Structural and Hirshfeld Surface Analysis of Thallium(I) and Indium(III) Complexes of a Soft Scorpionate Ligand
Abstract
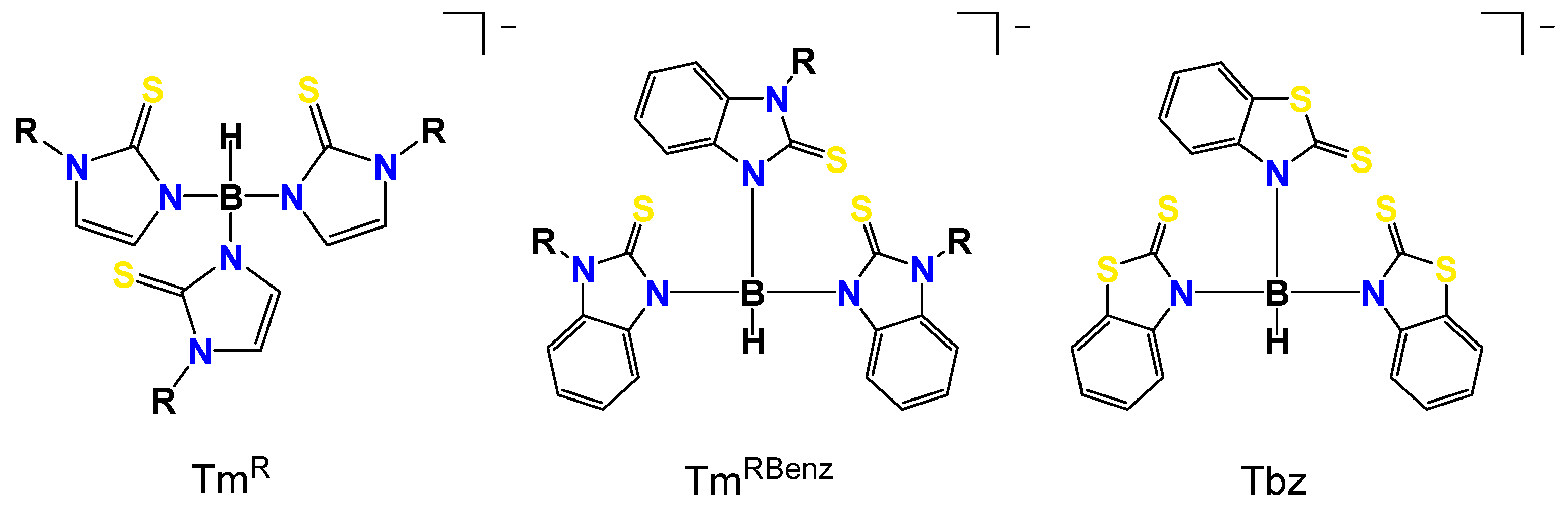
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Instrumentation
2.3. Preparation of Complexes
2.3.1. [Tl(TmtBu)]2∙2H2O
2.3.2. [In(TmtBu)2](InCl4)
2.4. X-ray Crystallography
3. Results and Discussion
3.1. Synthesis and Characterization
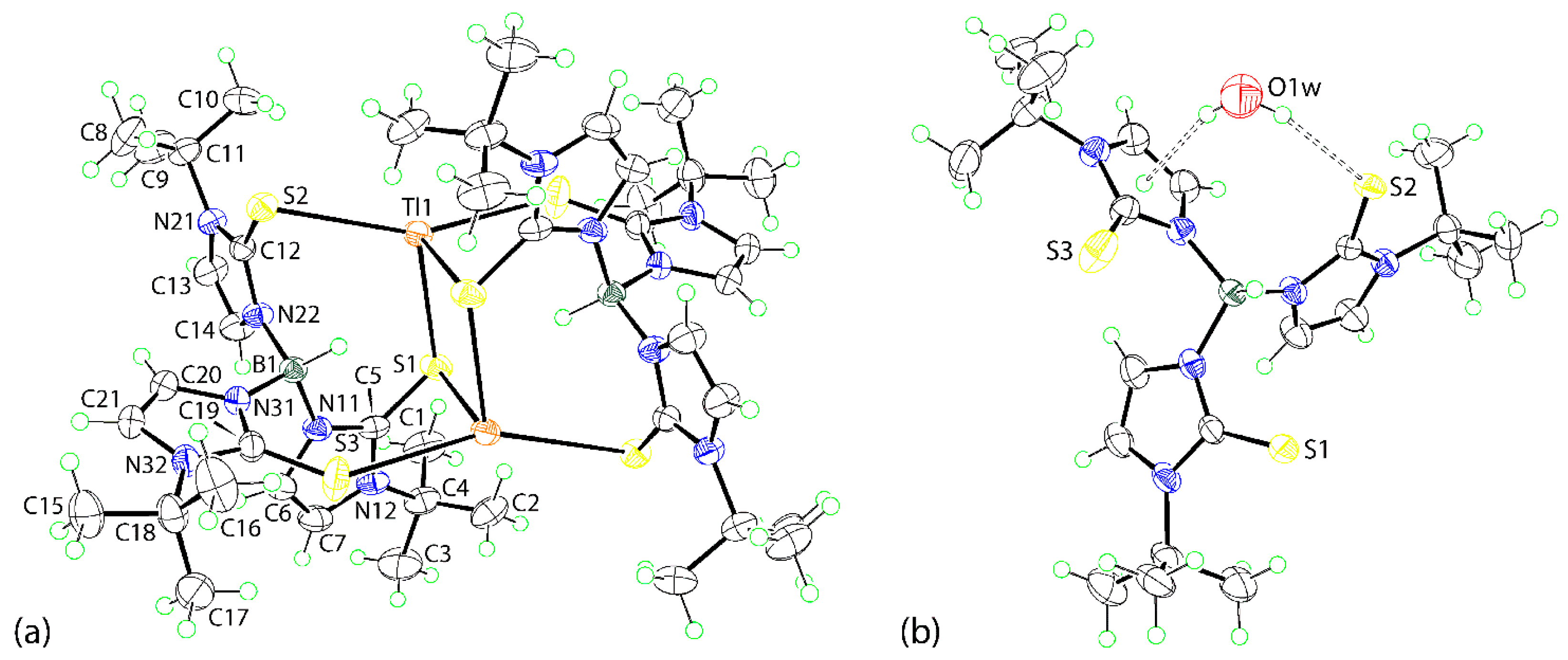
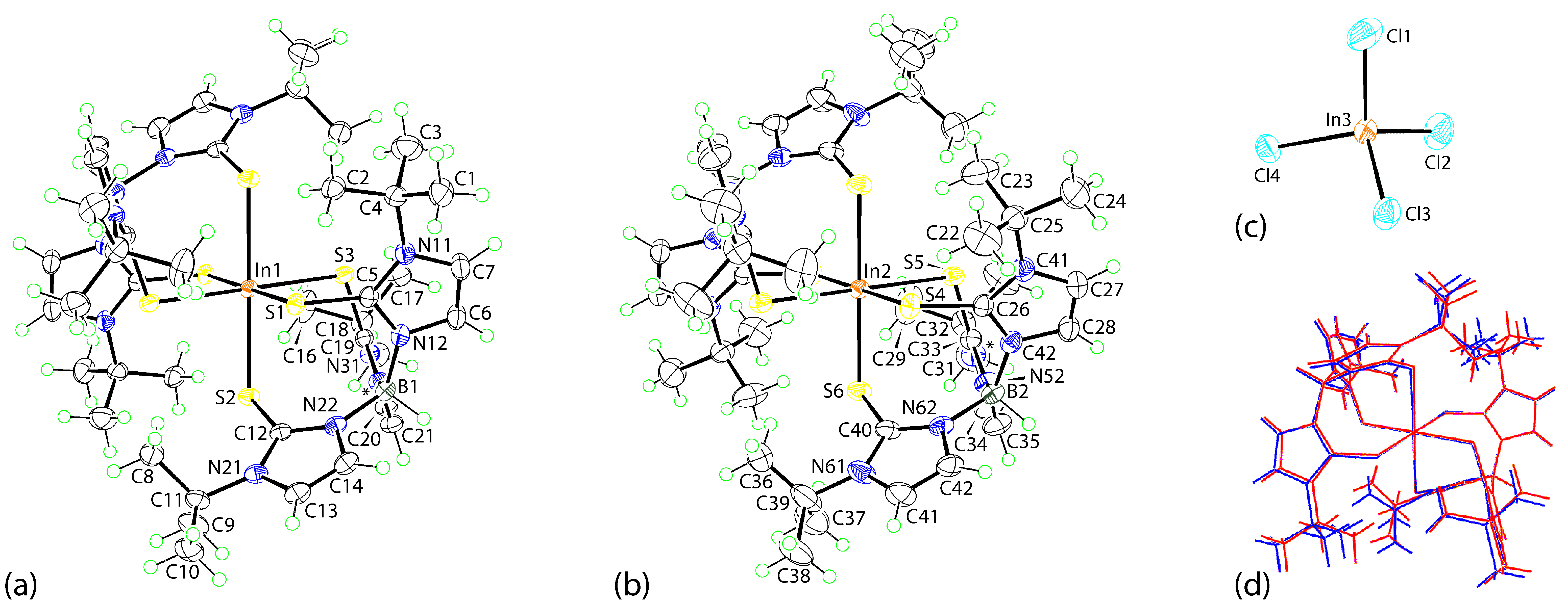
3.2. Crystal and Molecular Structures
3.2.1. Molecular Structures
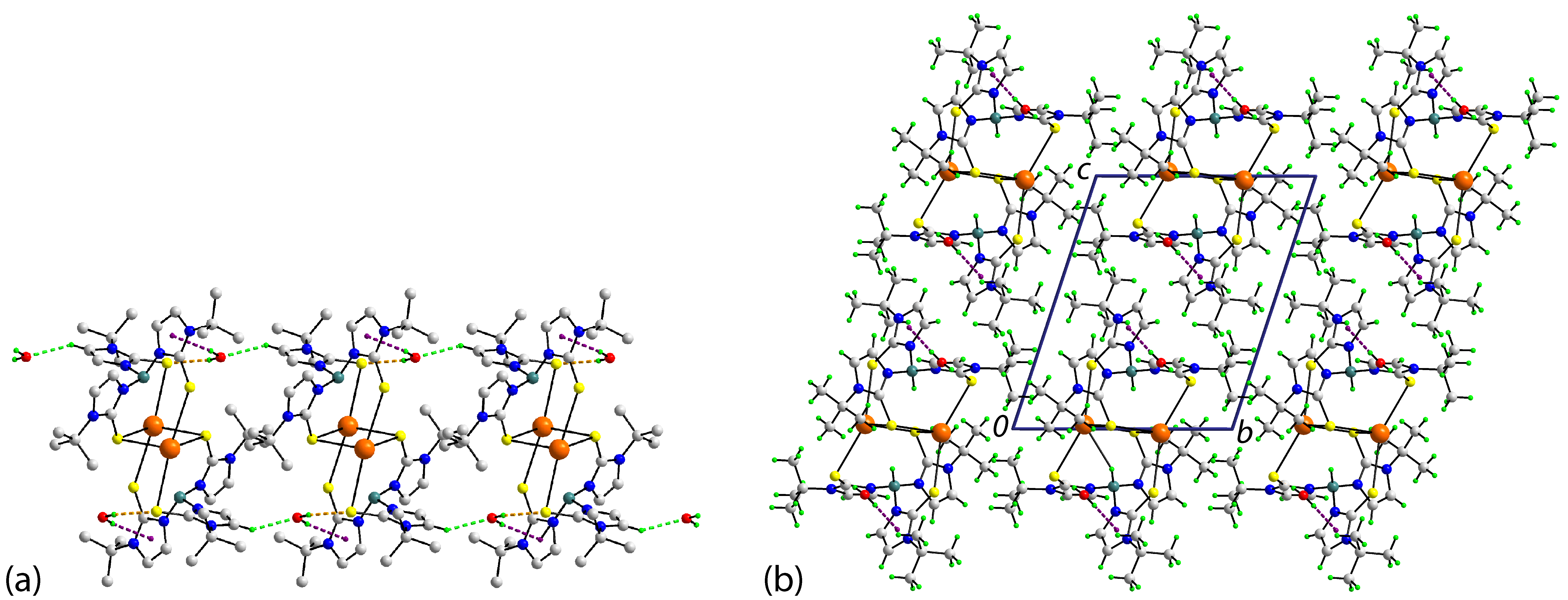
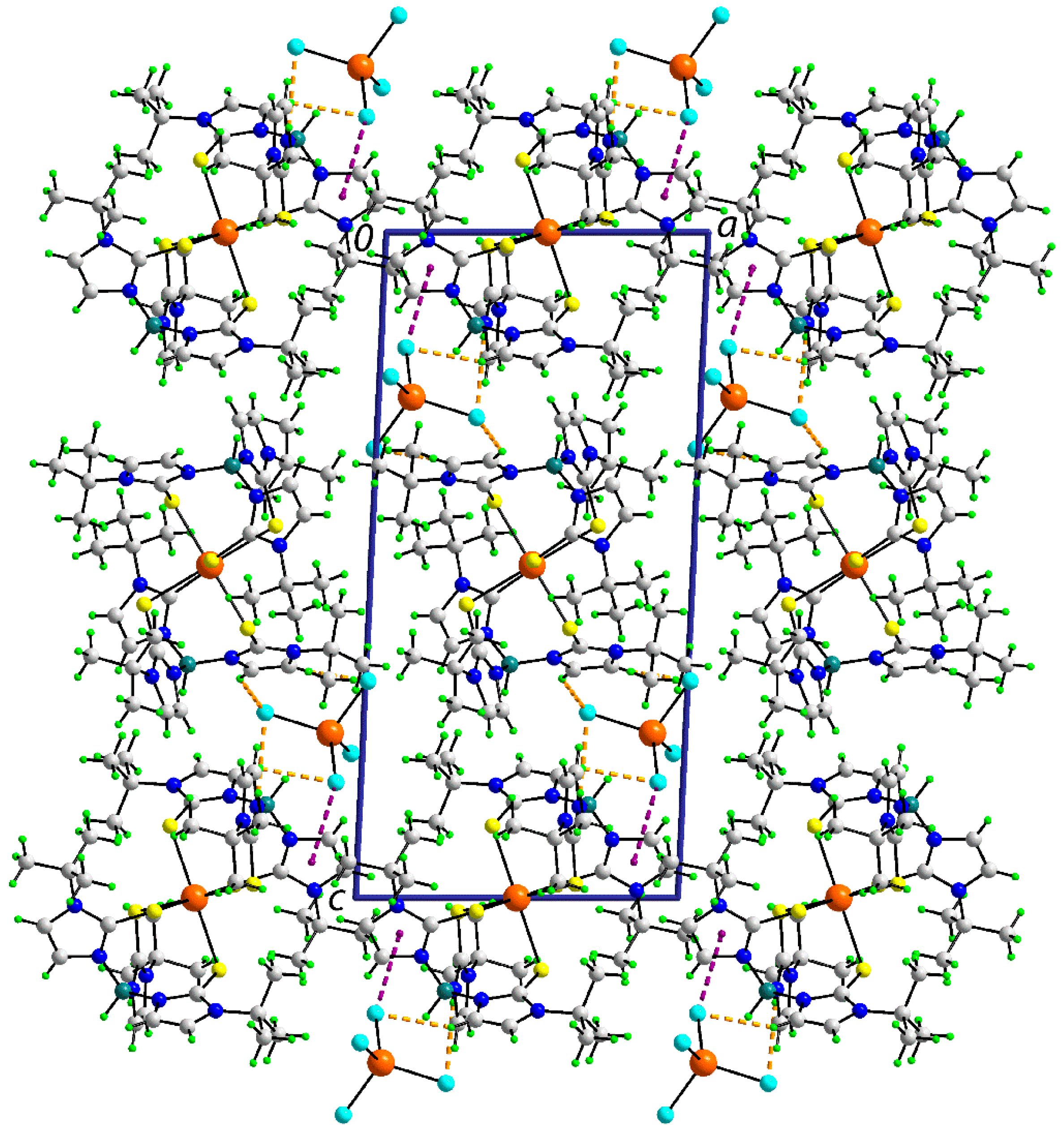
3.2.2. Molecular Packing
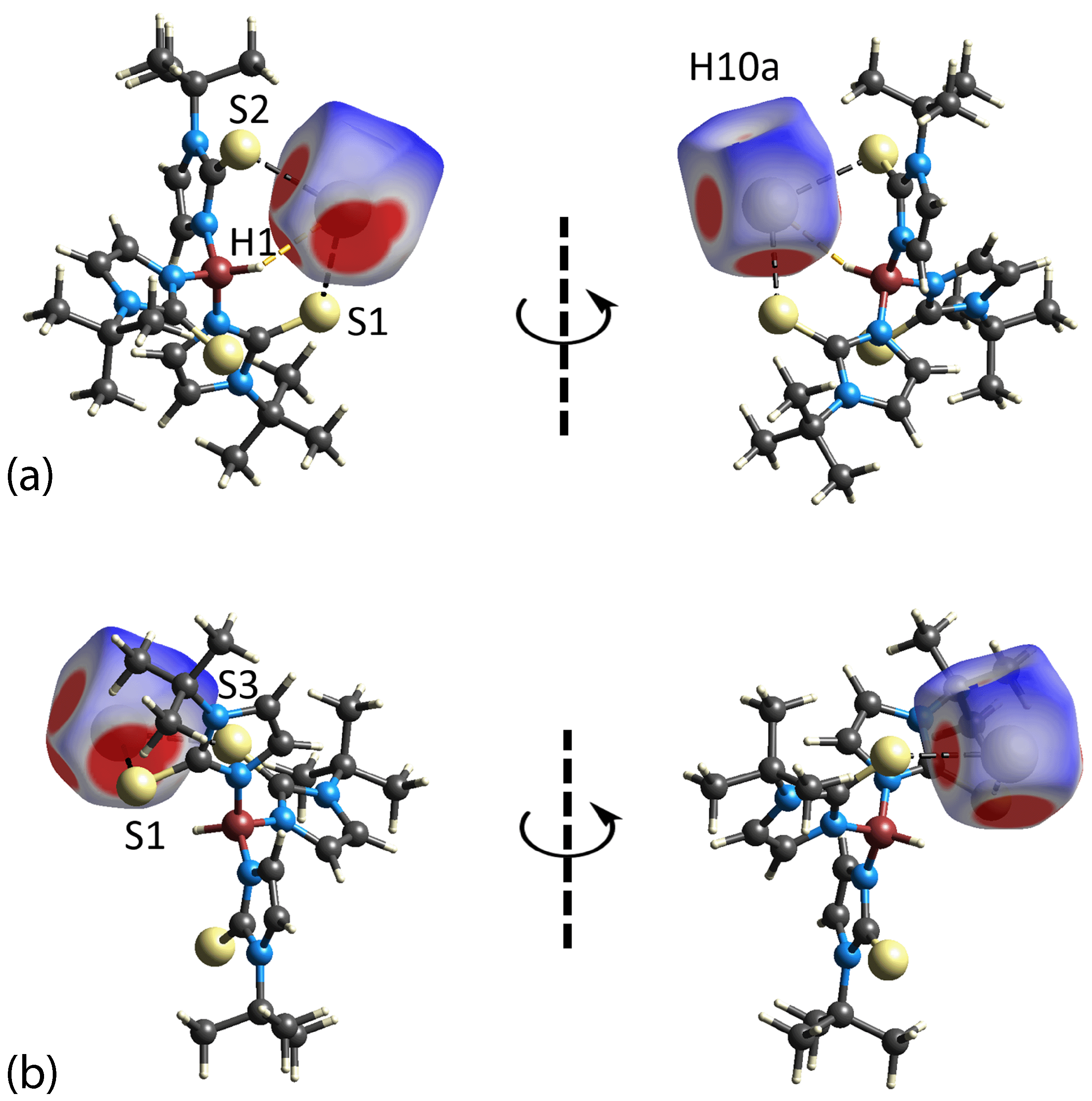
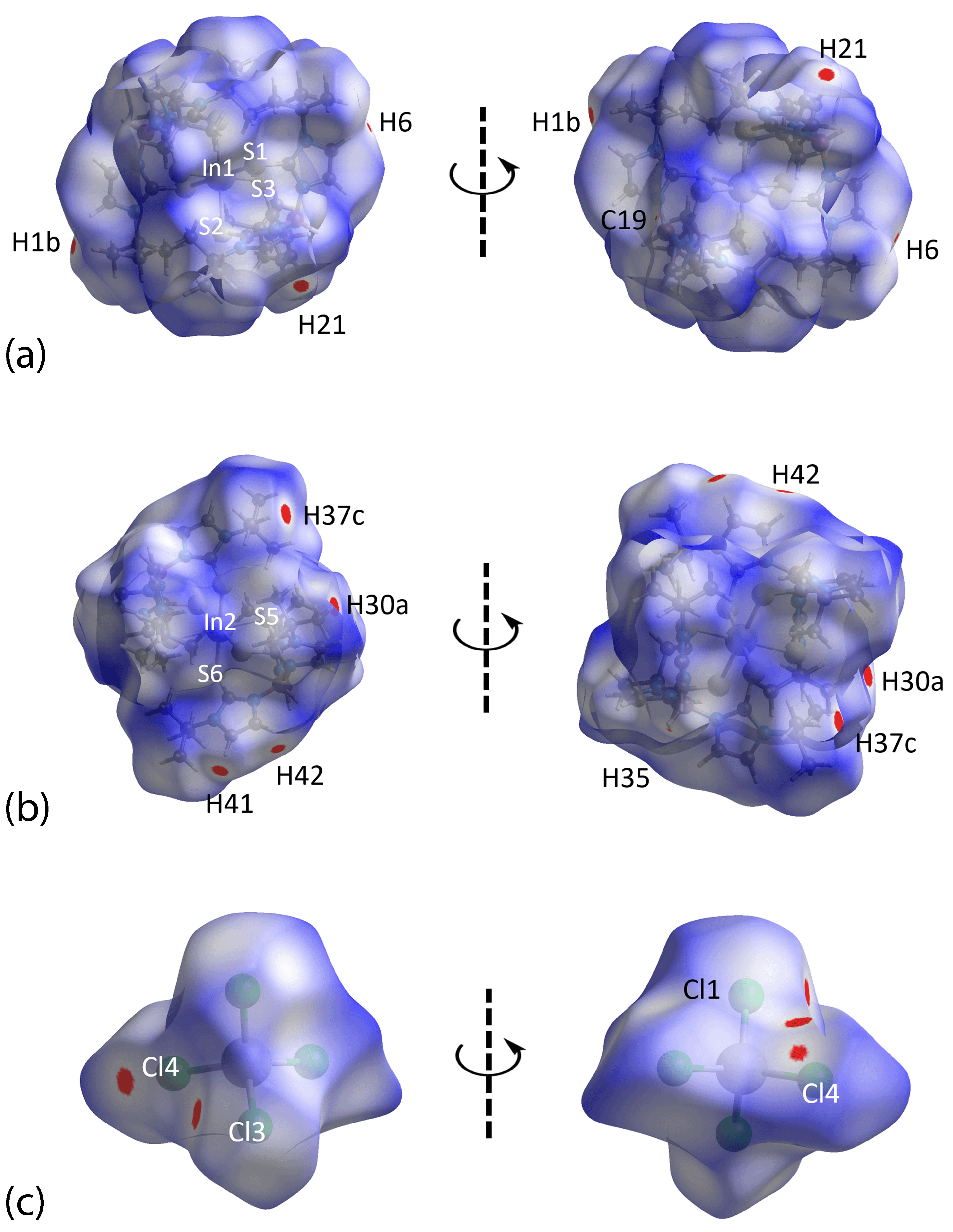
3.3. Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trofimenko, S. Scorpionates: The Coordination Chemistry of Polypyrazolylborate Ligands; Imperial College Press: London, UK, 1999. [Google Scholar]
- Pettinari, C. Scorpionates II: Chelating Borate Ligands—Dedicated to Swiatoslaw Trofimenko; Imperial College Press: London, UK, 2008. [Google Scholar]
- Fujisawa, K.; Tobita, K.; Sakuma, S.; Savard, D.; Leznoff, D.L. Binuclear and mononuclear copper(II) chlorido complexes with hindered neutral N3 type ligands: Influence of ligand framework and charge on their structure and physicochemical properties. Inorg. Chim. Acta 2019, 486, 582–588. [Google Scholar] [CrossRef]
- Lehnert, N.; Fujisawa, K.; Camarena, S.; Dong, H.T.; White, C.J. Activation of non-heme iron-nitrosyl complexes: Turning up the heat. ACS Catal. 2019, 9, 10499–10518. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ono, T.; Okamura, M. Synthesis and characterization of catecholato copper(II) complexes with sterically hindered neutral and anionic N3 type ligands: Tris(3,5-diisopropyl-1-pyrazolyl)methane and hydrotris(3,5-diisopropyl-1-pyrazolyl)borate. Inorganics 2020, 8, 37. [Google Scholar] [CrossRef]
- Fujisawa, K.; Sakuma, S.; Ikarugi, R.; Jose, A.; Solomon, E.I. Thermally stable manganese(III) peroxido complexes with hindered N3 tripodal ligands: Structures and their physicochemical properties. J. Inorg. Biochem. 2021, 225, 111597. [Google Scholar] [CrossRef]
- Trofimenko, S. Scorpionates: Genesis, milestones, prognosis. Polyhedron 2004, 23, 197–203. [Google Scholar] [CrossRef]
- Lennartson, A. Toxic thallium. Nat. Chem. 2015, 7, 610–611. [Google Scholar] [CrossRef]
- Renouf, C. A touch of indium. Nat. Chem. 2012, 4, 862. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, K.; Kuboniwa, A.; Kiss, M.; Szilagyi, R.K. Mono- and binuclear tris(3-tert-butyl-2-sulfanylidene-1H-imidazol-1-yl)hydroborate bismuth(III) dichloride complexes: A soft scorpionate ligand can coordinate to p-block elements. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Steel, G.; Rajasekharan-Nair, R.; Stepek, I.A.; Kennedy, A.R.; Reglinski, J.; Spicer, M.D. Observations on the steric impact of N- and S-donor scorpionate ligands. Eur. J. Inorg. Chem. 2016, 2016, 2409–2412. [Google Scholar] [CrossRef] [Green Version]
- Reglinski, J.; Spicer, M.D. Chemistry of the p-block elements with anionic scorpionate ligands. Coord. Chem. Rev. 2015, 297–298, 181–207. [Google Scholar] [CrossRef] [Green Version]
- Spicer, M.D.; Reglinski, J. Soft scorpionate ligands based on imidazole-2-thione donors. Eur. J. Inorg. Chem. 2009, 2009, 1553–1574. [Google Scholar] [CrossRef]
- Rong, Y.; Palmer, J.H.; Parkin, G. Benzannulated tris(2-mercapto-1-imidazolyl)hydroborato ligands: Tetradentate κ4-S3H binding and access to monomeric monovalent thallium in an [S3] coordination environment. Dalton Trans. 2014, 43, 1397–1407. [Google Scholar] [CrossRef] [Green Version]
- Yurkerwich, K.; Buccella, D.; Melnick, J.G.; Parkin, G. Monovalent indium in a sulfur-rich coordination environment: Synthesis, structure and reactivity of tris(2-mercapto-1-tert-butylimidazolyl)hydroborato indium, [TmBut]In. Chem. Commun. 2008, 28, 3305–3307. [Google Scholar] [CrossRef]
- Kimblin, C.; Bridgewater, B.M.; Hascall, T.; Parkin, G. The synthesis and structural characterization of bis(mercaptoimidazolyl)(pyrazolyl)hydroborato and tris(mercaptoimidazolyl)hydroborato complexes of thallium(I) and thallium(III). J. Chem. Soc. Dalton Trans. 2000, 1267–1274. [Google Scholar] [CrossRef]
- Ojo, J.F.; Slavin, P.A.; Reglinski, J.; Garner, M.; Spicer, M.D.; Kennedy, A.R.; Teat, S.J. The synthesis of soft tripodal ligands: Restrictions on the preparation of hydrotris(thiazolyl)borate anions from borohydride melts. Inorg. Chim. Acta 2001, 313, 15–20. [Google Scholar] [CrossRef]
- Slavin, P.A.; Reglinski, J.; Spicera, M.D.; Kennedy, A.R. Preparation and crystal structure of the [bis{hydrotris(methimazolyl)borato}thallium(III)] cation: Modulated chemistry resulting from the use of soft and hard tripodal ligands. J. Chem. Soc. Dalton Trans. 2000, 239–240. [Google Scholar] [CrossRef]
- Yurkerwich, K.; Yurkerwich, M.; Parkin, G. Synthesis and structural characterization of tris(2-mercapto-1-adamantylimidazolyl)hydroborato complexes: A sterically demanding tripodal [S3] donor ligand. Inorg. Chem. 2011, 50, 12284–12295. [Google Scholar] [CrossRef]
- Dodds, C.A.; Reglinski, J.; Spicer, M.D. Lower main-group element complexes with a soft scorpionate ligand: The structural influence of stereochemically active lone pairs. Chem. Eur. J. 2006, 12, 931–939. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2012. [Google Scholar]
- Kreider-Mueller, A.; Rong, Y.; Owen, J.S.; Parkin, G. Molecular structures of tris(2-mercapto-1-tert-butylimidazolyl)hydroborato and tris(2-mercapto-1-adamantylimidazolyl)hydroborato sodium complexes: Analysis of [TmR] ligand coordination modes and conformations. Dalton Trans. 2014, 43, 10852–10865. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; White, J.L.; Tanski, J.M.; Zakharov, L.N.; Yap, G.P.A.; Incarvito, C.D.; Rheingold, A.L.; Rabinovich, D. Cobalt tris(mercaptoimidazolyl)borate complexes: Synthetic studies and the structure of the first cobaltaboratrane. Dalton Trans. 2004, 1626–1634. [Google Scholar] [CrossRef]
- Kimblin, C.; Bridgewater, B.M.; Churchill, D.G.; Parkin, G. Mononuclear tris(2-mercapto-1-arylimidazolyl)hydroborato complexes of zinc, [TmAr]ZnX: Structural evidence that a sulfur rich coordination environment promotes the formation of a tetrahedral alcohol complex in a synthetic analogue of LADH. Chem. Commun. 1999, 2301–2302. [Google Scholar] [CrossRef]
- Reglinski, J.; Garner, M.; Cassidy, I.D.; Slavin, P.A.; Spicer, M.D.; Armstrong, D.R. Sodium hydrotris(methimazolyl)borate, a novel soft, tridentate ligand: Preparation, structure and comparisons with sodium hydrotris(pyrazolyl)borate. J. Chem. Soc. Dalton Trans. 1999, 2119–2126. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, CrysAlis PRO; Oxford Diffraction Ltd.: Oxfordshire, UK, 2015.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND, Crystal Impact GbR; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Schollmeyer, D.; Shishkin, O.V.; Rühl, T.; Vysotsky, M.O. OH–π and halogen–π interactions as driving forces in the crystal organisations of tri-bromo and tri-iodo trityl alcohols. CrystEngComm 2008, 10, 715–723. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystal. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R.T. Utilizing Hirshfeld surface calculations, non-covalent inter action (NCI) plots and the calculation of inter action energies in the analysis of molecular packing. Acta Crystallogr. Sect. E Crystallogr. Commun. 2019, 75, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]

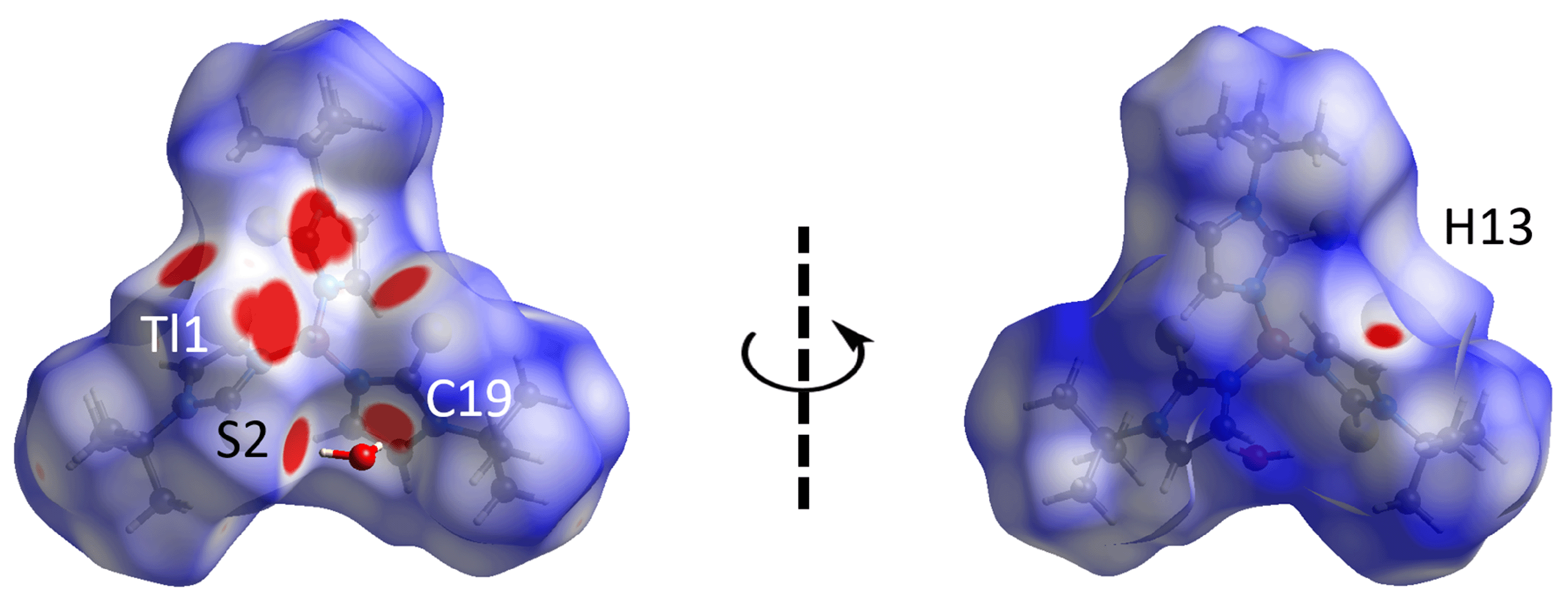
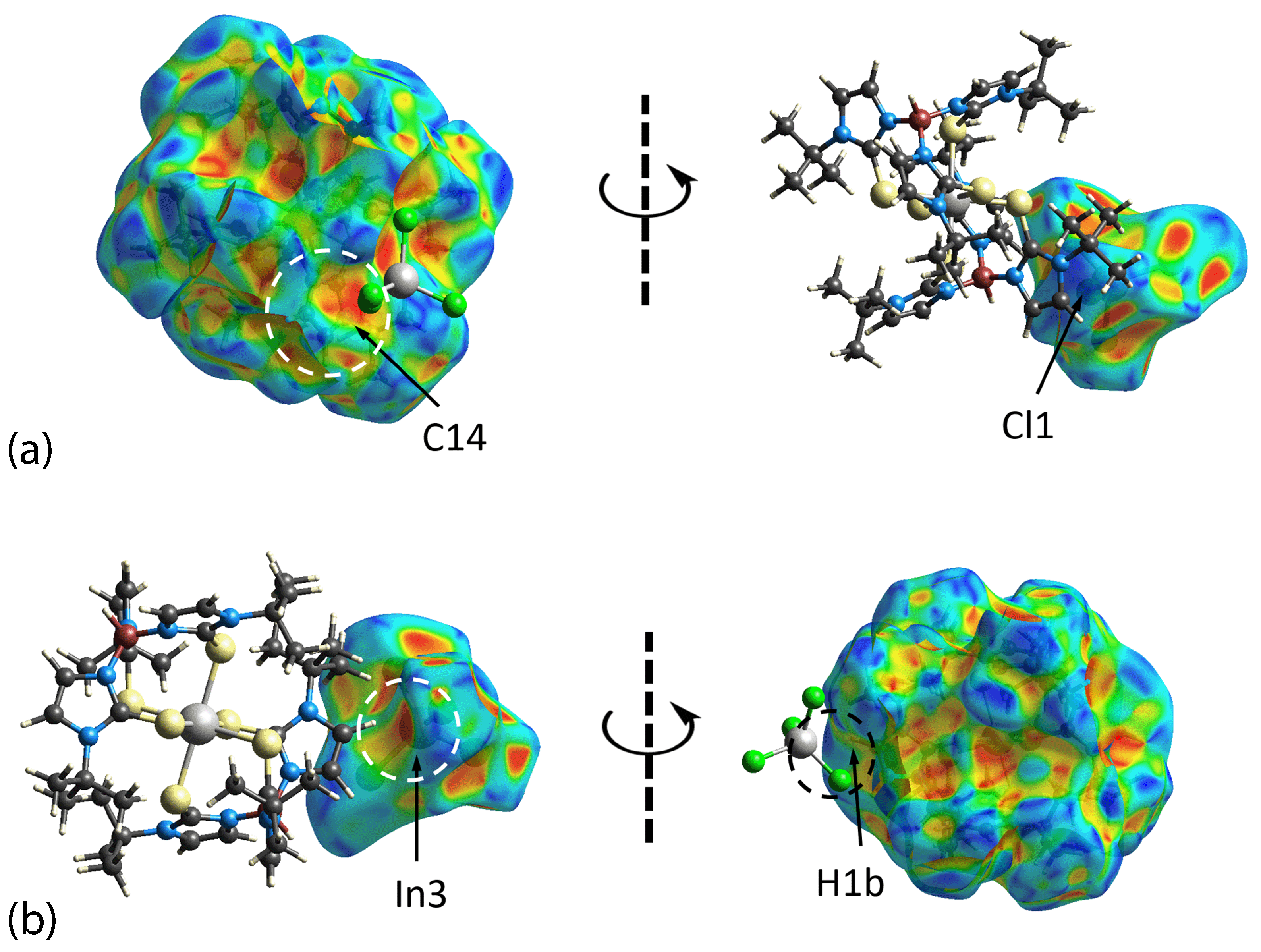
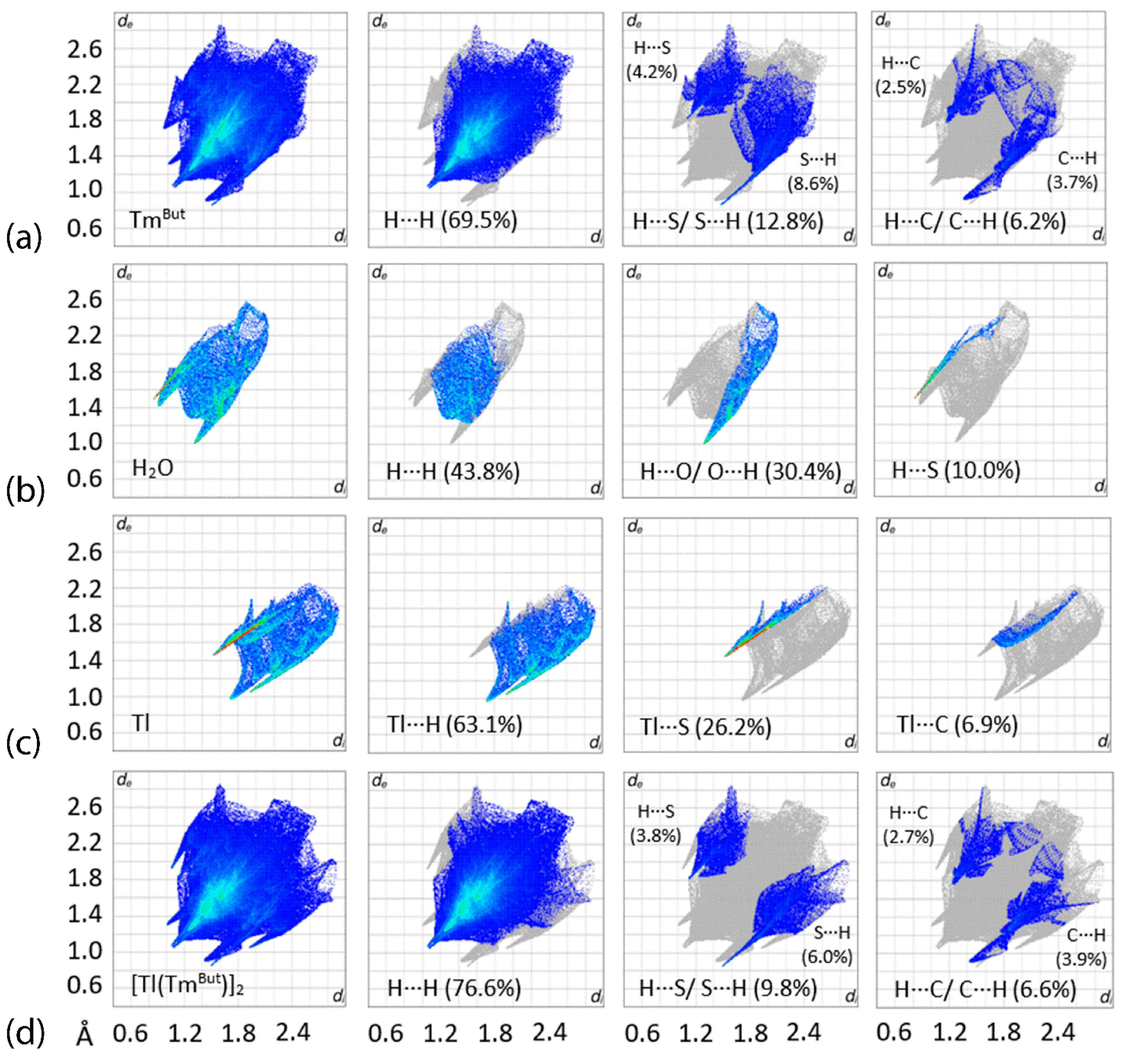
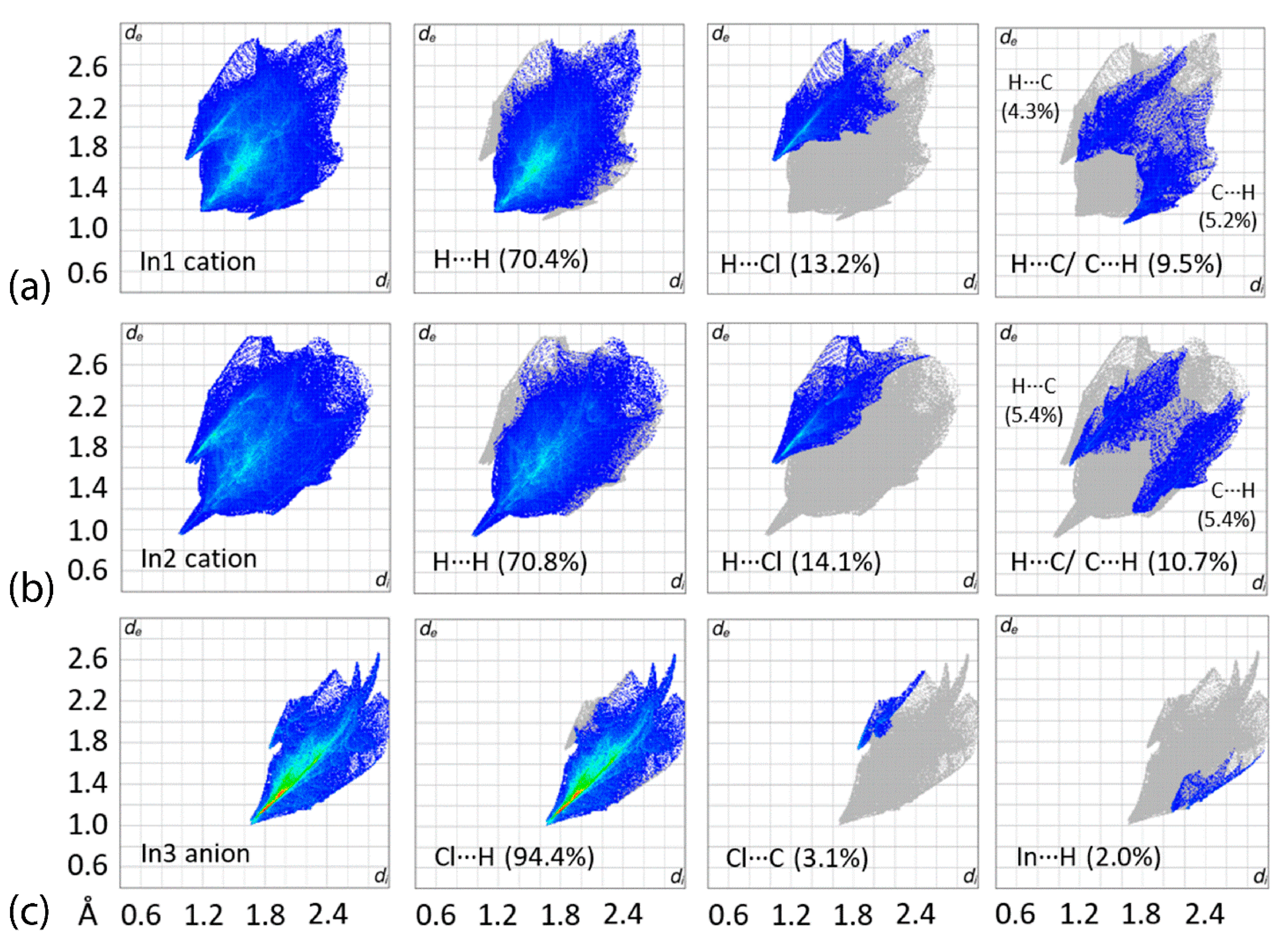
| Complex | [Tl(TmtBu)]2∙2H2O | [In(TmtBu)2](InCl4) |
|---|---|---|
| Formula | C42H68B2N12S6Tl2, 2(H2O) | C42H68B2InN12S6, InCl4 |
| Molecular weight | 1399.86 | 1326.50 |
| Crystal size/mm3 | 0.03 × 0.10 × 0.13 | 0.13 × 0.22 × 0.26 |
| Colour | colorless | colorless |
| Crystal system | triclinic | triclinic |
| Space group | P | P |
| a/Å | 9.5421(2) | 11.1900(1) |
| b/Å | 11.8656(2) | 11.4449(2) |
| c/Å | 14.5839(3) | 23.2153(3) |
| a/° | 67.963(2) | 94.421(1) |
| β/° | 71.203(2) | 92.606(1) |
| γ/° | 73.428(2) | 90.262(1) |
| V/Å3 | 1423.33(6) | 2961.14(7) |
| Z | 1 | 2 |
| Dc/g cm−3 | 1.633 | 1.488 |
| μ/mm−1 | 5.918 | 1.212 |
| Measured data | 48,875 | 101474 |
| θ range/° | 2.5–29.9 | 2.6–29.8 |
| Unique data | 7633 | 15775 |
| Observed data (I ≥ 2.0σ(I)) | 6786 | 14551 |
| No. of parameters | 298 | 616 |
| R, obs. data; all data | 0.032; 0.038 | 0.028; 0.030 |
| a; b in weighting scheme | 0.055; 1.294 | 0.041; 2.674 |
| Rw, obs. data; all data | 0.085; 0.087 | 0.075; 0.076 |
| Range of residual electron density peaks/eÅ−3 | −1.38–2.58 | −0.78–1.76 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Tl1–S1 | 3.0408(9) | S1–Tl1–S3i | 82.04(3) |
| Tl1–S2 | 3.1696(9) | S2–Tl1–S1i | 91.89(2) |
| Tl1–S1i a | 2.9993(10) | S2–Tl1–S3i | 156.32(3) |
| Tl1–S3i a | 3.2764(11) | S1i–Tl1–S3i | 104.23(3) |
| C5–S1 | 1.721(4) | Tl1–S1–Tl1i | 86.10(2) |
| C12–S2 | 1.700(3) | Tl1–S1–C5 | 120.26(12) |
| C19–S3 | 1.694(4) | Tl1–S2–C12 | 83.37(12) |
| S1–Tl1–S2 | 114.53(2) | Tl1–S1i–C5i | 86.11(12) |
| S1–Tl1–S1i a | 93.90(2) | Tl1–S3i–C19i | 127.75(14) |
| (N11,N12,C5-C7)/(N21,N22,C12-C14) | 88.2(3) | (N21,N22,C12-C14)/(N31,N32,C19-C21) | 78.7(3) |
| (N11,N12,C5-C7)/(N31,N32,C19-C21) | 83.2(3) |
| A | H | B | H···B | A···B | A–H···B | Symmetry Operation |
|---|---|---|---|---|---|---|
| O1w | H1w | S2 | 2.47 | 3.2779(10) | 160 | x, y, z |
| O1w | H2w | Cg (1) | 2.47 | 3.2816(18) | 161 | x, y, z |
| C13 | H13 | O1w | 2.46 | 3.214(5) | 136 | −1 + x, y, z |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| In1–S1 | 2.5682(4) | In2–S4 | 2.6110(4) |
| In1–S2 | 2.6597(4) | In2–S5 | 2.6709(5) |
| In1–S3 | 2.6429(4) | In2–S6 | 2.5905(5) |
| C5–S1 | 1.7236(17) | C26–S4 | 1.7223(19) |
| C12–S2 | 1.7279(17) | C33–S5 | 1.7249(19) |
| C19–S3 | 1.7253(17) | C40–S6 | 1.725(2) |
| S1–In1–S2 | 91.715(13) | S4–In2–S5 | 92.567(14) |
| S1–In1–S3 | 93.179(13) | S4–In2–S6 | 95.416(15) |
| S2–In1–S3 | 92.142(13) | S5–In2–S6 | 89.139(15) |
| In1–S1–C5 | 105.90(6) | In2–S4–C26 | 109.61(6) |
| In1–S2–C12 | 107.64(6) | In2–S5–C33 | 106.11(7) |
| In1–S3–C19 | 106.57(6) | In2–S6–C40 | 106.41(7) |
| (N11,N12,C5-C7)/(N21,N22,C12-C14) | 83.99(11) | (N41,N42,C26-C28)/(N21,N22,C12-C14) | 82.84(12) |
| (N11,N12,C5-C7)/(N31,N32,C19-C21) | 89.18(11) | (N41,N42,C26-C28)/(N51,N52,C33-C35) | 88.91(13) |
| (N21,N22,C12-C14)/(N31,N32,C19-C21) | 87.98(11) | (N51,N52,C33-C35)/(N61,N62,C40-C42) | 84.43(13) |
| A | H | B | H···B | A···B | A–H···B | Symmetry Operation |
|---|---|---|---|---|---|---|
| C21 | H21 | Cl1 | 2.85 | 3.723 (2) | 154 | x, −1 + y, z |
| C41 | H41 | Cl3 | 2.83 | 3.773 (2) | 174 | 1 − x, − y, 1 − z |
| C42 | H42 | Cl4 | 2.81 | 3.562 (2) | 137 | 1 − x, − y, 1 − z |
| C1 | H1b | Cl4 | 2.83 | 3.797 (2) | 168 | x, y, z |
| In3 | Cl1 | Cg(1) | 3.7819 (11) | 6.0631 (9) | 162.17 (3) | x, −1 + y, z |
| Contact | Distance (Å) | ΣvdW (Å) | Δ(ΣvdW—Distance) | Symmetry Operation |
|---|---|---|---|---|
| [Tl(TmtBu)]2∙2H2O | ||||
| H1w···S2 | 2.37 | 2.89 | 0.52 | x, y, z |
| H2w···C19 | 2.35 | 2.79 | 0.44 | x, y, z |
| H13···O1w | 2.34 | 2.61 | 0.27 | − 1 + x, y, z |
| [In(TmtBu)2](InCl4) | ||||
| H41···Cl3 | 2.40 | 2.84 | 0.44 | 1 − x, − y, 1 − z |
| H21···Cl1 | 2.49 | 2.84 | 0.35 | x, − 1 + y, z |
| H37c···H30a | 1.93 | 2.18 | 0.25 | 1 + x, y, z |
| H42···Cl4 | 2.72 | 2.84 | 0.12 | 1 − x, − y, 1 − z |
| H1b···Cl4 | 2.73 | 2.84 | 0.11 | x, y, z |
| H6···Cl4 | 2.78 | 2.84 | 0.06 | x, y, z |
| H35···C19 | 2.76 | 2.79 | 0.03 | x, y, z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Kuboniwa, A.; Tan, S.L.; Tiekink, E.R.T. Structural and Hirshfeld Surface Analysis of Thallium(I) and Indium(III) Complexes of a Soft Scorpionate Ligand. Crystals 2023, 13, 745. https://doi.org/10.3390/cryst13050745
Fujisawa K, Kuboniwa A, Tan SL, Tiekink ERT. Structural and Hirshfeld Surface Analysis of Thallium(I) and Indium(III) Complexes of a Soft Scorpionate Ligand. Crystals. 2023; 13(5):745. https://doi.org/10.3390/cryst13050745
Chicago/Turabian StyleFujisawa, Kiyoshi, Ayaka Kuboniwa, Sang Loon Tan, and Edward R. T. Tiekink. 2023. "Structural and Hirshfeld Surface Analysis of Thallium(I) and Indium(III) Complexes of a Soft Scorpionate Ligand" Crystals 13, no. 5: 745. https://doi.org/10.3390/cryst13050745
APA StyleFujisawa, K., Kuboniwa, A., Tan, S. L., & Tiekink, E. R. T. (2023). Structural and Hirshfeld Surface Analysis of Thallium(I) and Indium(III) Complexes of a Soft Scorpionate Ligand. Crystals, 13(5), 745. https://doi.org/10.3390/cryst13050745







