Synthesis, Characterization and Single Crystal X-ray Diffraction Analysis of Fused Triazolo/Thiadiazole Clubbed with Indole Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. General Procedures
2.3. X-ray Structure Determinations
2.4. Hirshfeld Surface Analysis
3. Results and Discussion
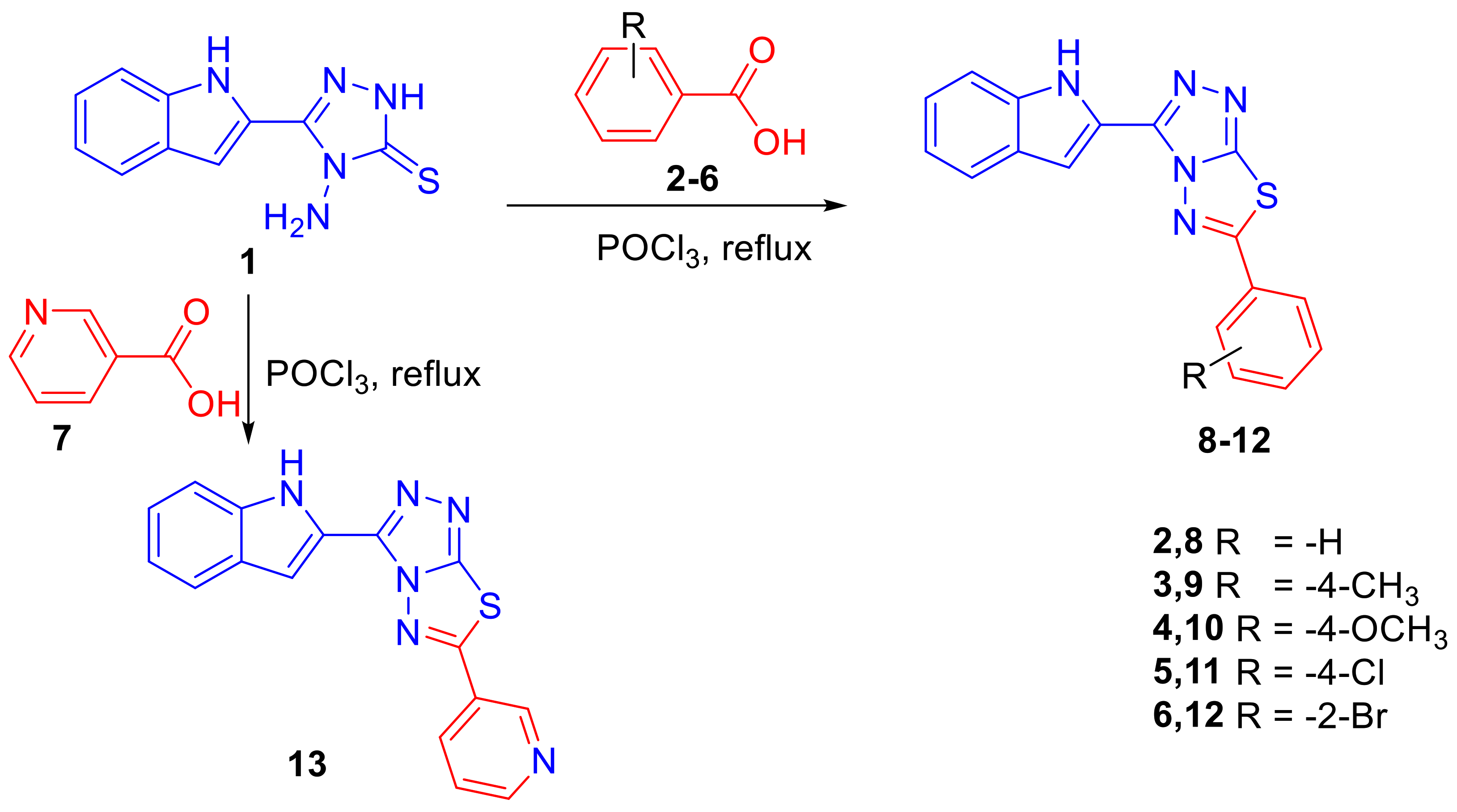
3.1. Chemistry and Characterizations
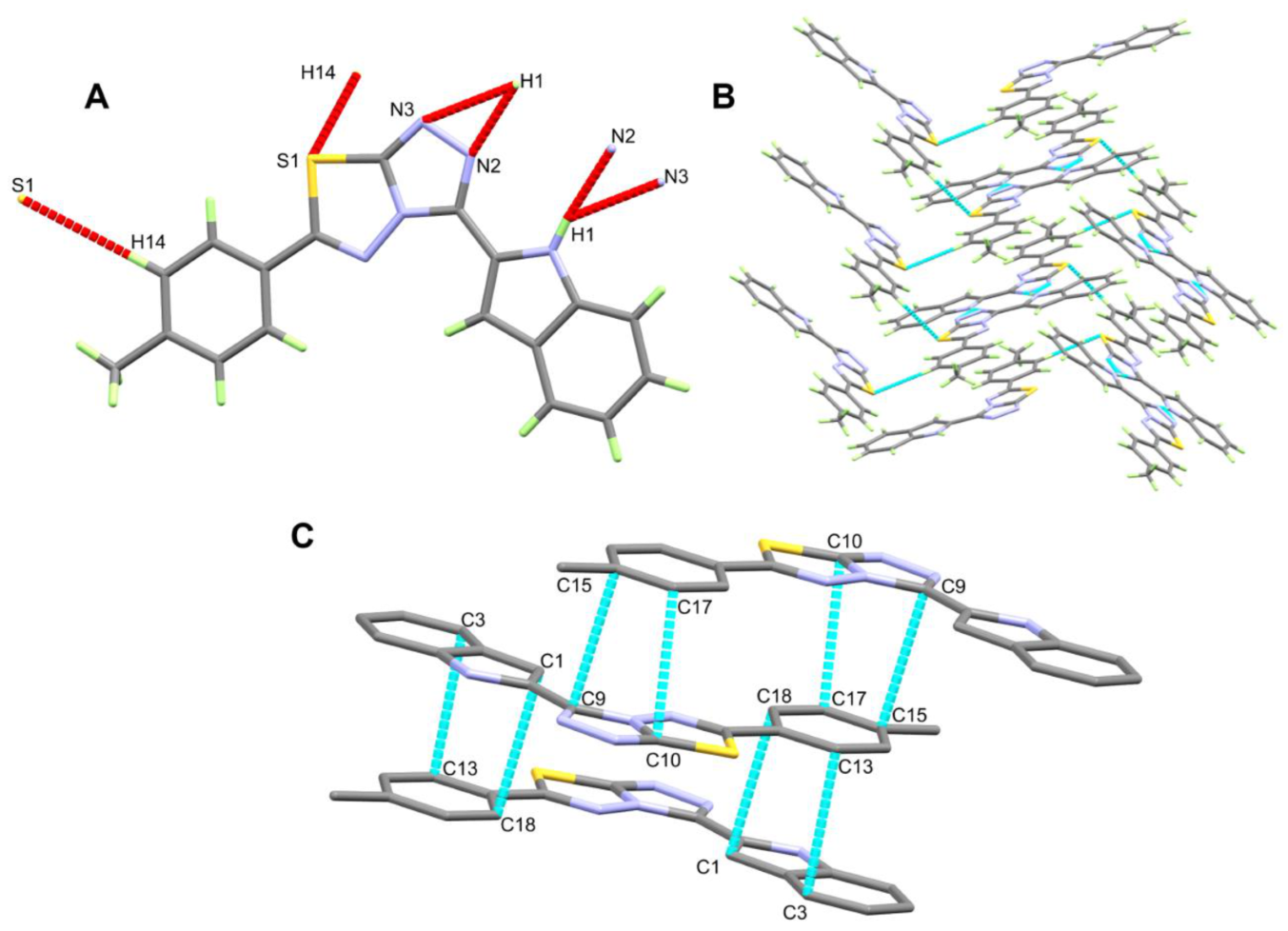
3.2. Crystal Structure Description
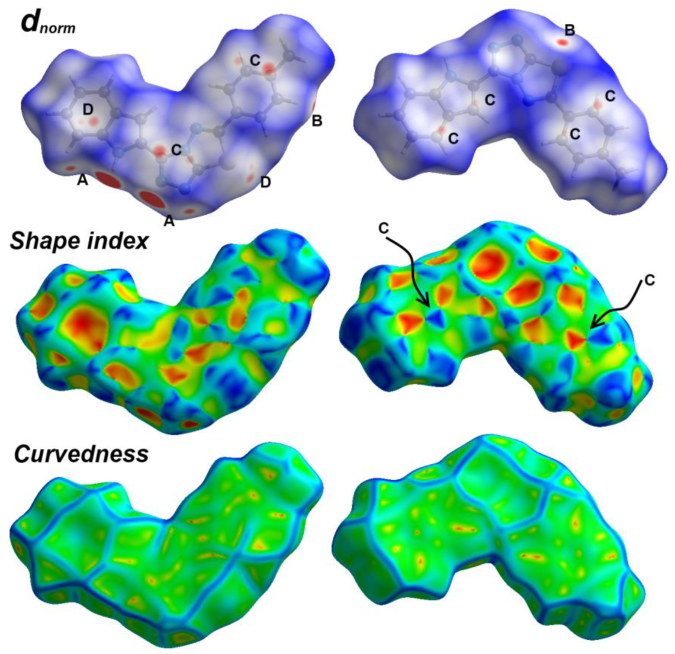
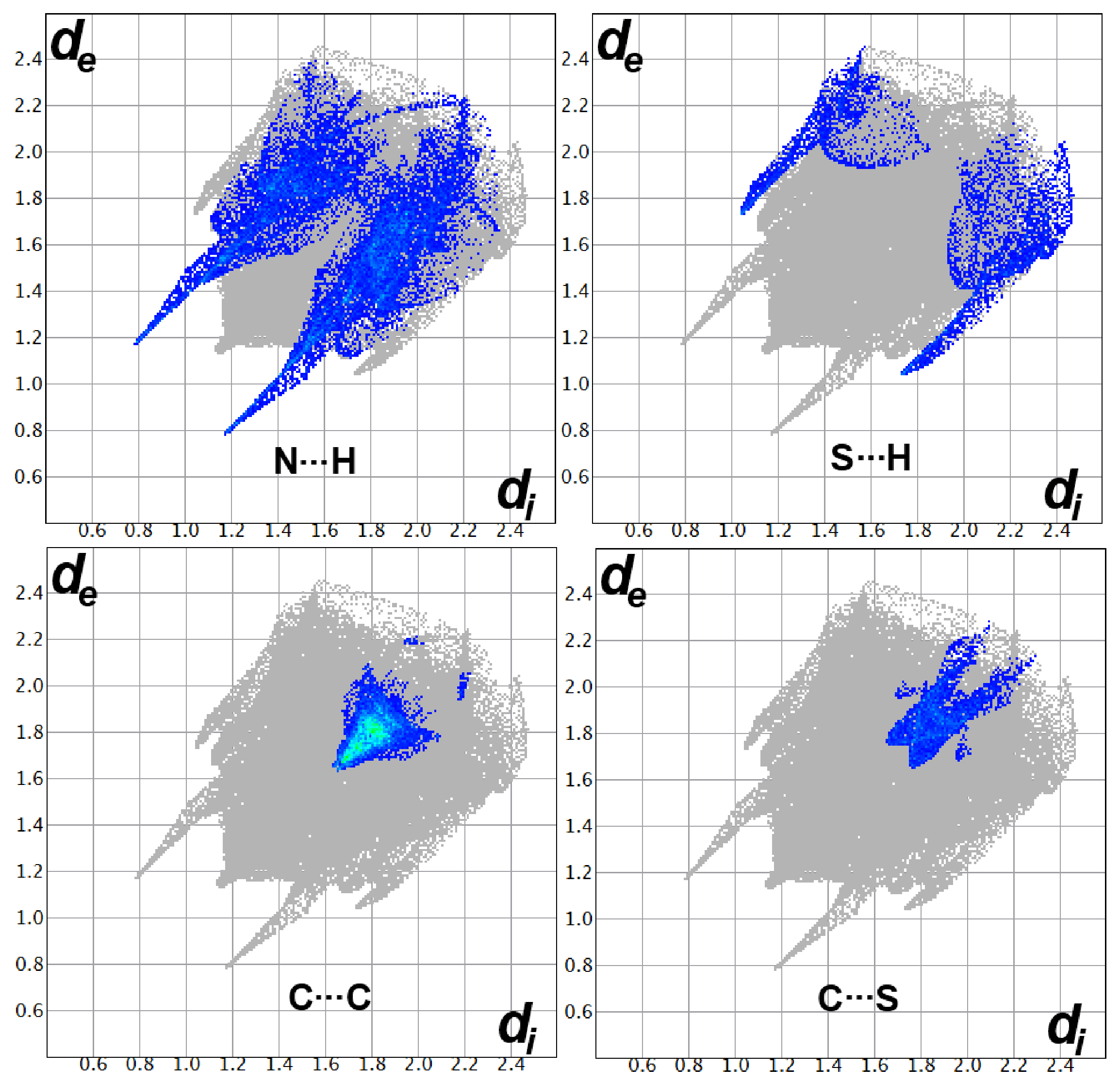
3.3. Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elshaier, Y.A.; Nemr, M.T.; Al Refaey, M.; Fadaly, W.A.; Barakat, A. Chemistry of 2-vinylindoles: Synthesis and applications. New J. Chem. 2022, 46, 13383–13400. [Google Scholar] [CrossRef]
- Boraei, A.T.; Ghabbour, H.A.; Gomaa, M.S.; El Ashry, E.S.H.; Barakat, A. Synthesis and anti-proliferative assessment of triazolo-thiadiazepine and triazolo-thiadiazine scaffolds. Molecules 2019, 24, 4471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boraei, A.T.A.; Gomaa, M.S.; El Sayed, E.S.H.; Duerkop, A. Design, selective alkylation and X-ray crystal structure determination of dihydro-indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogue as potent anticancer agents. Eur. J. Med. Chem. 2017, 125, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.; Ashour, H.K.; El Sayed, H.; Abdelmoaty, N.; El-Falouji, A.I.; Gomaa, M.S. Design and synthesis of new phthalazine-based derivatives as potential EGFR inhibitors for the treatment of hepatocellular carcinoma. Bioorg. Chem. 2019, 85, 293–307. [Google Scholar] [CrossRef]
- Islam, M.S.; Barakat, A.; Al-Majid, A.M.; Ali, M.; Yousuf, S.; Choudhary, M.I.; Khalil, R.; Ul-Haq, Z. Catalytic asymmetric synthesis of indole derivatives as novel α-glucosidase inhibitors in vitro. Bioorg. Chem. 2018, 79, 350–354. [Google Scholar] [CrossRef]
- Sechi, M.; Derudas, M.; Dallocchio, R.; Dessi, A.; Bacchi, A.; Sannia, L.; Carta, F.; Palomba, M.; Ragab, O.; Chan, C.; et al. Design and synthesis of novel indole β-diketo acid derivatives as HIV-1 integrase inhibitors. J. Med. Chem. 2014, 47, 5298–5310. [Google Scholar] [CrossRef]
- Al-Quawasmeh, R.A.; Huesca, M.; Nedunuri, V.; Peralta, R.; Wright, J.; Lee, Y.; Young, A. Potent antimicrobial activity of 3-(4,5-diaryl-1H-imidazol-2-yl)-1H-indole derivatives against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2010, 20, 3518–3520. [Google Scholar] [CrossRef]
- Narayana, B.; Ashalatha, B.V.; Vijayaraj, K.K.; Fernandes, J.; Sarojini, B.K. Synthesis of some new biologically active 1, 3, 4-oxadiazolyl nitroindoles and a modified Fischer indole synthesis of ethyl nitro indole-2-carboxylates. Bioorg. Med. Chem. 2005, 13, 4638–4644. [Google Scholar] [CrossRef]
- Ty, N.; Dupeyre, G.; Chabot, G.G.; Seguin, J.; Tillequin, F.; Scherman, D.; Michel, S.; Cachet, X. Synthesis and biological evaluation of new disubstituted analogues of 6-methoxy-3-(3′,4′,5′-trimethoxybenzoyl)-1H-indole (BPR0L075), as potential antivascular agents. Bioorg. Med. Chem. 2008, 16, 7494–7503. [Google Scholar] [CrossRef]
- Bi, W.; Bi, Y.; Xue, P.; Zhang, Y.; Gao, X.; Wang, Z.; Li, M.; Baudy-Floc’h, M.; Ngerebara, N.; Gibson, K.M.; et al. Synthesis and characterization of novel indole derivatives reveal improved therapeutic agents for treatment of ischemia/reperfusion (I/R) injury. J. Med. Chem. 2010, 53, 6763–6767. [Google Scholar] [CrossRef]
- Mascal, M.; Modes, K.V.; Durmus, A. Concise photochemical synthesis of the antimalarial indole alkaloid decursivine. Angew. Chem. Int. Ed. 2011, 50, 4445–4446. [Google Scholar] [CrossRef]
- Huang, M.; Deng, Z.; Tian, J.; Liu, T. Synthesis and biological evaluation of salinomycin triazole analogues as anticancer agents. Eur. J. Med. Chem. 2017, 127, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Gujjar, R.; Marwaha, A.; El Mazouni, F.; White, J.; White, K.L.; Creason, S.; Shackleford, D.M.; Baldwin, J.; Charman, W.N.; Buckner, F.S.; et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J. Med. Chem. 2009, 52, 1864–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Lu, S.; Yuan, G.; Yang, S.; Du, X. Synthesis and antibacterial activity of some heterocyclic β-enamino ester derivatives with 1,2,3-triazole. Heterocycl. Commun. 2000, 6, 421–426. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Foroumadi, A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016, 109, 380–392. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, Y.; Fazili, K.M.; Bhat, K.A.; Ara, T. Synthesis and biological evaluation of novel 3-O-tethered triazoles of diosgenin as potent antiproliferative agents. Steroids 2017, 118, 1–8. [Google Scholar]
- Baburajeev, C.P.; Mohan, C.D.; Ananda, H.; Rangappa, S.; Fuchs, J.E.; Jagadish, S.; Siveen, K.S.; Chinnathambi, A.; Ali Alharbi, S.A.; Zayed, M.E.; et al. Development of novel triazolo-thiadiazoles from heterogeneous “green” catalysis as protein tyrosine phosphatase 1B inhibitors. Sci. Rep. 2015, 5, 14195. [Google Scholar] [CrossRef] [Green Version]
- Timur, İ.; Kocyigit, Ü.M.; Dastan, T.; Sandal, S.; Ceribası, A.O.; Taslimi, P.; Gulcin, İ.; Koparir, M.; Karatepe, M.; Çiftçi, M. In vitro cytotoxic and in vivo antitumoral activities of some aminomethyl derivatives of 2,4-dihydro-3H-1,2,4-triazole-3-thiones—Evaluation of their acetylcholinesterase and carbonic anhydrase enzymes inhibition profiles. J. Biochem. Mol. Toxic. 2019, 33, e22239. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, T.; Hameed, S.; Khan, K.M.; Choudhary, M.I. Syntheses, urease inhibition, and antimicrobial studies of some chiral 3-substituted-4-amino-5-thioxo-1H,4H-1,2,4-triazoles. Med. Chem. 2008, 4, 539–543. [Google Scholar] [CrossRef]
- Sevaille, L.; Gavara, L.; Bebrone, C.; De Luca, F.; Nauton, L.; Achard, M.; Mercuri, P.; Tanfoni, S.; Borgianni, L.; Guyon, C.; et al. 1,2,4-Triazole-3-thione compounds as inhibitors of dizinc metallo-β-lactamases. ChemMedChem 2017, 12, 972–985. [Google Scholar] [CrossRef] [Green Version]
- Gavara, L.; Sevaille, L.; De Luca, F.; Mercuri, P.; Bebrone, C.; Feller, G.; Legru, A.; Cerboni, G.; Tanfoni, S.; Baud, D.; et al. 4-Amino-1, 2, 4-triazole-3-thione-derived Schiff bases as metallo-β-lactamase inhibitors. Eur. J. Med. Chem. 2020, 208, 112720. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.A.; Soliman, S.M.; Yousuf, S.; Bibi, M.; Barakat, A. N-Acetyl Indole Linked to a Fused Triazolo/Thiadiazole Scaffold: Synthesis, Single Crystal X-Ray Structure, and Molecular Insight. Crystals 2020, 10, 600. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Abolibda, T.Z.; Sayed, A.R.; Gomha, S.M. Synthetic utility of aminomercapto [1,2,4] triazoles in the preparation of fused triazoles. Curr. Org. Chem. 2022, 26, 693–714. [Google Scholar]
- Zhu, L.; Tang, S.Y.; Chen, D.P.; Li, C.P.; Shao, L.H.; Ouyang, G.P.; Wang, Z.C.; Li, Z.R. Synthesis and antibacterial activity of indole 3-substituted-[1,2,4] triazole derivatives. Chem. Pap. 2022, 77, 895–907. [Google Scholar] [CrossRef]
- Dadlani, V.G.; Chhabhaiya, H.; Somani, R.R.; Tripathi, P.K. Synthesis, molecular docking, and biological evaluation of novel 1, 2, 4-triazole-isatin derivatives as potential Mycobacterium tuberculosis shikimate kinase inhibitors. Chem. Biol. Drug Des. 2022, 100, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Trafalis, D.T.; Sagredou, S.; Dalezis, P.; Voura, M.; Fountoulaki, S.; Nikoleousakos, N.; Almpanakis, K.; Deligiorgi, M.V.; Sarli, V. Anticancer Activity of Triazolo-Thiadiazole Derivatives and Inhibition of AKT1 and AKT2 Activation. Pharmaceutics 2021, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Liu, H.Y.; Wang, H.X.; Shi, Y.P.; Tang, R.; Zhang, S.; Chen, B.Q. Synthesis and antitumor evaluation of novel fused heterocyclic 1, 2, 4-triazolo [3, 4-b]-1, 3, 4-thiadiazole derivatives. Med. Chem. Res. 2019, 28, 1718–1725. [Google Scholar] [CrossRef]
- Boraei, A.T.; Singh, P.K.; Sechi, M.; Satta, S. Discovery of novel functionalized 1, 2, 4-triazoles as PARP-1 inhibitors in breast cancer: Design, synthesis and antitumor activity evaluation. Eur. J. Med. Chem. 2019, 182, 111621. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Sarhan, A.A.M.; Yousuf, S.; Barakat, A. Synthesis of a New Series of Nitrogen/Sulfur Heterocycles by Linking Four Rings: Indole; 1,2,4-Triazole; Pyridazine; and Quinoxaline. Molecules 2020, 25, 450. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, A.A.; Boraei, A.T.; Barakat, A.; Nafie, M.S. Discovery of hydrazide-based pyridazino [4,5-b] indole scaffold as a new phosphoinositide 3-kinase (PI3K) inhibitor for breast cancer therapy. RSC Adv. 2020, 10, 19534–19541. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Rikagu Oxford Diffraction Inc.: Yarnton, UK, 2020. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B.J. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17 University of Western Australia. 2017. Available online: http://hirshfeldsurface.net (accessed on 31 July 2021).
- Dey, D.; Bhandary, S.; Thomas, S.P.; Spackman, M.A.; Chopra, D. Energy frameworks and a topological analysis of the supramolecular features in in situ cryocrystallized liquids: Tuning the weak interaction landscape via fluorination. Phys. Chem. Chem. Phys. 2016, 18, 31811–31820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miroslaw, B.; Demchuk, O.M.; Luboradzki, R.; Tyszczuk-Rotko, K. Low-Molecular-Weight Organogelators Based on N-dodecanoyl-L-amino Acids—Energy Frameworks and Supramolecular Synthons. Materials 2023, 16, 702. [Google Scholar] [CrossRef] [PubMed]


| 9 | |
|---|---|
| CCDC | 2239222 |
| empirical formula | C18H13N5S |
| fw | 331.39 |
| temp. (K) | 120(2) K |
| λ(Å) | 0.71073 Å |
| cryst syst | Monoclinic |
| space group | P21/n |
| a (Å) | a = 7.8707(2) Å |
| b (Å) | b = 15.9681(4) Å |
| c (Å) | c = 11.9798(4) Å |
| β (deg) | 100.283(3)° |
| V (Å3) | 1481.44(7) Å3 |
| Z | 4 |
| ρcalc (Mg/m3) | 1.486 Mg/m3 |
| μ(Mo Kα) (mm−1) | 0.228 mm−1 |
| No. reflns. | 7305 |
| Unique reflns. | 3997 |
| Completeness to θ = 25.242° | 100% |
| GOOF (F2) | 1.031 |
| Rint | 0.0213 |
| R1 a (I ≥ 2σ) | 0.0454 |
| wR2 b (I ≥ 2σ) | 0.1095 |
| Bond | Length/Å | Bond | Length/Å |
|---|---|---|---|
| S(1)-C(10) | 1.7275(18) | N(3)-C(10) | 1.306(2) |
| S(1)-C(11) | 1.7734(17) | N(4)-C(10) | 1.360(2) |
| N(1)-C(7) | 1.376(2) | N(4)-C(9) | 1.370(2) |
| N(1)-C(8) | 1.381(2) | N(4)-N(5) | 1.3747(19) |
| N(2)-C(9) | 1.324(2) | N(5)-C(11) | 1.304(2) |
| N(2)-N(3) | 1.402(2) | ||
| Bonds | Angle/° | Bonds | Angle/° |
| C(10)-S(1)-C(11) | 87.61(8) | N(1)-C(7)-C(6) | 130.16(16) |
| C(7)-N(1)-C(8) | 108.31(14) | N(1)-C(7)-C(2) | 107.94(15) |
| C(9)-N(2)-N(3) | 109.54(14) | N(1)-C(8)-C(9) | 120.90(15) |
| C(10)-N(3)-N(2) | 104.79(14) | N(2)-C(9)-N(4) | 107.75(14) |
| C(10)-N(4)-C(9) | 105.87(14) | N(2)-C(9)-C(8) | 127.46(16) |
| C(10)-N(4)-N(5) | 118.88(14) | N(4)-C(9)-C(8) | 124.79(15) |
| C(9)-N(4)-N(5) | 135.17(14) | N(3)-C(10)-N(4) | 112.04(15) |
| C(11)-N(5)-N(4) | 107.48(14) | N(3)-C(10)-S(1) | 138.55(14) |
| N(5)-C(11)-S(1) | 116.67(13) | N(4)-C(10)-S(1) | 109.37(12) |
| C(1)-C(8)-N(1) | 109.98(15) | N(5)-C(11)-C(12) | 123.27(15) |
| D-H…A | d(D-H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| N1-H1…N2#1 | 0.85(2) | 2.12(2) | 2.959(2) | 169(2) |
| N1-H1…N3#1 | 0.85(2) | 2.60(2) | 3.292(2) | 139(2) |
| C14-H14…S1#2 | 0.95 | 2.905 | 2.905 | 179.35 |
| Contact | Distance | Symmetry Code |
|---|---|---|
| C1…C18 | 3.371 | 1 − x,1 − y,1 − z |
| C3…C13 | 3.342 | 1 − x,1 − y,1 − z |
| C9…C15 | 3.288 | 2 − x,1 − y,1 − z |
| C10…C17 | 3.349 | 2 − x,1 − y,1 − z |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| N2…H1 | 1.963 | C9…C15 | 3.288 |
| N3…H1 | 2.483 | C10…C17 | 3.349 |
| N3…H6 | 2.512 | C6…S1 | 3.469 |
| S1…H14 | 2.772 | C7…S1 | 3.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Haukka, M.; Soliman, S.M.; Barakat, A.; Alaswad, S.O.; Boraei, A.T.A.; Gad, E.M.; Youssef, M.F. Synthesis, Characterization and Single Crystal X-ray Diffraction Analysis of Fused Triazolo/Thiadiazole Clubbed with Indole Scaffold. Crystals 2023, 13, 423. https://doi.org/10.3390/cryst13030423
Altowyan MS, Haukka M, Soliman SM, Barakat A, Alaswad SO, Boraei ATA, Gad EM, Youssef MF. Synthesis, Characterization and Single Crystal X-ray Diffraction Analysis of Fused Triazolo/Thiadiazole Clubbed with Indole Scaffold. Crystals. 2023; 13(3):423. https://doi.org/10.3390/cryst13030423
Chicago/Turabian StyleAltowyan, Mezna Saleh, Matti Haukka, Saied M. Soliman, Assem Barakat, Saleh O. Alaswad, Ahmed T. A. Boraei, Emad M. Gad, and Mohamed F. Youssef. 2023. "Synthesis, Characterization and Single Crystal X-ray Diffraction Analysis of Fused Triazolo/Thiadiazole Clubbed with Indole Scaffold" Crystals 13, no. 3: 423. https://doi.org/10.3390/cryst13030423








