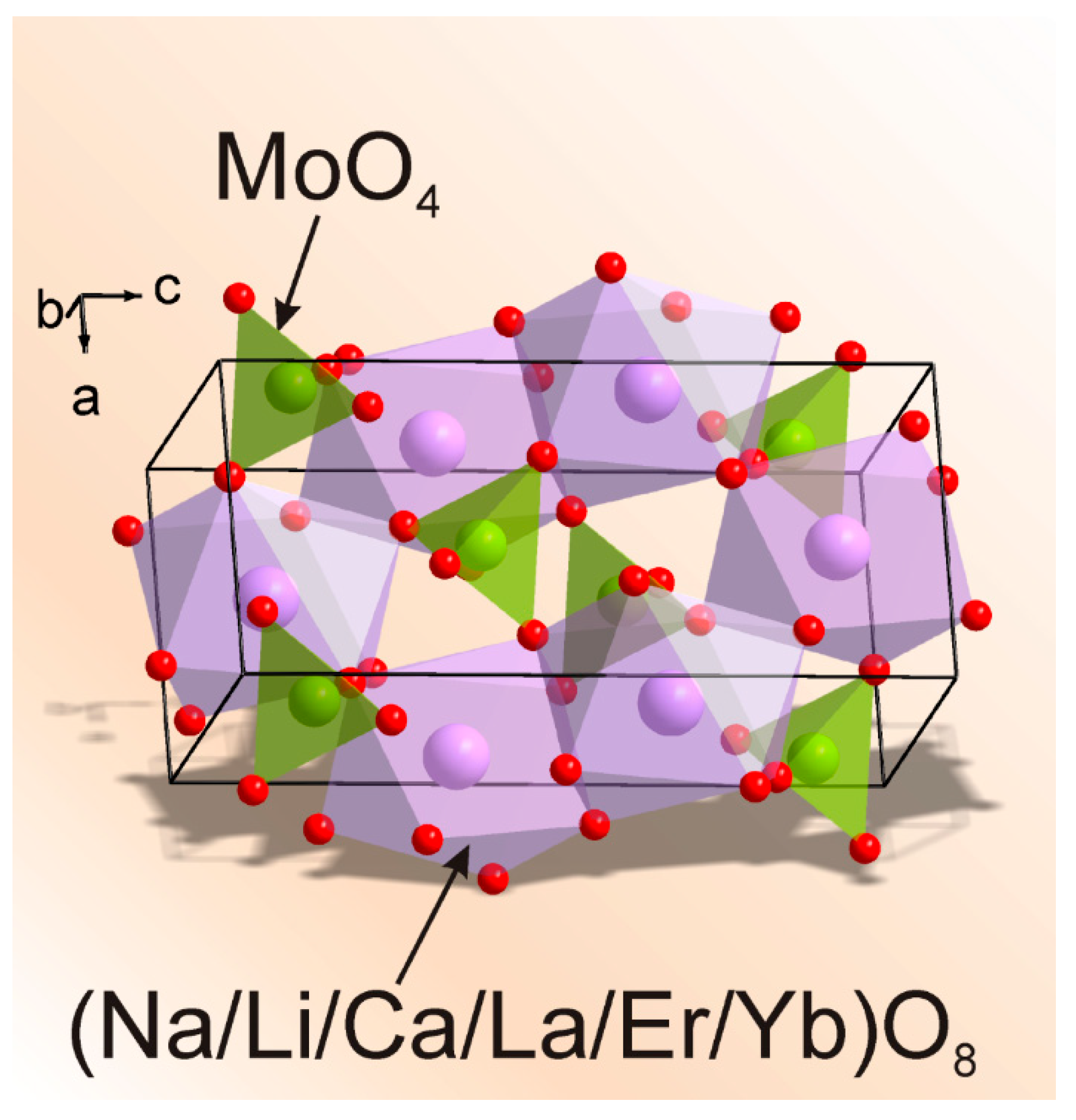
3.1. Particle Morphology and Phase Composition
3.2. Upconversion Properties
3.3. Raman Spectroscopy
3.4. Calculation of Band Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jendoubi, I.; Smail, R.B.; Maczka, M.; Zid, M.F. Optical and electrical properties of the yavapaiite-like molybdate NaAl(MoO4)2. Ionics 2018, 24, 3515–3533. [Google Scholar] [CrossRef]
- Goulas, A.; Chi-Tangyie, G.; Wang, D.; Zhang, S.; Ketharam, A.; Vaidhyanathan, B.; Reaney, I.M.; Cadman, D.A.; Whittow, W.G.; Vardaxoglou, C.; et al. Microstructure and microwave dielectric properties of 3D printed low loss Bi2Mo2O9 ceramics for LTCC applications. Appl. Mater. Today 2020, 21, 100862. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.; Atuchin, V.; Molokeev, M.; Oreshonkov, A. Microwave-employed sol–gel synthesis of scheelite-type microcrystalline AgGd(MoO4)2:Yb3+/Ho3+ upconversion yellow phosphors and their spectroscopic properties. Crystals 2020, 10, 1000. [Google Scholar]
- Sha, X.; Chen, B.; Zhang, X.; Zhang, J.; Xu, S.; Li, X.; Sun, J.; Zhang, Y.; Wang, X.; Zhang, Y.; et al. Pre-assessments of optical transition, gain performance and temperature sensing of Er3+ in NaLn(MoO4)2 (Ln = Y, La, Gd and Lu) single crystals by using their powder-formed samples derived from traditional solid state reaction. Opt. Laser Technol. 2021, 140, 107012. [Google Scholar] [CrossRef]
- Chimitova, O.D.; Bazarov, B.G.; Bazarova, J.G.; Atuchin, V.V.; Azmi, R.; Sarapulova, A.E.; Mikhailova, D.; Balachandran, G.; Fiedler, A.; Geckle, U.; et al. The crystal growth and properties of novel magnetic double molybdate RbFe5(MoO4)7 with mixed Fe3+/Fe2+ states and 1D negative thermal expansion. CrystEngComm 2021, 23, 3297–3307. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, D.; Du, C.; Xu, D.-M.; Li, R.-T.; Shi, Z.-Q.; Darwish, M.A.; Zhou, T.; Jantunen, H. Design and fabrication of a satellite communication dielectric resonator antenna with novel low loss and temperature-stabilized (Sm1−xCax) (Nb1−xMox)O4 (x = 0.15–0.7) microwave ceramics. Chem. Mater. 2023, 35, 104–115. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Grossman, V.G.; Adichtchev, S.V.; Surovtsev, N.V.; Gavrilova, T.A.; Bazarov, B.G. Structural and vibrational properties of microcrystalline TlM(MoO4)2 (M = Nd, Pr) molybdates. Opt. Mater. 2012, 34, 812–816. [Google Scholar] [CrossRef]
- Li, T.; Guo, C.; Wua, Y.; Li, L.; Jeong, J.H. Green upconversion luminescence in Yb3+/Er3+ co-doped ALn(MoO4)2 (A = Li, Na and K, Ln = La, Gd and Y). J. Alloys Compd. 2012, 540, 107–112. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Chimitova, O.D.; Adichtchev, S.V.; Bazarov, J.G.; Gavrilova, T.A.; Molokeev, M.S.; Surovtsev, N.V.; Bazarova, Z.G. Synthesis, structural and vibrational properties of microcrystalline β-RbSm(MoO4)2. Mater. Lett. 2013, 106, 26–29. [Google Scholar] [CrossRef]
- Abakumov, A.M.; Morozov, V.A.; Tsirlin, A.A.; Verbeeck, J.; Hadermann, J. Cation ordering and flexibility of the BO42− tetrahedra in incommensurately modulated CaEu2(BO4)4 (B = Mo, W) scheelites. Inorg. Chem. 2014, 53, 9407–9415. [Google Scholar] [CrossRef]
- Hao, S.-Z.; Zhou, D.; Hussain, F.; Su, J.-Z.; Liu, W.-F.; Wang, D.-W.; Wang, Q.-P.; Qi, Z.-M. Novel scheelite-type [Ca0.55(Nd1−xBix)0.3]MoO4 (0.2 ≤ x ≤ 0.95) microwave dielectric ceramics with low sintering temperature. J. Am. Ceram. Soc. 2020, 103, 7259–7266. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.S.; Atuchin, V.V.; Molokeev, M.S.; Oreshonkov, A.S. Microwave sol-gel synthesis, microstructural and spectroscopic properties of scheelite-type ternary molybdate upconversion phosphor NaPbLa(MoO4)3:Er3+/Yb3+. J. Alloys Compd. 2020, 826, 152095. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Xia, Z.; Molokeev, M.S.; Jing, X. Effect of composition modulation on the luminescence properties of Eu3+ doped Li1−xAgxLu(MoO4)2 solid-solution phosphors. Dalton Trans. 2015, 44, 18078–18089. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. The modulated structure and frequency upconversion properties of CaLa2(MoO4)4:Ho3+/Yb3+ phosphors prepared by microwave synthesis. Phys. Chem. Chem. Phys. 2015, 17, 19278–19287. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, W.; Wang, T.; Zhang, J.; Du, H. Photoluminescence enhancement in a Na5Y(MoO4)4:Dy3+ white-emitting phosphor by partial replacement of MoO42− with WO42− or VO43−. Ceram. Int. 2021, 47, 12028–12037. [Google Scholar] [CrossRef]
- Yu, Y.; Shao, K.; Niu, C.; Liu, L.; Zhu, X.; Wang, Z.; Ma, Z.; Wang, G. Crystal growth, first-principle calculations, optical properties and laser performances toward a molybdate Er+3:KBaGd(MoO4)3 crystal. J. Lumin. 2022, 252, 119324. [Google Scholar] [CrossRef]
- Lim, C.S.; Atuchin, V.V.; Aleksandrovsky, A.S.; Molokeev, M.S. Preparation of NaSrLa(WO4)3:Ho3+/Yb3+ ternary tungstates and their upconversion photoluminescence properties. Mater. Lett. 2016, 181, 38–41. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Atuchin, V.V. Microwave synthesis and spectroscopic properties of ternary scheelite-type molybdate phosphors NaSrLa(MoO4)3:Er3+, Yb3+. J. Alloys Compd. 2017, 713, 156–163. [Google Scholar] [CrossRef]
- Wang, H.; Yamg, T.; Feng, L.; Ning, Z.; Liu, M.; Lai, X.; Gao, D.; Bi, J. Energy transfer and multicolor tunable luminescence properties of NaGd0.5Tb0.5−xEux(MoO4)2 phosphors for UV-LED. J. Electron. Mater. 2018, 47, 6494–6506. [Google Scholar] [CrossRef]
- Xie, J.; Cheng, L.; Tang, H.; Wang, Z.; Sun, H.; Lu, L.; Mi, X.; Liu, Q.; Zhang, X. Wide range color tunability and efficient energy transfer of novel NaCaGd(WO4)3:Tb3+, Eu3+ phosphors with excellent thermal stability for pc-WLEDs. Inorg. Chem. Front. 2021, 8, 4517–4527. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Microwave sol–gel synthesis and upconversion photoluminescence properties of CaGd2(WO4)4:Er3+/Yb3+ phosphors with incommensurately modulated structure. J. Solid State Chem. 2015, 228, 160–166. [Google Scholar] [CrossRef]
- Lim, C.S.; Atuchin, V.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A. Microwave sol–gel synthesis of CaGd2(MoO4)4:Er3+/Yb3+ phosphors and their upconversion photoluminescence properties. J. Am. Ceram. Soc. 2015, 98, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, J.P.C.; Sales, A.J.M.; Sousa, D.G.; da Silva, M.A.S.; Moreira, S.G.C.; Pavani, K.; Soares, M.J.; Graça, M.P.F.; Kumar, J.S.; Sombra, A.S.B. Temperature-, power-, and concentration-dependent two and three photon upconversion in Er3+/Yb3+ co-doped lanthanum ortho-niobate phosphors. RSC Adv. 2016, 6, 68160–68169. [Google Scholar] [CrossRef]
- Krut’ko, V.A.; Komova, M.G.; Pominova, D.V. Synthesis and luminescence of Gd2−x−yYbxEr(Ho)yGeMoO8 germanate-molybdates with scheelite structure. J. Solid State Chem. 2020, 292, 121704. [Google Scholar] [CrossRef]
- Singh, P.; Jain, N.; Shukla, S.; Tiwari, A.K.; Kumar, K.; Singh, J.; Pandey, A.C. Luminescence nanothermometry using a trivalent lanthanide co-doped perovskite. RSC Adv. 2023, 13, 2939–2948. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Z.; Liu, S.; Zhang, S.; Zhang, W.; Feng, X.; Zhao, P.; He, J.; Tao, X. Simultaneous dual-wavelength operation of β-BaTeMo2O9 Raman laser at 1320 and 1500 nm. Appl. Phys. Express 2013, 6, 072702. [Google Scholar] [CrossRef]
- Shi, P.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V. Crystal chemistry and luminescence properties of red-emitting CsGd1−xEux(MoO4)2 solid-solution phosphors. Dalton Trans. 2014, 43, 9669–9676. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, H.; Jiang, H.; Li, J.; Wang, J. Flux growth, structure, and physical characterization of new disordered laser crystal LiNd(MoO4)2. J. Cryst. Growth 2015, 423, 1–8. [Google Scholar] [CrossRef]
- Reshak, A.H.; Alahmed, Z.A.; Bila, J.; Atuchin, V.V.; Bazarov, B.G.; Chimitova, O.D.; Molokeev, M.S.; Prosvirin, I.P.; Yelisseyev, A.P. Exploration of the electronic structure of monoclinic α-Eu2(MoO4)3: DFT-based study and X-ray photoelectron spectroscopy. J. Phys. Chem. C 2016, 120, 10559–10568. [Google Scholar] [CrossRef]
- Khyzhun, O.Y.; Bekenev, V.L.; Atuchinb, V.V.; Pokrovsky, L.D.; Shlegel, V.N.; Ivannikova, N.V. The electronic structure of Pb2MoO5: First-principles DFT calculations and X-ray spectroscopy measurements. Mater. Des. 2016, 105, 315–322. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Molokeev, M.S.; Krylov, A.S.; Oreshonkov, A.S.; Zhou, D. Structural and spectroscopic properties of self-activated monoclinic molybdate BaSm2(MoO4)4. J. Alloys Compd. 2017, 729, 843–849. [Google Scholar] [CrossRef] [Green Version]
- Solodovnikov, S.F.; Atuchin, V.V.; Solodovnikova, Z.A.; Khyzhun, O.Y.; Danylenko, M.I.; Pishchur, D.P.; Plyusnin, P.E.; Pugachev, A.M.; Gavrilova, T.A.; Yelisseyev, A.P.; et al. Synthesis, structural, thermal, and electronic properties of palmierite-related double molybdate α-Cs2Pb(MoO4)2. Inorg. Chem. 2017, 56, 3276–3286. [Google Scholar] [CrossRef]
- Ren, H.; Li, H.; Zou, Y.; Deng, H.; Peng, Z.; Ma, T.; Ding, S. Growth and properties of Pr3+-doped NaGd(MoO4)2 single crystal: A promising InGaN laser-diode pumped orange-red laser crystal. J. Lumin. 2022, 249, 119034. [Google Scholar] [CrossRef]
- Savina, A.A.; Atuchin, V.V.; Solodovnikov, S.F.; Solodovnikova, Z.A.; Krylov, A.S.; Maximovskiy, E.A.; Molokeev, M.S.; Oreshonkov, A.S.; Pugachev, A.M.; Khaikina, E.G. Synthesis, structural and spectroscopic properties of acentric triple molybdate Cs2NaBi(MoO4)3. J. Solid State Chem. 2015, 225, 53–58. [Google Scholar] [CrossRef]
- Mhiri, M.; Badri, A.; Amara, M.B. Synthesis and crystal structure of NaMgFe(MoO4)3. Acta Crystallogr. Sect. E Crystallogr. Commun. 2016, E72, 864–867. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.S. Synthesis of NaCaLa(MoO4)3:Ho3+/Yb3+ phosphors via microwave sol-gel route and their upconversion photoluminescence properties. Korean J. Mater. Res. 2016, 26, 363–369. [Google Scholar] [CrossRef]
- Rajendran, M.; Vaidyanathan, S. New red emitting phosphors NaSrLa(MO4)3:Eu3+ [M = Mo and W] for white LEDs: Synthesis, structural and optical study. J. Alloys Compd. 2019, 789, 919–931. [Google Scholar] [CrossRef]
- Lim, C.S. Synthesis of microcrystalline LiNaCaLa(MoO4)3:Yb3+/Ho3+ upconversion phosphors and effect of Li+ on their spectroscopic properties. Trans. Electr. Electron. Mater. 2019, 20, 60–66. [Google Scholar] [CrossRef]
- Lim, C.-S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Structural and spectroscopic effects of Li+ substitution for Na+ in LixNa1−xCaGd0.5Ho0.05Yb0.45(MoO4)3 scheelite-type upconversion phosphors. Molecules 2021, 26, 7357. [Google Scholar] [CrossRef]
- Yu, Y.; Shao, K.; Zhu, X.; Zhang, X.; Qiu, H.; Wu, S.; Wang, G. Research on a novel molybdate Er3+:KBaY(MoO4)3 crystal as a prominent 1.55 μm laser medium. J. Lumin. 2021, 237, 118194. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoon, J.-W.; Lim, C.S.; Shim, K.B. Microwave-assisted synthesis of barium molybdate by a citrate complex method and oriented aggregation. Mater. Res. Bull. 2005, 40, 1468–1476. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.A.; Krikun, E.V. Microwave sintering: Fundamentals and modeling. J. Am. Ceram. Soc. 2013, 96, 1003–1020. [Google Scholar] [CrossRef]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef]
- Perera, S.S.; Munasinghe, H.N.; Yatooma, E.N.; Rabuffetti, F.A. Microwave-assisted solid-state synthesis of NaRE(MO4)2 phosphors (RE = La, Pr, Eu, Dy; M = Mo, W). Dalton Trans. 2020, 49, 7914–7919. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Ikonnikovg, D.A.; Atuchin, V.V. Triple molybdate scheelite-type upconversion phosphor NaCaLa(MoO4)3:Er3+/Yb3+: Structural and spectroscopic properties. Dalton Trans. 2016, 45, 15541–15551. [Google Scholar] [CrossRef] [Green Version]
- Bruker. Bruker AXS TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Oreshonkov, A.S. SI: Advances in density functional theory (DFT) studies of solids. Materials 2022, 15, 2099. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Für Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Bartók, A.P.; Yates, J.R. Regularized SCAN functional. J. Chem. Phys. 2019, 150, 161101. [Google Scholar] [CrossRef]
- Sun, J.; Remsing, R.C.; Zhang, Y.; Sun, Z.; Ruzsinszky, A.; Peng, H.; Yang, Z.; Paul, A.; Waghmare, U.; Wu, X.; et al. Accurate first-principles structures and energies of diversely bonded systems from an efficient density functional. Nat. Chem. 2016, 8, 831–836. [Google Scholar] [CrossRef]
- Lee, M.H. Improved Optimised Pseudopotentials and Application to Disorder in γ-Al2O3. Ph.D. Thesis, Cambridge University, Cambridge, UK, 1996. [Google Scholar]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Bellaiche, L.; Vanderbilt, D. Virtual crystal approximation revisited: Application to dielectric and piezoelectric properties of perovskites. Phys. Rev. B 2000, 61, 7877. [Google Scholar] [CrossRef] [Green Version]
- Atuchin, V.V.; Gavrilova, T.A.; Grivel, J.-C.; Kesler, V.G. Electronic structure of layered titanate Nd2Ti2O7. Surf. Sci. 2008, 602, 3095–3099. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Bazarov, B.G.; Gavrilova, T.A.; Grossman, V.G.; Molokeev, M.S.; Bazarova, Z.G. Preparation and structural properties of nonlinear optical borates K2(1−x)Rb2xAl2B2O7, 0 < x < 0.75. J. Alloys Compd. 2012, 515, 119–122. [Google Scholar]
- Hazen, R.M.; Finger, L.W.; Mariathasan, J.W.E. High pressure crystal chemistry of scheelite-type tungstates and molybdates. J. Phys. Chem. Solids 1985, 46, 253–263. [Google Scholar] [CrossRef]
- Stevens, S.B.; Morrison, C.A.; Allik, T.H.; Rheingold, A.L.; Haggerty, B.S. NaLa(MoO4)2 as a laser host material. Phys. Rev. B Condens. Matter. 1991, 43, 7386–7394. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, C.; Horchani-Naifer, K.; Khadraoui, Z.; Elhouichet, H.; Ferid, M. Synthesis, characterization and DFT calculations of electronic and optical properties of CaMoO4. Phys. B Condens. Matter 2016, 497, 34–38. [Google Scholar] [CrossRef]
- Marques, V.S.; Cavalcante, L.S.; Sczancoski, J.C.; Alcantara, A.F.P.; Orlandi, M.O.; Moraes, E.; Longo, E.; Varela, J.A.; Li, M.S.; Santos, M.R.M.C. Effect of Different Solvent Ratios(Water/Ethylene Glycol) on the Growth Process of CaMoO4 Crystals and Their Optical Properties. Cryst. Growth Des. 2010, 10, 4752–4768. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sahu, M.; Ghosh, P.S.; Tyagi, D.; Saxena, M.K.; Kadam, R.M. Energy transfer dynamics and luminescence properties of Eu3+ in CaMoO4 and SrMoO4. Dalton Trans. 2015, 44, 18957–18969. [Google Scholar] [CrossRef]
- Tranquilin, R.L.; Oliveira, M.C.; Santiago, A.A.G.; Lovisa, L.X.; Ribeiro, R.A.P.; Longo, E.; de Lazaro, S.R.; Almeida, C.R.R.; Paskocimas, C.A.; Motta, F.V.; et al. Presence of excited electronic states on terbium incorporation in CaMoO4: Insights from experimental synthesis and first-principles calculations. J. Phys. Chem. Solids 2021, 149, 109790. [Google Scholar] [CrossRef]

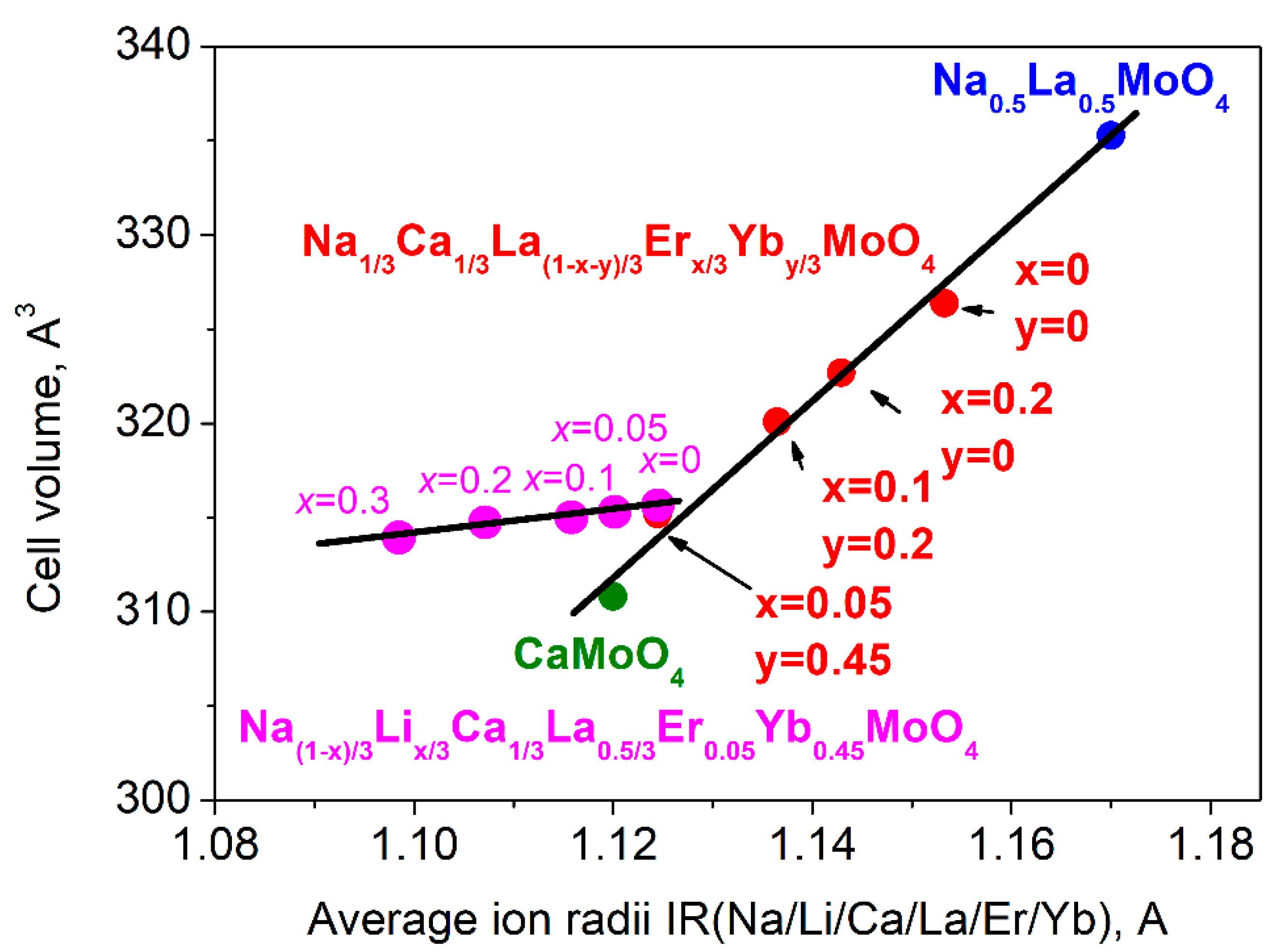
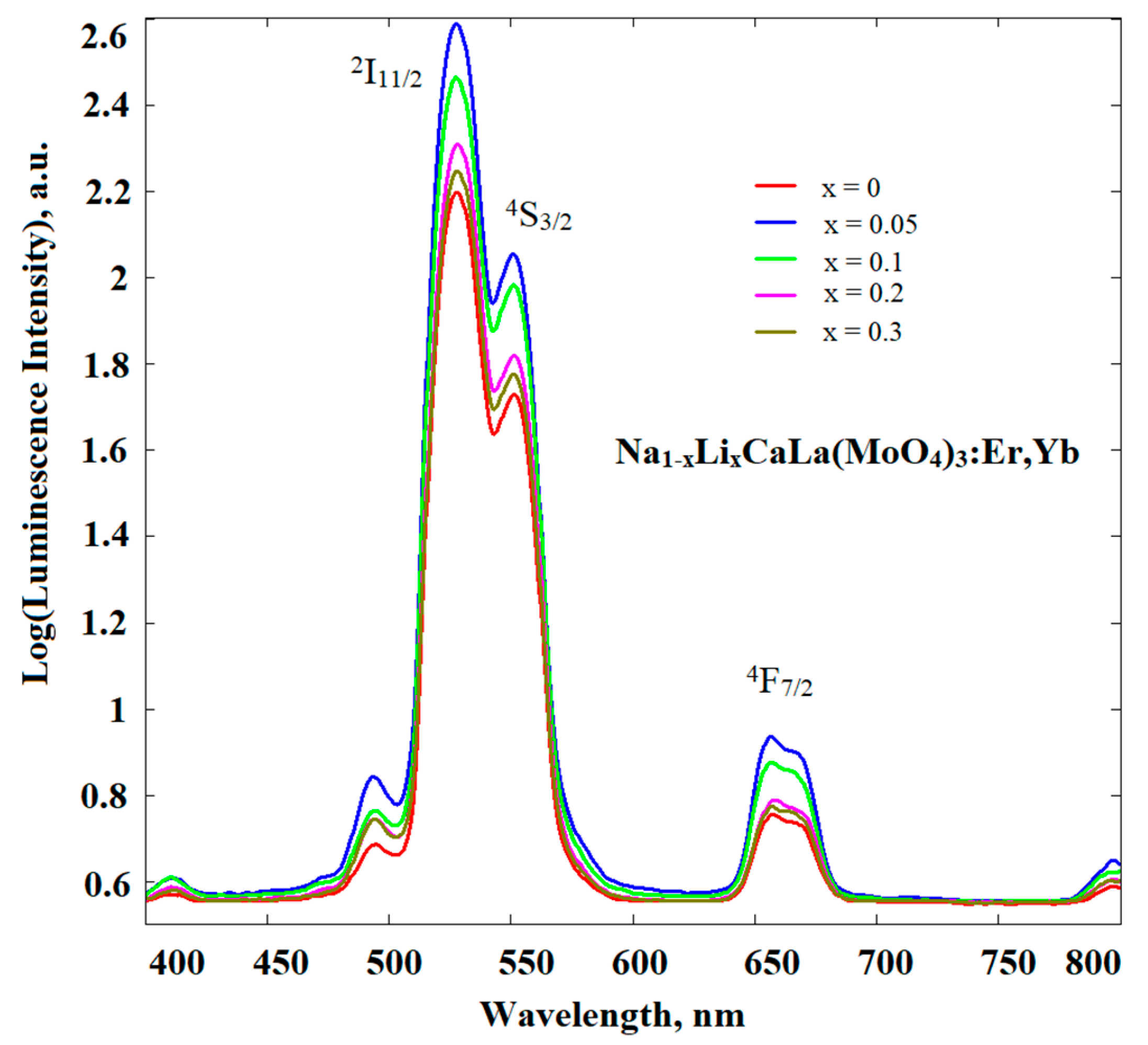
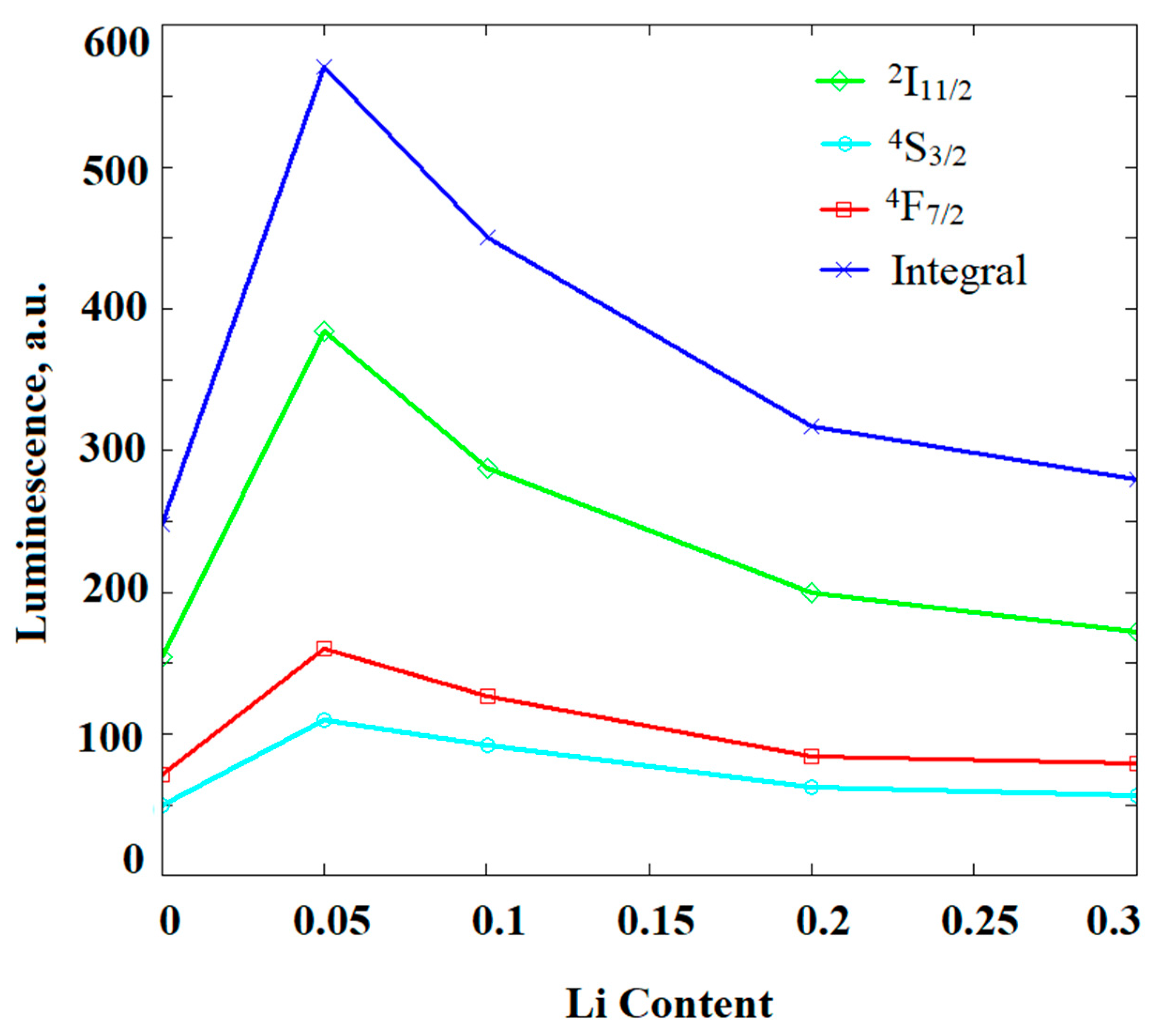


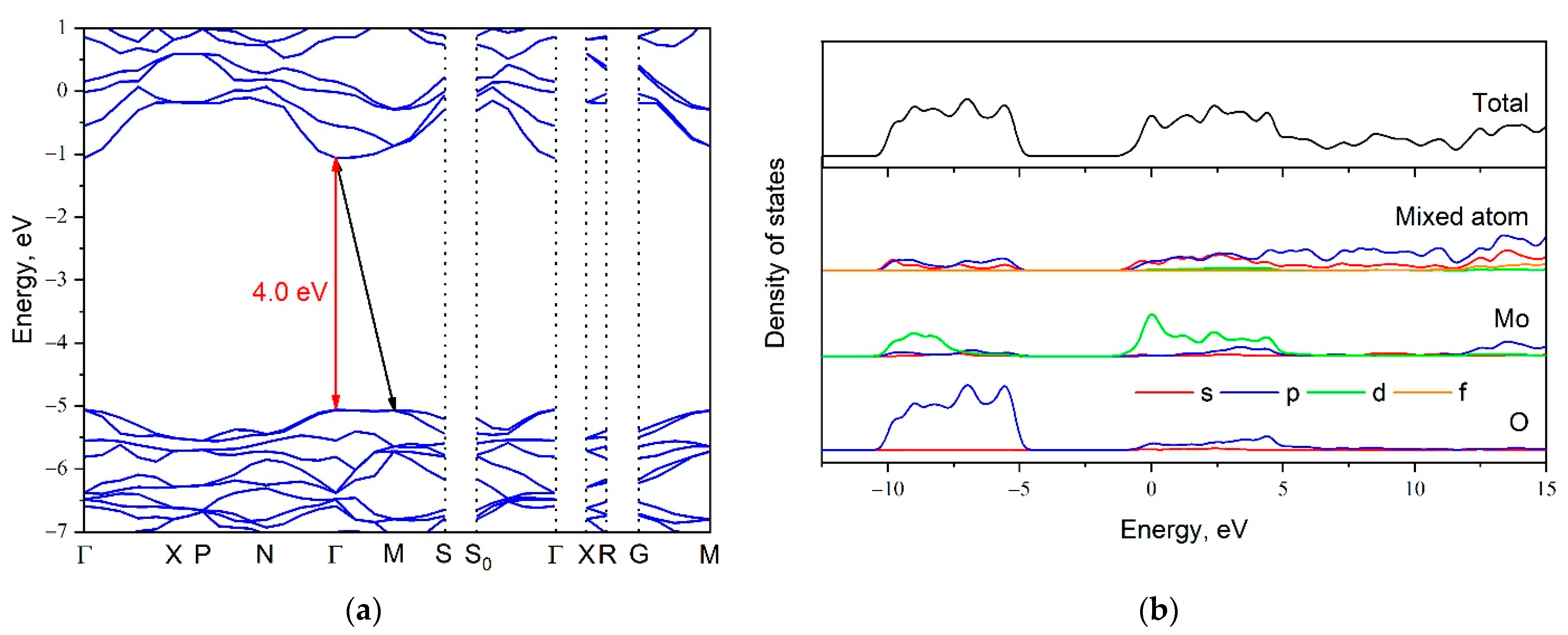
| Abbreviation | Chemical Composition |
|---|---|
| NCLM:EY | NaCaLa0.5Er0.05Yb0.45(MoO4)3 |
| LiNCLM:EY-0.05 | Li0.05Na0.95CaLa0.5Er0.05Yb0.45(MoO4)3 |
| LiNCLM:EY-0.1 | Li0.1Na0.9CaLa0.5Er0.05Yb0.45(MoO4)3 |
| LiNCLM:EY-0.2 | Li0.2Na0.8CaLa0.5Er0.05Yb0.45(MoO4)3 |
| LiNCLM:EY-0.3 | Li0.3Na0.7CaLa0.5Er0.05Yb0.45(MoO4)3 |
| Compound | Space Group | Z | Cell Parameters (Å), Cell Volume (Å3) | Rp, RB (%) χ2 |
|---|---|---|---|---|
| NCLM | I41/a | 4 | a = 5.2452 (1) c = 11.4731 (4) V = 315.65 (2) | 10.94, 2.04 1.11 |
| Na0.95Li0.05CLM: 0.05Er, 0.45Yb | I41/a | 4 | a = 5.2432 (1) c = 11.4690 (3) V = 315.30 (2) | 9.26, 1.93 1.10 |
| Na0.9Li0.1CLM: 0.05Er, 0.45Yb | I41/a | 4 | a = 5.2418 (2) c = 11.4646 (4) V = 315.01 (2) | 11.10, 2.95 1.12 |
| Na0.8Li0.2CLM: 0.05Er, 0.45Yb | I41/a | 4 | a = 5.2405 (1) c = 11.4609 (3) V = 314.75 (1) | 11.22, 2.82 1.10 |
| Na0.7Li0.3CLM: 0.05Er, 0.45Yb | I41/a | 4 | a = 5.2364 (1) c = 11.4494 (3) V = 313.94 (2) | 10.11, 2.64 1.11 |
| Compound | Source | Calc./Exp. | Band Gap, eV |
|---|---|---|---|
| NCLM:EY | this work | calc. | 4.00 |
| LiNCLM:EY-0.05 | this work | calc. | 4.02 |
| LiNCLM:EY-0.1 | this work | calc. | 4.12 |
| LiNCLM:EY-0.2 | this work | calc. | 3.99 |
| LiNCLM:EY-0.3 | this work | calc. | 4.137 |
| CaMoO4 | [62] | calc | 2.95 |
| CaMoO4 | [60] | calc. | 3.2 |
| CaMoO4:Eu3+ | [62] | calc | 3.35 |
| CaMoO4:Tb3+ | [63] | calc | 3.96 |
| CaMoO4 | [61] | calc. | 4.64 |
| CaMoO4 | [63] | exp. | 3.87 |
| CaMoO4 | [60] | exp. | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Structural and Spectroscopic Effects of Li+ Substitution for Na+ in LixNa1−xCaLa0.5Er0.05Yb0.45(MoO4)3 Upconversion Scheelite-Type Phosphors. Crystals 2023, 13, 362. https://doi.org/10.3390/cryst13020362
Lim CS, Aleksandrovsky A, Molokeev M, Oreshonkov A, Atuchin V. Structural and Spectroscopic Effects of Li+ Substitution for Na+ in LixNa1−xCaLa0.5Er0.05Yb0.45(MoO4)3 Upconversion Scheelite-Type Phosphors. Crystals. 2023; 13(2):362. https://doi.org/10.3390/cryst13020362
Chicago/Turabian StyleLim, Chang Sung, Aleksandr Aleksandrovsky, Maxim Molokeev, Aleksandr Oreshonkov, and Victor Atuchin. 2023. "Structural and Spectroscopic Effects of Li+ Substitution for Na+ in LixNa1−xCaLa0.5Er0.05Yb0.45(MoO4)3 Upconversion Scheelite-Type Phosphors" Crystals 13, no. 2: 362. https://doi.org/10.3390/cryst13020362






