Reactive Ceramic Membrane for Efficient Micropollutant Purification with High Flux by LED Visible-Light Photocatalysis: Device Level Attempts
Abstract
1. Introduction
2. Experiment and Methods
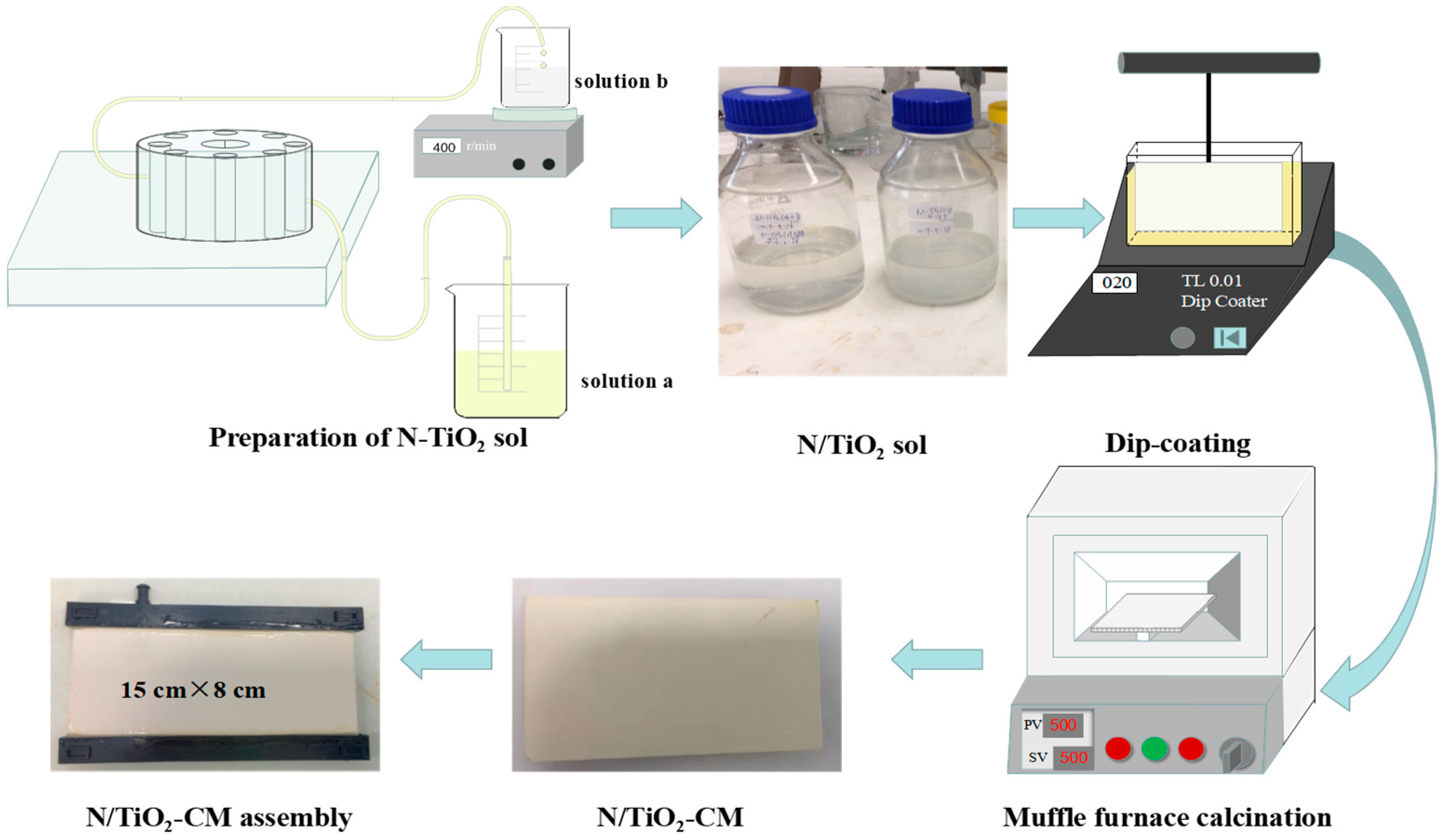
2.1. Preparation of the N-TiO2-CM
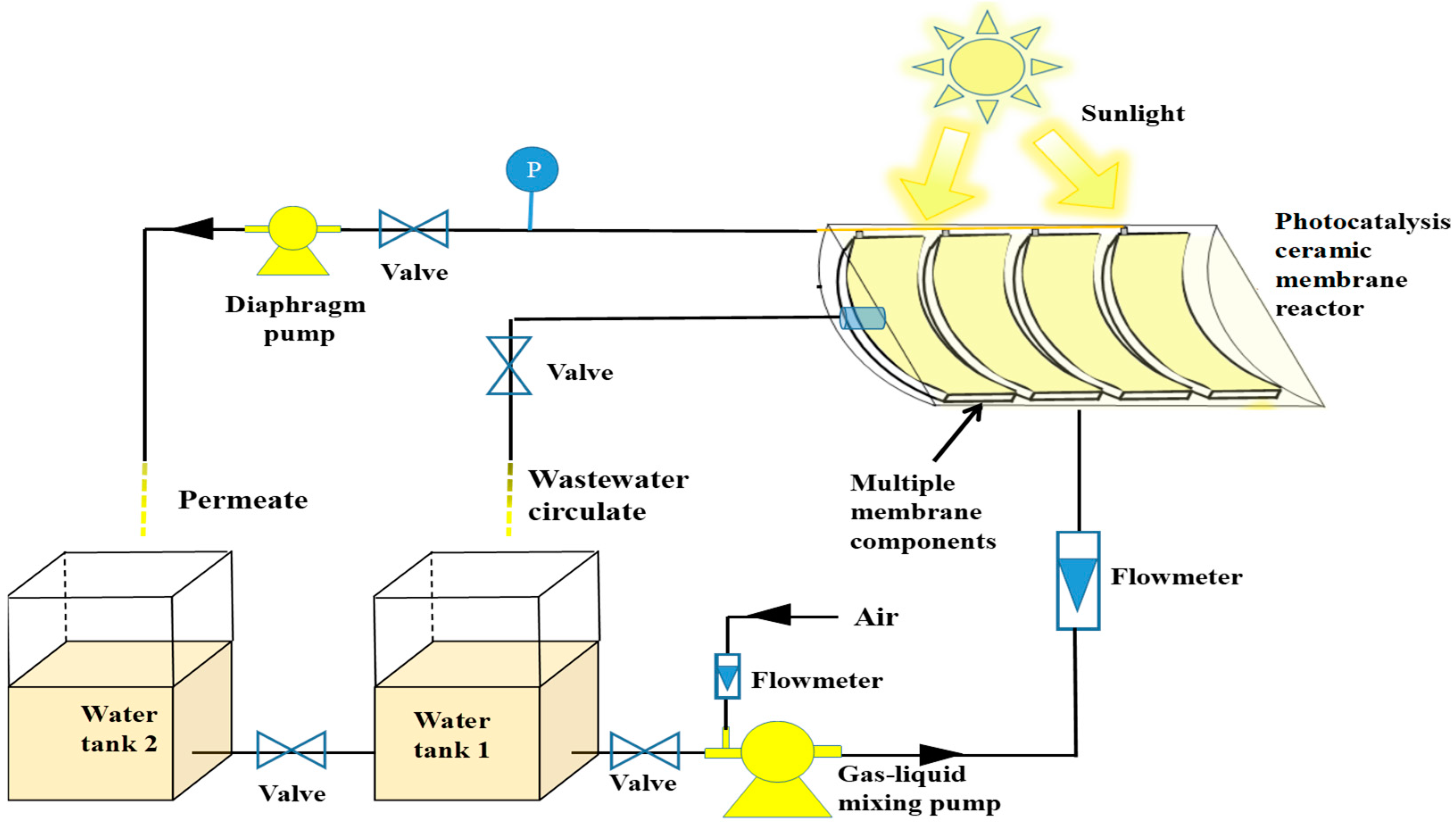
2.2. The Experimental Setup of PRCM
2.3. Removal of MP by the Fixed Bed PRCM System
2.4. Analysis Methods
3. Results and Discussion
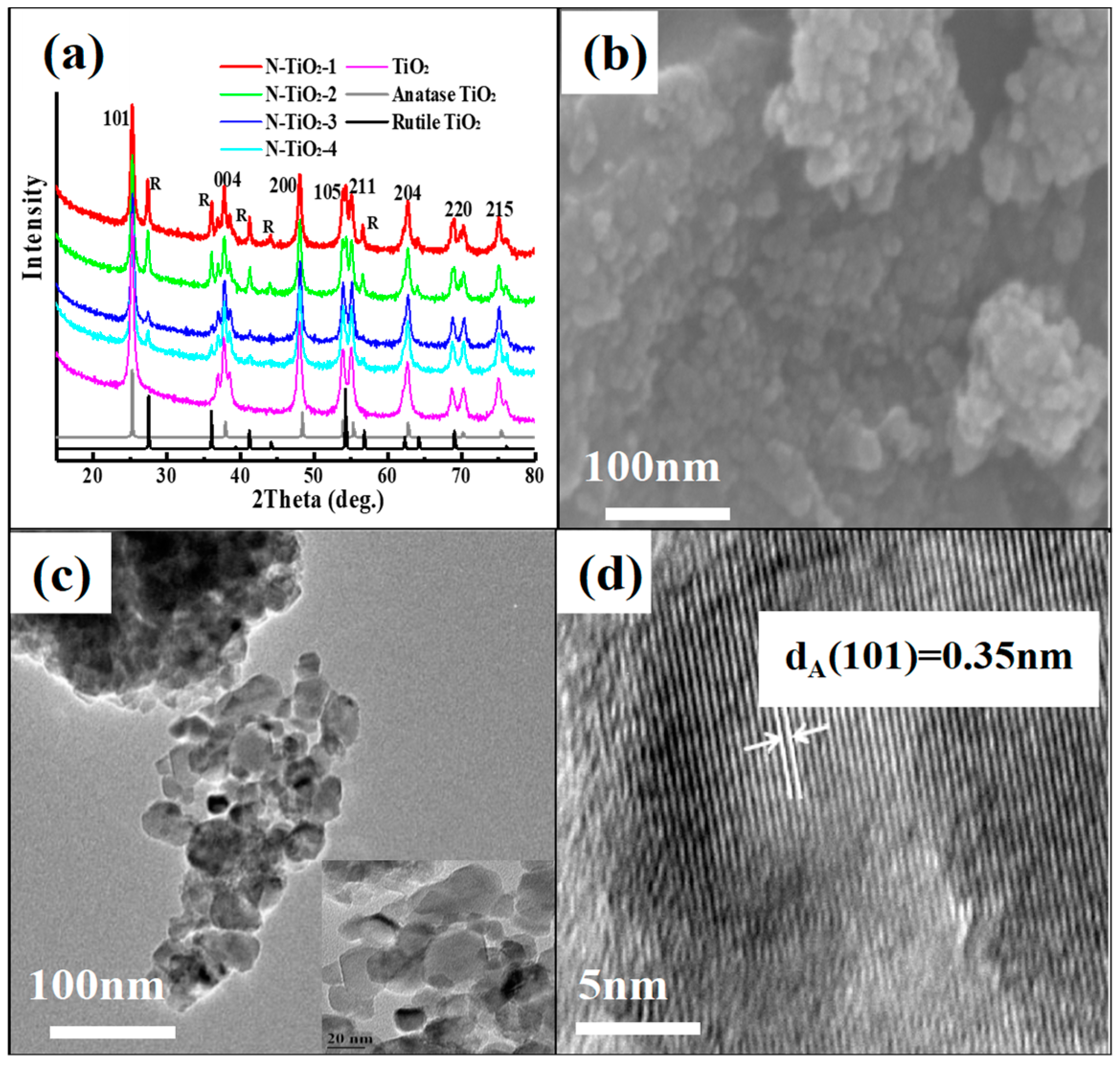
3.1. Characterization of the N-TiO2 Nanoparticles
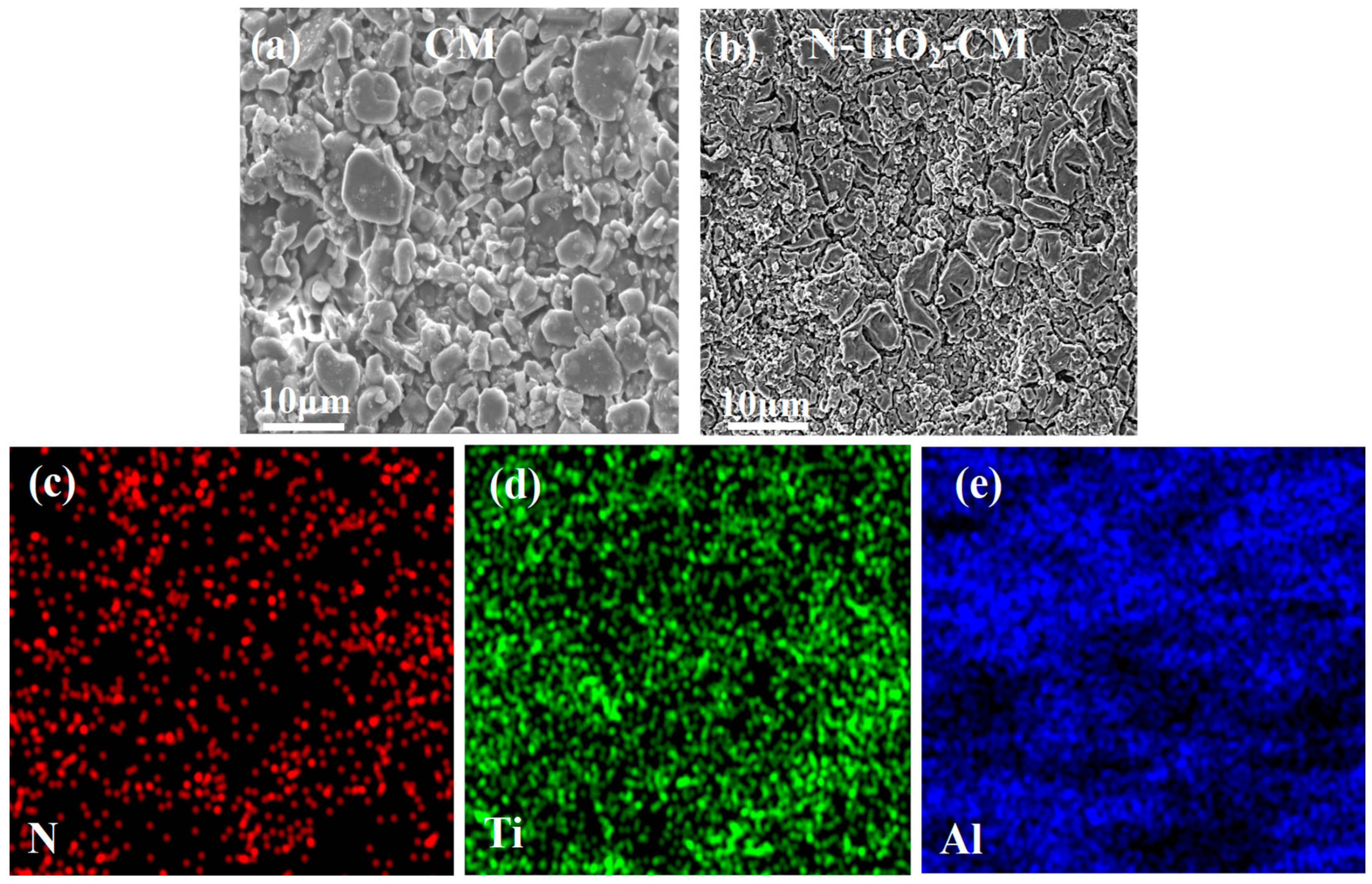
3.2. Characterization of PRCM
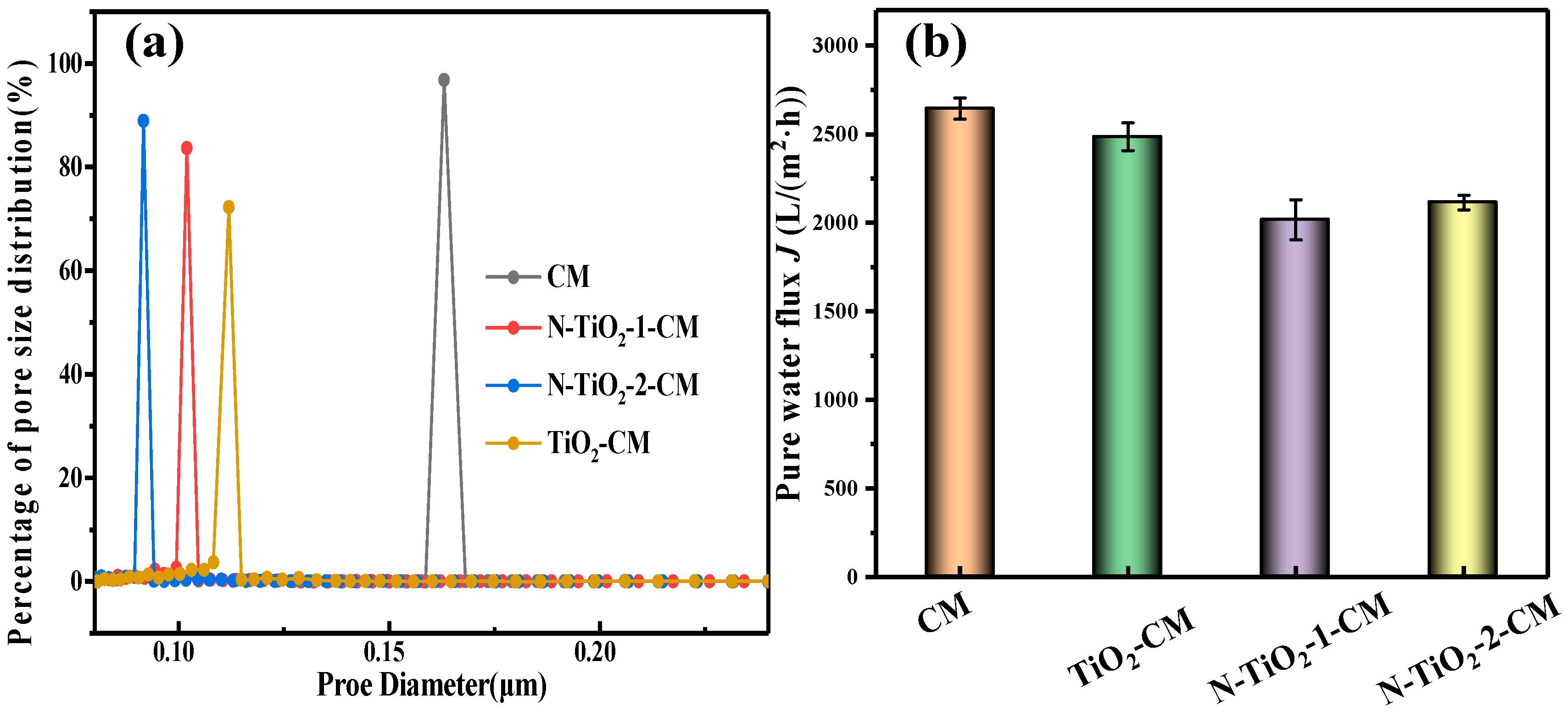
3.3. Performance Evaluation of PRCM
| Membrane Type | Loading Method | Pollutants | Other Conditions | Shape and Size of PRCM | Flux of Pure Water | Treatment Capacity | Reaction Time | Removal Efficiency | Reference |
|---|---|---|---|---|---|---|---|---|---|
| CuO/TiO2-CM | Extrusion method | Ciprofloxacin (C0 = 0.5 mg/L) | One LED UVA lamp TMP = 0.2 MPa | 4.7 × 10−3 m2 Tubular PRCM (D × L = 1 cm × 15 cm) | 218 L/(m2 × h) | 25.5 L/(m2 × h) | 60 min | 99.5% | [49] |
| TiO2-CM | Atomic layer deposition | Methylene blue (C0 = 1 mg/L) | One UV lights of 365 nm with 10 mW/cm2 TMP = 0.1 MPa | 1.8 × 10−4 m2 Disc PRCM (D = 1.5 cm) | 150 L/(m2 × h) | 1666.7 L/(m2 × h) | 100 min | 50% | [50] |
| Ag-TiO2- CM | Dip-coating method | Bisphenol A (C0 = 10 mg/L) | One 100 W Xe lamp TMP = 0.2 MPa | 1.4 × 10−3 m2 Tubular PRCM (D × L = 1.5 cm × 8 cm) | 325 L/(m2 × h) | 157.1 L/(m2 × h) | 270 min | 88% | [51] |
| Graphene-TiO2-CM | Dip-coating method | Estradiol (C0 = 0.5 mg/L) | One 6 W LED UVA lamp TMP = 10 MPa | 8.8 × 10−3 m2 Tubular PRCM (D × L = 1.4 cm × 20 cm) | 90 L/(m2 × h) | 90.9 L/(m2 × h) | 210 min | 42.1% | [52] |
| N-TiO2- CM | Dip-coating method | Tetracycline (C0 = 20 mg/L) | Two 45 W LED UV lamps with 24 µmol/(m2 s) TMP = −0.092 MPa | 1.2 × 10−2 m2 Planar PRCM (L × W = 15 × 8 cm) | 2114.2 L/(m2 × h) | 475.8 L/(m2 × h) | 270 min | 92% | This work |
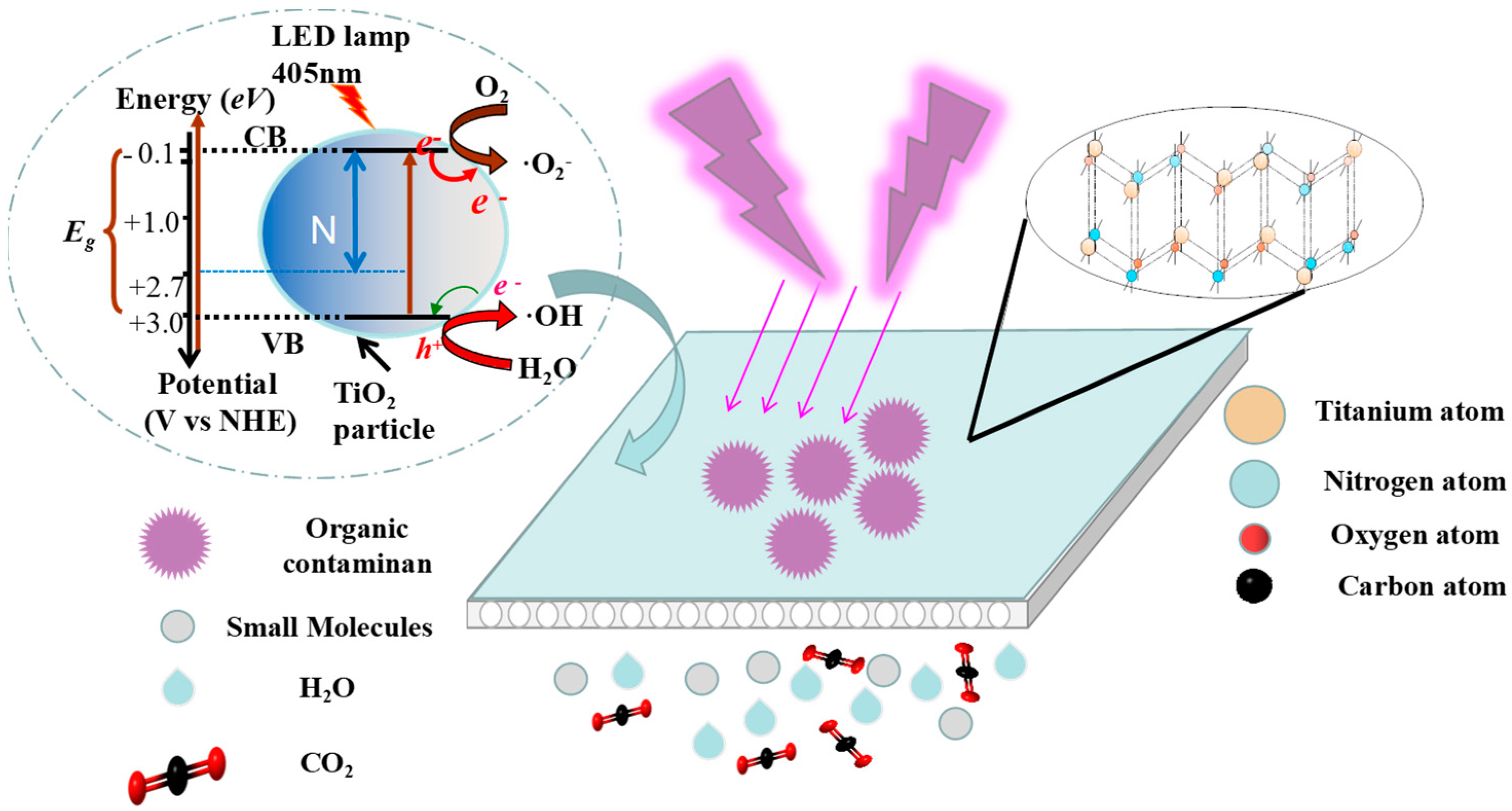
3.4. Photocatalytic Mechanism of PRCM
3.5. Utilization Proposal of Scale-up N-TiO2-CM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, W.; Chen, S.; Yu, H.; Xu, T.; Liang, H. Photocatalytic ozonation of organic pollutants in wastewater using a flowing through reactor. J. Hazard. Mater. 2020, 405, 124277. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.J.; Emond, C.; Hardy, E.M.; Sauvageot, N.; Appenzeller, B. Population-based biomonitoring of exposure to persistent and non-persistent organic pollutants in the Grand Duchy of Luxembourg: Results from hair analysis. Environ. Int. 2021, 153, 106526. [Google Scholar] [CrossRef] [PubMed]
- Martí-Calatayud, M.C.; Heßler, R.; Schneider, S.; Bohner, C.; Yüce, S.; Wessling, M.; de Sena, R.F.; Athayde Júnior, G.B. Transients of micropollutant removal from high-strength wastewaters in PAC-assisted MBR and MBR coupled with high-retention membranes. Sep. Purif. Technol. 2020, 246, 116863. [Google Scholar] [CrossRef]
- Egea-Corbacho, A.; Martín-García, A.P.; Franco, A.A.; Quiroga, J.M.; Andreasen, R.R.; Jørgensen, M.K.; Christensen, M.L. Occurrence, identification and removal of microplastics in a Wastewater Treatment Plant compared to an advanced MBR technology: Full-scale pilot plant. J. Environ. Chem. Eng. 2023, 11, 109644. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Liu, C.; Li, P.; Tang, X.; Korshin, G.V. Ozonation effects on emerging micropollutants and effluent organic matter in wastewater: Characterization using changes of three-dimensional HP-SEC and EEM fluorescence data. Environ. Sci. Pollut. Res. 2016, 23, 20567–20579. [Google Scholar] [CrossRef]
- Chavoshani, A.; Hashemi, M.; Amin, M.M.; Ameta, S.C. Pharmaceuticals as emerging micropollutants in aquatic environments. In Micropollutants and Challenges; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–90. [Google Scholar]
- Büning, B.; Rechtenbach, D.; Behrendt, J.; Otterpohl, R. Removal of Emerging Micropollutants from Wastewater by Nanofiltration and Biofilm Reactor (MicroStop). Environ. Prog. Sustain. Energy 2020, 40, 13587. [Google Scholar] [CrossRef]
- Ws, A.; Lai, Y.; Hyna, B. Nanofiltration and reverse osmosis processes for the removal of micro-pollutants. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 527–552. [Google Scholar]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, R.; Huang, D.; Shen, Y.; Shi, W. Membrane fouling in microfiltration of alkali/surfactant/polymer flooding oilfield wastewater: Effect of interactions of key foulants. J. Colloid Interface Sci. 2020, 570, 20–30. [Google Scholar] [CrossRef]
- PhD, A.M.N.; Adam, M.R.; Kamal, S.N.E.A.; Jaafar, J.; Othman, M.H.D. A review of the potential of conventional and advanced membrane technology in the removal of pathogens from wastewater. Sep. Purif. Technol. 2022, 286, 120454. [Google Scholar]
- Abdel-Shafy, H.I.; El-Khateeb, M.A.; Mansour, M.S. Treatment of leather industrial wastewater via combined advanced oxidation and membrane filtration. Water Sci. Technol. 2016, 74, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Hu, X.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues: A review on its potential applications, advances, and challenges. Sci. Total Environ. 2021, 784, 146912. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cheng, Z.; Wei, J.; Xu, Q.; Zhang, X.; Wei, Z. Efficient degradation of antibiotics by photo-Fenton reactive ceramic membrane with high flux by a facile spraying method under visible LED light. J. Clean. Prod. 2022, 366, 132849. [Google Scholar] [CrossRef]
- Oliveira, C.; Viana, M.M.; Amaral, M. Coupling photocatalytic degradation using a green TiO2 catalyst to membrane bioreactor for petroleum refinery wastewater reclamation. J. Water Process Eng. 2020, 34, 101093. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A. Photocatalytic-membrane technology: A critical review for membrane fouling mitigation. J. Ind. Eng. Chem. 2021, 93, 101–116. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Hou, Y.; Li, P.; Sun, H.; Niu, Q.J. Photo-Fenton assisted self-cleaning hybrid ultrafiltration membranes with high-efficient flux recovery for wastewater remediation. Sep. Purif. Technol. 2020, 249, 117159. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2020, 408, 124461. [Google Scholar] [CrossRef]
- Scaratti, G.; De Noni Júnior, A.; José, H.J.; de Fatima Peralta Muniz Moreira, R. 1,4-Dioxane removal from water and membrane fouling elimination using CuO-coated ceramic membrane coupled with ozone. Environ. Sci. Pollut. Res. 2020, 27, 22144–22154. [Google Scholar] [CrossRef]
- Xu, T.; Cui, L.; Li, H.; Zou, P.; Liang, J. Effects of electrolytic oxidation for mitigating ultrafiltration membrane fouling caused by different natural organic matter fractions. Environ. Sci. Water Res. Technol. 2020, 6, 645–655. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, Z.; Yan, C.; Zhang, X.; Tian, Y.; Zhang, X.; Quan, X. Simultaneous Removal of Nitrogen and Refractory Organics from a Biologically Treated Leachate by Pulse Electrochemical Oxidation in a Multi-channel Flow Reactor. ACS Omega 2021, 6, 25539–25550. [Google Scholar] [CrossRef]
- Le, D.T.; Luong, N.S.; Ngo, V.D.; Tien, N.M.; Dung, T.N.; Nghia, N.M.; Loc, N.T.; Thu, V.T.; Lam, T.D. Highly Visible Light Activity of Nitrogen Doped TiO2 Prepared by Sol-Gel Approach. J. Electron. Mater. 2016, 46, 158–166. [Google Scholar]
- Wei, W.A.; Mot, A.; Zsa, B. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar]
- Yu, C.; Fan, C.; Meng, X.; Kai, Y.; Cao, F.; Xin, L. A novel Ag/BiOBr nanoplate catalyst with high photocatalytic activity in the decomposition of dyes. React. Kinet. Mech. Catal. 2011, 103, 141–151. [Google Scholar] [CrossRef]
- Lecorre, D.; Bras, J.; Dufresne, A. Ceramic membrane filtration for isolating starch nanocrystals-ScienceDirect. Carbohyd. Polym. 2011, 86, 1565–1572. [Google Scholar] [CrossRef]
- Biswas, N.K.; Srivastav, A.; Saxena, S.; Verma, A.; Dutta, R. N-TiO2 crystal seeds incorporated in amorphous matrix for enhanced solar hydrogen generation: Experimental & first-principles analysis. Int. J. Hydrogen Energy 2022, 47, 22415–22429. [Google Scholar]
- He, Y.; Zhang, X.; Wei, Y.; Chen, X.; Wang, Z.; Yu, R. Ti-MOF derived N-Doped TiO2 nanostructure as visible-light-driven photocatalyst. Chem. Res. Chin. Univ. 2020, 36, 447–452. [Google Scholar] [CrossRef]
- Assayehegn, E.; Solaiappan, A.; Chebude, Y.; Alemayehu, E. Fabrication of tunable anatase/rutile heterojunction N/TiO2 nanophotocatalyst for enhanced visible light degradation activity. Appl. Surf. Sci. 2020, 515, 145966. [Google Scholar] [CrossRef]
- Mehdizadeh, P.; Tavangar, Z.; Shabani, N.; Hamadanian, M. Visible light activity of nitrogen-doped TiO2 by sol-gel method using various nitrogen sources. J. Nanostruct. 2020, 10, 307–316. [Google Scholar]
- Divya, G.; Jaishree, G.; Rao, T.S.; Chippada, M.L.V.P.; Lakshmi, K.V.D.; Supriya, S.S. Improved catalytic efficiency by N-doped TiO2 via sol gel under microwave irradiation: Dual applications in degradation of dye and microbes. Hybrid Adv. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Yu, Y.; Xia, J.; Chen, C.; Chen, H.; Geng, J.; Li, H. One-step synthesis of a visible-light driven C@N-TiO2 porous nanocomposite: Enhanced absorption, photocatalytic and photoelectrochemical performance. J. Phys. Chem. Solids 2020, 136, 109169. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, X.; Wang, Y. Photocatalytic degradation of flumequine by N-doped TiO2 catalysts under simulated sunlight. Environ. Eng. Res. 2021, 26, 200524. [Google Scholar] [CrossRef]
- Berktaş, A.; Kartal, Ö.E. Decolorization of Reactive Black 5 Using N-Doped TiO2. Gazi Univ. J. Sci. 2022, 35, 360–370. [Google Scholar] [CrossRef]
- Chng, E.; Sahrin, N.T.; Nawaz, R.; Kait, C.F.; Lee, S.L. Glycerol-assisted sol-gel synthesis of Nitrogen-doped Titanium dioxide for visible light-driven photodegradation of indoor gaseous formaldehyed. Platf. J. Sci. Technol. 2021, 4, 17–31. [Google Scholar]
- Yang, X.; Jia, Q.; Pang, J.; Yang, Y.; Zheng, S.; Jia, J.; Qin, Z. Hierarchical porous N-TiO2/carbon foam composite for enhancement of photodegradation activity under simulated sunlight. Diam. Relat. Mater. 2022, 128, 109234. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Zuo, M.; Liang, Y.; Qin, P.; Wang, H.; Wu, Z.; Luo, L.; Liu, C.; Leng, L. Preparation of photocatalysts decorated by carbon quantum dots (CQDs) and their applications: A review. J. Environ. Chem. Eng. 2023, 11, 109487. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, H.; Rana, A.; Saini, V.K. Facile synthesis of boron and nitrogen doped TiO2 as effective catalysts for photocatalytic degradation of emerging micro-pollutants. J. Ind. Eng. Chem. 2022, 107, 126–136. [Google Scholar] [CrossRef]
- Fazio, E.; Mezzasalma, A.M.; Urso, L.D.; Spadaro, S.; Barreca, F.; Gallo, G.; Neri, F.; Compagnini, G. N-TiO2-x nanocatalysts: PLAL synthesis and photocatalytic activity. J. Nanomater. 2020, 2020, 2901516. [Google Scholar] [CrossRef]
- Mhemid, R.K.S.; Salman, M.S.; Mohammed, N.A. Comparing the efficiency of N-doped TiO2 and commercial TiO2 as photo catalysts for amoxicillin and ciprofloxacin photo-degradation under solar irradiation. J. Environ. Sci. Health Part A 2022, 57, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Lee, M.; Chun, S.; Lee, G.; Kim, J.; Jeong, B.; Kim, T.; Hong-Dae, K. Promotional effect of surface treated N-TiO2 as support for VOx-based catalysts on the selective catalytic reduction of NO using NH3. Appl. Surf. Sci. 2021, 560, 149934. [Google Scholar] [CrossRef]
- Krishnan, S.; Karim, A.V.; Shriwastav, A. Visible light responsive Cu-N/TiO2 nanoparticles for the photocatalytic degradation of bisphenol A. Water Sci. Technol. 2022, 86, 1527–1539. [Google Scholar] [CrossRef]
- Liu, F.; Cao, H.; Xu, L.; Fu, H.; Sun, S.; Xiao, Z.; Sun, C.; Long, X.; Xia, Y.; Wang, S. Design and preparation of highly active TiO2 photocatalysts by modulating their band structure. J. Colloid Interface Sci. 2023, 629, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Raza, N.; Raza, W.; Gul, H.; Azam, M.; Kim, K.H. Solar-light-active silver phosphate/titanium dioxide/silica heterostructures for photocatalytic removal of organic dye. J. Clean. Prod. 2020, 254, 120031. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Tala-Ighil, R.; Tounane, M.; Iratni, A.; Souier, T.; Liu, S.; Cai, W. Optical, structural and photocatalysis properties of Cu-doped TiO2 thin films. Appl. Surf. Sci. 2017, 395, 110–116. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012, 211, 88–94. [Google Scholar] [CrossRef]
- Pathania, D.; Katwal, R.; Sharma, G.; Naushad, M.; Khan, M.R.; Ala’a, H. Novel guar gum/Al2O3 nanocomposite as an effective photocatalyst for the degradation of malachite green dye. Int. J. Biol. Macromol. 2016, 87, 366–374. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, D.; Dey, S.; Ghosh, S.; Banerjee, S. Development and performance evaluation of a novel CuO/TiO2 ceramic ultrafiltration membrane for ciprofloxacin removal. Mater. Chem. Phys. 2019, 229, 106–116. [Google Scholar] [CrossRef]
- Berger, T.E.; Regmi, C.; Schfer, A.I.; Richards, B.S. Photocatalytic degradation of organic dye via atomic layer deposited TiO2-ceramic membranes in single-pass flow-through operation. J. Membr. Sci. 2020, 604, 118015. [Google Scholar] [CrossRef]
- Shareef, U.; Othman, M.; Ismail, A.F.; Jilani, A. Facile removal of bisphenol A from water through novel Ag-doped TiO2 photocatalytic hollow fiber ceramic membrane. J. Australas. Ceram. Soc. 2020, 56, 29–39. [Google Scholar] [CrossRef]
- Presumido, P.H.; Santos, L.; Neuparth, T.; Santos, M.M.; Feliciano, M.; Primo, A.; Garcia, H.; B-Oli, M.; Vilar, V. A Novel ceramic tubular membrane coated with a continuous graphene-TiO2 nanocomposite thin-film for CECs mitigation. Chem. Eng. J. 2022, 430, 132639. [Google Scholar] [CrossRef]
- Verinda, S.B.; Muniroh, M.; Yulianto, E.; Maharani, N.; Gunawan, G.; Amalia, N.F.; Hobley, J.; Usman, A.; Nur, M. Degradation of ciprofloxacin in aqueous solution using ozone microbubbles: Spectroscopic, kinetics, and antibacterial analysis. Heliyon 2022, 8, 10137. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Frontistis, Z.; Nika, M.C.; Aalizadeh, R.; Thomaidis, N.S.; Mantzavinos, D. Sonochemical degradation of trimethoprim in water matrices: Effect of operating conditions, identification of transformation products and toxicity assessment. Ultrason. Sonochem. 2020, 67, 105139. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.R.D.; Ganiyu, S.O.; Santos, E.V.D.; Oturan, M.A.; Martínez-Huitle, C.A. Removal of antibiotic rifampicin from aqueous media by advanced electrochemical oxidation: Role of electrode materials, electrolytes and real water matrices. Electrochim. Acta 2021, 396, 139254. [Google Scholar] [CrossRef]
- Dhawle, R.; Mantzavinos, D.; Lianos, P. UV/H2O2 degradation of diclofenac in a photocatalytic fuel cell. Appl. Catal. B Environ. 2021, 299, 120706. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, H.; Wu, Y.; Dong, W. Discrepant oxidation behavior of ferric ion and hydroxyl radical on syringic acid and vanillic acid in atmospheric Fenton-like system. Chemosphere 2022, 287, 132022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Liu, Y.; Hao, J. One-pot hydrothermal synthesis of dual Z-scheme BiOBr/g-C3N4/Bi2WO6 and photocatalytic degradation of tetracycline under visible light. Mater. Lett. 2020, 281, 128463. [Google Scholar] [CrossRef]
- Xue, L.; An, F.; Yang, Y.; Ma, Y. Synthesis of N-TiO2/BiOI/RGO composites with significantly enhanced visible light photocatalytic activity. J. Mater. Res. 2020, 35, 153–161. [Google Scholar] [CrossRef]
- Hadi, H.M.; Wahab, H.S. Visible light photocatalytic decolourization of methyl orange using N-doped TiO2 nanoparticles. Al-Nahrain J. Sci. 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Wang, S.Q.; Liu, W.B.; Fu, P.; Cheng, W.L. Enhanced photoactivity of N-doped TiO2 for Cr (VI) removal: Influencing factors and mechanism. Korean J. Chem. Eng. 2017, 34, 1584–1590. [Google Scholar] [CrossRef]




| Treatment Methods | Pollutants | Removal Efficiency | Treatment Time | Other Conditions | Treatment Capacity | Reference |
|---|---|---|---|---|---|---|
| Photo-Fenton (α-Fe2O3-CM) | 20 mg/L Tetracycline | 82% | 200 min | H2O2 concentration 19.4 mmol/L, TMP 8 kPa, LED light 405 nm, light intensity 24 µmol/(m2·s). | 0.600 L/h | [15] |
| Ozonation | 10.94 mg/L ciprofloxacin | 84% | 600 min | O3 concentration 1.92 g/L, dissolved O3 concentration in solution 34.56 g/h | 1.000 L/h | [53] |
| Sonochemistry oxidation | 0.5 mg/L Trimethoprim | 90% | 90 min | power density = 36 W/L, pH = 6 | 0.133 L/h | [54] |
| Electrochemical oxidation (Carbon-felt) | 164.6 mg/L Rifampicin | 100% | 360 min | Current density = 50 mA/cm2, Potentials = 1.36 V, pH = 6.8, 50 mmol/L Na2SO4 | 0.025 L/h | [55] |
| UV/H2O2 | 5.92 × 10−4 mg/L Diclofenac | 90% | 90 min | H2O2 concentration 0.1 mmol/L, one Xe lamp with 100 mW/cm2, | 0.100 L/h | [56] |
| PRCM (N-TiO2-CM) | 20 mg/L Tetracycline | 92% | 270 min | Two 45 W LED UV lamps with 24 µmol/(m2 s) TMP = −0.092 MPa | 0.571 L/h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, X.; Fang, R.; Cheng, Z.; Xu, Q.; Ma, S.; Xiong, J.; Chen, P.; Feng, G. Reactive Ceramic Membrane for Efficient Micropollutant Purification with High Flux by LED Visible-Light Photocatalysis: Device Level Attempts. Crystals 2023, 13, 651. https://doi.org/10.3390/cryst13040651
Li S, Zhang X, Fang R, Cheng Z, Xu Q, Ma S, Xiong J, Chen P, Feng G. Reactive Ceramic Membrane for Efficient Micropollutant Purification with High Flux by LED Visible-Light Photocatalysis: Device Level Attempts. Crystals. 2023; 13(4):651. https://doi.org/10.3390/cryst13040651
Chicago/Turabian StyleLi, Shuo, Xuan Zhang, Rui Fang, Zhiliang Cheng, Qian Xu, Shu Ma, Jie Xiong, Peng Chen, and Guangjie Feng. 2023. "Reactive Ceramic Membrane for Efficient Micropollutant Purification with High Flux by LED Visible-Light Photocatalysis: Device Level Attempts" Crystals 13, no. 4: 651. https://doi.org/10.3390/cryst13040651
APA StyleLi, S., Zhang, X., Fang, R., Cheng, Z., Xu, Q., Ma, S., Xiong, J., Chen, P., & Feng, G. (2023). Reactive Ceramic Membrane for Efficient Micropollutant Purification with High Flux by LED Visible-Light Photocatalysis: Device Level Attempts. Crystals, 13(4), 651. https://doi.org/10.3390/cryst13040651








